INTRODUCTION
In 2010, the number of individuals under custody in US correctional facilities exceeded 2·3 million [Reference Glaze1] and, considering that more than 600 000 prisoners are released into the community each year [Reference Glaze1–Reference Lagan and Levin3], correctional facilities are likely to play an integral role in community health by serving as important reservoirs for infection [Reference Freudenberg2, Reference Hota4, Reference Okano and Blower5]. Studies have shown that high-risk populations such as those incarcerated have increased incidence of skin and soft tissue infection associated with methicillin-resistant Staphylococcus aureus (MRSA) [6–Reference David8]. However, it is necessary to further clarify what factors have contributed to these increases [6, 7, Reference Maree9, Reference Wootton10].
The majority of investigations of MRSA in jails and prisons have been conducted during outbreaks [6, 7, Reference Maree9, Reference Wootton10], but risk factors associated with endemic colonization in correctional facilities have not been well defined [Reference David8, Reference Farley11, Reference Lowy12]. Furthermore, there is limited information on how MRSA and methicillin-sensitive Staphylococcus aureus (MSSA) strains are introduced into prison facilities. To address these gaps in knowledge, the present study adds important new information on the prevalence of, and risk factors for, colonization with MSSA and MRSA in inmates upon entry into two New York State (NYS) maximum-security prisons.
METHODS
Sample and setting
Participants were inmates entering two NYS maximum-security prisons, Sing Sing Correctional Facility and Bedford Hills Correctional Facility, between 2 November 2009 and 10 January 2011. Sing Sing, located in Ossining, NY, USA, has the capacity to house about 1800 male inmates. Bedford Hills, located in Bedford Hills, NY, USA, has the capacity to house about 900 female inmates. Bedford Hills is a reception centre and therefore the majority of inmates entering this facility come from county and city jails. In contrast, the majority of inmates entering Sing Sing are transferred from other NYS prisons. Jails house inmates awaiting trial and/or sentencing of <1 year. Prisons are state- or federally operated facilities housing convicted felons and individuals with a sentence >1 year [Reference Harrison and Beck13]. Eligible inmates for this study were those able to provide informed consent, aged ⩾16 years, and entering the initial intake process at either facility (i.e. newly entering that facility). Recidivist inmates at either facility who had previously been incarcerated were also included. Participation was voluntary, each participant signed an informed consent document, and compensation was not provided. This study was approved by the Institutional Review Boards of the NYS Department of Corrections and Community Supervision (DOCCS) and Columbia University Medical Center.
Data collection procedure
Data were collected by four trained research associates from Columbia University. Eighty-nine per cent of eligible inmates entering Bedford Hills and 80% of eligible inmates entering Sing Sing provided written informed consent and agreed to participate in the study. After obtaining informed consent, anterior nares and oropharyngeal samples were collected using rayon-tipped swabs (Becton Dickinson, USA) and interviews were conducted via a structured questionnaire.
The questionnaire was designed based on previously identified risk factors for S. aureus colonization and infection in the community and in incarcerated populations [6, Reference David8, Reference Farley11, 14]. Demographic information, as well as information about medical history and risk behaviours, was collected. Specifically, questionnaire items were designed to obtain information about type and location of residence, occupation, and use of athletic equipment during the previous 6 months; sharing of personal items including towels, clothing, razors, and soap; and number of showers taken per week. Items pertaining to medical history included self-perceived general health; diagnoses of diabetes, heart condition, pulmonary condition, kidney disease, liver disease, cancer, HIV/AIDS, and skin condition (eczema, acne, dermatitis, psoriasis); history of any skin infection which was defined as either having answered yes to one of three questions (i.e. having a skin boil that drained pus, an insect bite which caused a boil or sore, or having a skin infection similar to one pictured in the information pamphlet that was distributed to the inmates); history of S. aureus infection; and use of oral or topical antibiotics, steroids, and nasal spray during the previous 6 months. Additional items pertaining to personal history and risk behaviours included tattoo and piercing history; sexual activity in the previous 6 months; tobacco consumption and substance use, including injection drug use; participation in religious, social, educational, gang-related, and sports groups; and involvement in fights during the previous 6 months. The original questionnaire was pilot-tested for 1 month with 51 inmates; based on the pilot test, items were adjusted for clarity (data collected during the pilot test were not included in the analysis reported here).
Microbiological and molecular analyses
Microbiological and molecular characterization of isolates was carried out as previously described [Reference Lee15, Reference Milheirico, Oliveira and de Lencastre16]. Briefly, isolates were enriched in 6% salt broth overnight in order to maximize bacterial culture yield. Isolates confirmed as S. aureus using coagulase and protein A detection (Murex Staphaurex, USA) were characterized by spa typing. Ridom Staph Type software (Ridom GmbH, Germany) was used to compare the isolates [Reference Harmsen17] and isolates were clustered using parameters for Based Upon Repeat Patterns (BURP) as determined by Mellman et al. [Reference Mellmann18]. Staphylococcal chromosomal cassette mec typing was performed on isolates to determine methicillin resistance using multiplex polymerase chain reaction [Reference Milheirico, Oliveira and de Lencastre16].
Statistical analysis
Because inmates entering the men's and women's prisons were representative of two different populations, separate analyses were performed for each prison. S. aureus colonization prevalence and potential risk factors for S. aureus colonization were evaluated at the individual level; all recovered isolates were included in the spa type analysis (and therefore included more than one isolate per individual for those inmates colonized in both the nares and oropharynx) and these were evaluated at the isolate level.
Bivariate analyses were performed to assess differences in inmate characteristics by S. aureus colonization status using χ 2 tests, Fisher's exact tests or Student's t tests, as appropriate. Bivariate analyses were also conducted to compare colonization rate of S. aureus and MRSA by sending facility (for those facilities that had transferred ⩾10 inmates within the study population). To avoid expressing the association in odds ratios, which overestimates the prevalence ratio when the outcome is not rare (as is the case here), adjusted prevalence ratios of S. aureus colonization by the risk factors of interest and 95% confidence intervals were estimated using multivariable Poisson regression with a robust error variance. Variables associated with S. aureus colonization at P⩽0·20 based on bivariate analysis were included in the multivariable regression analysis, with S. aureus colonization as the outcome variable. In order to identify factors associated with overall S. aureus colonization, as well as factors specifically associated with MRSA colonization, two comparisons were conducted separately: (1) inmates colonized with any S. aureus were compared with non-colonized inmates, and (2) inmates colonized with MRSA were compared with inmates colonized with MSSA.
For the analyses, social group membership was defined as regularly playing sports with the same group of inmates, spending time socially with the same group of inmates, or being in a gang within the previous 6 months. The distributions of age were different at the two prisons. Age was included in the regression analyses in tertiles: 16 to <29 (reference group), 29 to <43, and ⩾43 years for women, and 17 to <28 (reference group), 28 to <38, and ⩾38 years for men. All tests were two-sided, and variables were considered significant at P ⩽0·05 in multivariable regression analysis. Analyses were conducted using SAS software version 9.2 (SAS Institute Inc., USA).
RESULTS
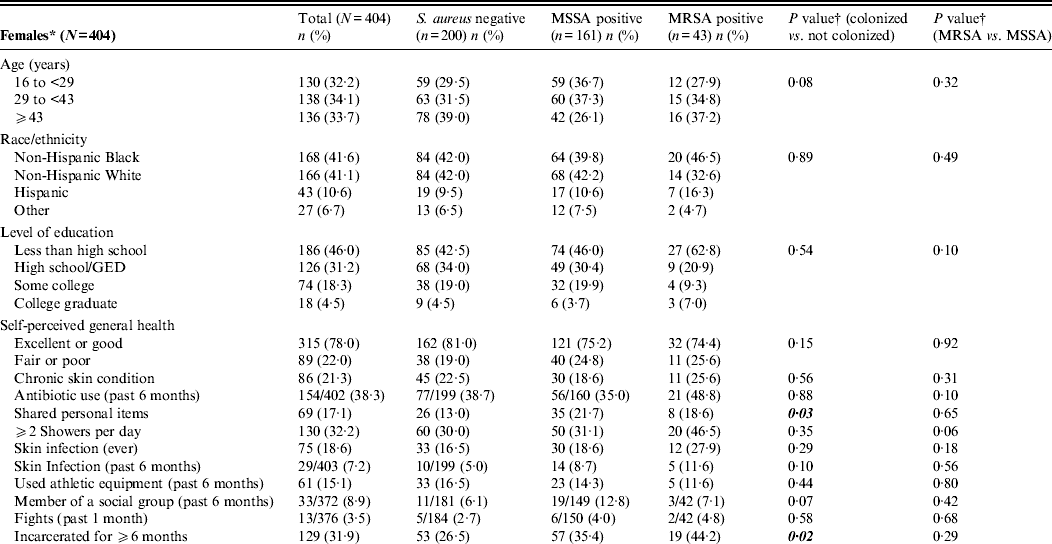
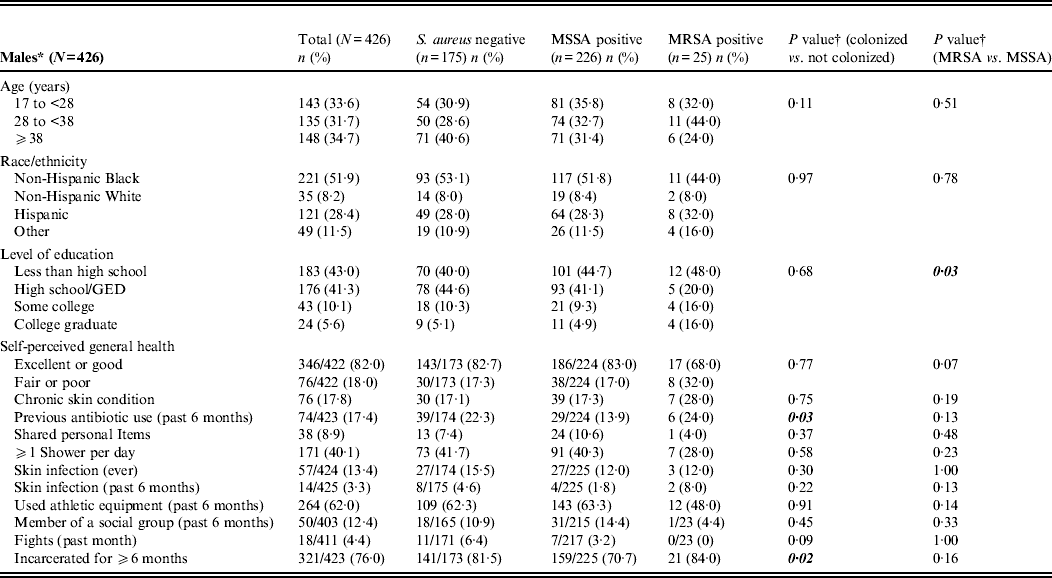
A total of 426 male and 404 female inmates were included in the analysis. The majority of inmates entering the female prison were transferred from city and county jails (96·3%). The remaining female inmates were transferred from other prisons. Of the inmates who were admitted from jails, the largest proportion came from the jail located near Manhattan (Rikers Island, 37·9%), 33·5% came from eight other county jails, and 28·5% were admitted from 38 other jails, each representing <2·2% of the inmates admitted to the female prison. In contrast, most inmates entering the male prison were transferred from other prisons (96·7%). Of the inmates who were admitted from prisons, 63·1% came from a centralized hub serving as one of the major processing centres for DOCCS in NYS, 19·8% came from seven other prisons, and 17·1% were admitted from 23 other prisons, each representing <2·4% of the inmates admitted to the male prison. The majority of female inmates were self-described as either non-Hispanic Black (41·6%) or non-Hispanic White (41·1%); male inmates were identified as being predominantly non-Hispanic Black (51·9%) or Hispanic (28·4%) (Table 1).
Table 1. Characteristics of female and male inmates entering prison with and without S. aureus colonization
MRSA, Methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; GED, Graduate Educational Development.
* Denominators are shown for missing data.
† Significant P values (P ⩽0·05) are in bold italics.
Prevalence of S. aureus colonization
The total prevalence of S. aureus nasal and/or oropharyngeal colonization in inmates entering prison was 50·5% for women and 58·3% for men; 10·6% of women and 5·9% of men were colonized with MRSA at either or both body sites. Of inmates colonized at only one site, the prevalence of S. aureus colonization in the oropharynx was higher than in the anterior nares (18·3% oropharyngeal and 13·6% nasal at the women's prison, and 24·7% oropharyngeal and 15·3% nasal at the men's prison) (Table 2).
Table 2. Prevalence of S. aureus colonization among inmates entering prison
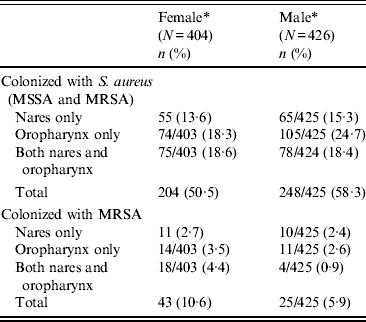
MRSA, Methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
* Denominators are shown for missing data.
Factors associated with S. aureus colonization
Frequency of S. aureus colonization did not vary by sending facility for either men or women (data not shown). Race/ethnicity and level of education did not differ significantly between female or male inmates who were or were not colonized with S. aureus. Based on bivariate analysis, self-reported risk factors for S. aureus colonization varied between male and female inmates (Table 1). Female inmates who were colonized with S. aureus upon entry to prison were more likely to report being incarcerated for at least 6 months (P = 0·02) and sharing personal items (P = 0·03) such as hair brushes, combs, clothing, towels, deodorant, footwear, and nail clippers. Colonized female inmates were also more likely to report being in a social group (P = 0·07). In the multivariable regression analysis, incarceration for at least 6 months was associated with S. aureus colonization (P = 0·008) in female inmates. In addition, colonized female inmates were younger (P = 0·04) and were more likely to report fair/poor general health (P = 0·02) (Table 3).
Table 3. Multivariable Poisson regression for S. aureus colonization (MRSA and MSSA) in female inmates entering prison

CI, Confidence interval; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
* Only significant findings are presented here. The adjusted multivariable regression model also included shared personal items, skin infection within the past 6 months, and social group membership.
† Significant P values (P ⩽0·05) are in bold italics.
Male inmates colonized with S. aureus were less likely to report being incarcerated for at least 6 months (P = 0·02) and to report taking oral antibiotics in the previous 6 months (P = 0·03), but those associations were not significant when evaluated using multivariable regression analysis (data not shown).
Factors associated with MRSA colonization
Although MRSA colonization was not significantly associated with any individual sending facility for men or women, the prevalence of MRSA colonization was greater in female inmates admitted to Bedford Hills from the major jail in the NY City metropolitan area (Rikers Island), compared to female inmates admitted from all other jails sending ⩾10 female inmates (P = 0·07). No variation in MRSA colonization by sending facility was found for men.
Differences in self-reported risk factors were not statistically significant for incoming female inmates who were colonized with MRSA compared to those who were colonized with MSSA. Female inmates colonized with MRSA were more likely to report taking at least two showers per day while incarcerated (P = 0·06). Multivariable regression analysis, however, showed no statistically significant associations between MRSA colonization and risk characteristics in women entering prison.
In bivariate analyses for men, higher education and fair/poor general health were significantly associated with MRSA colonization (P = 0·03, P = 0·07, respectively). As for women, no risk characteristics were found to be significantly associated with MRSA colonization when evaluated using multivariable regression analysis.
spa-type analysis
Overall, 90 MRSA isolates from both prison facilities had 26 different spa types; these were further classified using BURP analysis into eight spa clonal complexes (CCs); one isolate was not spa typable (Fig. 1). spa-CC008 was the most common spa-CC identified at both prisons, with spa type 008 (USA300) and USA300-related strains accounting for 64·3% of nasal and 75·0% of oropharyngeal MRSA isolates in women, and 71·4% of nasal and 73·3% of oropharyngeal MRSA isolates in men. In addition, spa-CC002 was another common spa-CC at the women's prison, with spa type 002 (USA100) and USA100-related strains accounting for 20·7% of nasal and 21·9% of oropharyngeal MRSA isolates in women.
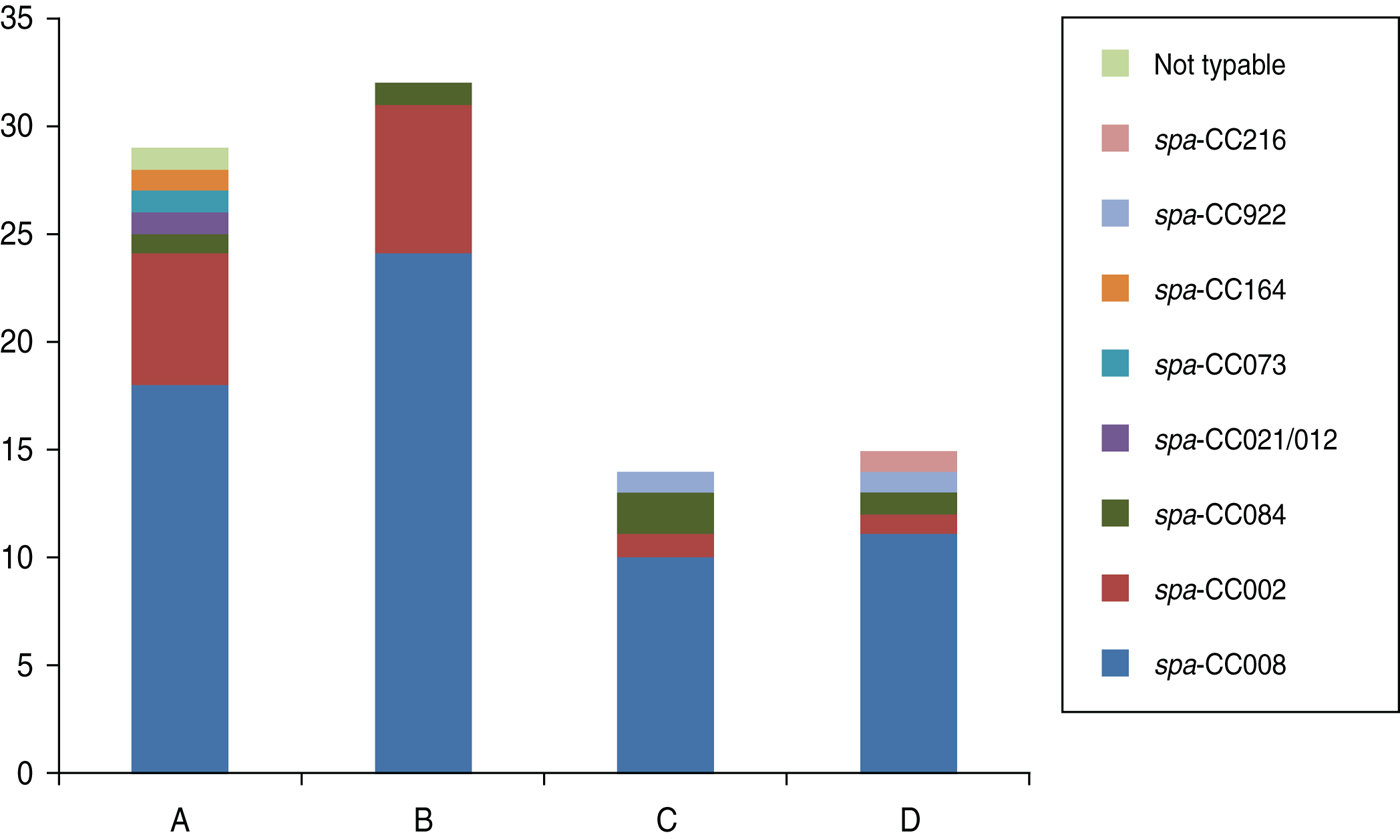
Fig. 1. Diversity of spa clonal complexes (CCs) for (A) nasal methicillin-resistant Staphylococcus aureus (MRSA) isolates from the women's prison (n = 29); (B) oropharyngeal MRSA isolates from the women's prison (n = 32); (C) nasal MRSA isolates from the men's prison (n = 14); (D) oropharyngeal MRSA isolates from the men's prison (n = 15). Based Upon Repeat Patterns (BURP) analysis was used to classify 26 spa types into eight spa-CCs.
Increased genetic diversity was observed for MSSA isolates. Overall, 521 MSSA isolates from both prison facilities were found to have 173 different spa types, and BURP analysis was used to further classify isolates into 11 spa-CCs, four clusters with no spa type founder, and 26 singletons (representing 13 unique spa types). Ten MSSA isolates (representing eight unique spa types) were excluded from the analysis because they contained fewer than five repeats; 16 MSSA isolates were not spa typable. The most common spa-CCs at both facilities were spa-CC008 (17·5% nasal and 10·8% oropharyngeal MSSA isolates in women, 19·7% nasal and 15·4% oropharyngeal MSSA isolates in men), spa-CC002 (14·4% nasal and 19·8% oropharyngeal MSSA isolates in women, 13·9% nasal and 20·4% oropharyngeal MSSA isolates in men) and spa-CC084 (24·7% nasal and 30·6% oropharyngeal MSSA isolates in women, 13·9% nasal and 25·3% oropharyngeal MSSA isolates in men). However, the distribution of other common spa-CCs varied according to colonization site and facility (data not shown).
DISCUSSION
This study provides a comprehensive analysis of prevalence and risk factors for colonization with S. aureus of incoming prisoners at one male and one female maximum-security prison in NYS. These findings extend, and are consistent with, our previously reported findings of disparate nasal and oropharyngeal S. aureus colonization in incoming prisoners [Reference Lee15]. Although several studies have described risk factors and prevalence of S. aureus colonization in incarcerated populations, the majority of those studies were conducted in jails, and the studies conducted in jails and prisons were limited to outbreak settings [6, 7, Reference Maree9, Reference Farley11, Reference Pan19].
In the present study, the prevalence of S. aureus nasal colonization was similar to that reported in earlier studies in the community and correctional facilities [Reference David8, Reference Farley11, Reference Lowy12, Reference Lee15, Reference Felkner20]. Moreover, when nasal and oropharyngeal colonization were combined, the prevalence of S. aureus was higher than what has been found in the community. This may reflect differences in sampling and/or processing of samples. The prevalence of MRSA colonization in inmates was high and exceeded that in the community, which has previously been estimated to be 1–1·5% [Reference Graham, Lin and Larson21]. Moreover, we found that colonization with S. aureus was higher in females than males and that risk factors for MSSA or MRSA colonization varied between male and female inmates.
David and colleagues found that inmates jailed for >2 months were significantly more likely to be colonized with MRSA compared to individuals who spent <2 months in jail [Reference David8]. In our study, the length of incarceration was significantly associated with S. aureus colonization in female inmates only. The association may reflect the fact that the women's prison is a reception centre where newly admitted inmates come from jails as opposed to other prisons. Since Bedford Hills serves as the only reception centre in NYS for female inmates admitted into the prison system from jails, length of previous incarceration for women in jail may explain its observed positive association with MRSA colonization [14]. In contrast, the male prison predominately receives inmates who have been within the prison system; the length of previous incarceration for men was typically time spent in other prisons. Additionally, prevalence of MRSA colonization was higher in female inmates from Rikers than from all other jail facilities, suggesting that jails may vary in their potential to serve as reservoirs. Jail facilities house inmates awaiting trial with short sentences while prison houses individuals that have been convicted and sentenced for long periods of time [Reference Harrison and Beck13]. On average the turnover rate for a 1000-bed jail is 36 times per year admitting a total population of 36 000 inmates. In contrast, for a 1000-bed prison, the turnover rate is usually 750 beds per year, totalling a population of 1750 inmates [22]. These findings suggest that because of the short sentences and rapid turnover, jails are a likely reservoir for MSSA or MRSA colonization and infection.
Previous research in jails has shown that inmates colonized with S. aureus were more likely to be younger [Reference Farley11, Reference Palavecino23]. In our study younger age of women was also associated with S. aureus colonization. Earlier studies in jails and the community suggest that particular race/ethnicities may have increased rates of colonization and infection with MRSA [Reference Farley11, 24, Reference Groom25]. In this study, however, race/ethnicity was not associated with colonization. Compared with other studies [6, Reference David8, Reference Farley11, 14], we identified fewer risk factors for MSSA or MRSA colonization, possibly because a large proportion of inmates in the current study already had a number of risk factors such as poor hygiene, illicit drug use, prior incarceration, and crowded living situations [Reference Hota4, Reference Lowy12, Reference Charlebois26].
Moreover, spa type 008 (USA300), the most common strain associated with MRSA infection and colonization in the community, was also the most common subtype of MRSA isolates in both prisons. Also, in the women's prison, spa type 002 (USA100), a frequent nosocomial pathogen, was another common strain. Taken together, this suggests the potential for transmission of spa type 008 (USA300) and spa type 002 (USA100) strains into and within prisons by newly admitted inmates from the community. There is also the potential for MRSA to be reintroduced into the community by the prison inmates being released or paroled.
One limitation of this study is that data regarding potential risk factors were self-reported. While it may have been possible to verify some data using prison medical records, such records were not consistently available for all inmates and some medical records had not been maintained for a sufficient period of time. Nevertheless, inmates' responses were unlikely to differ according to colonization status. Therefore, misclassification would have been non-differential thereby leading to an underestimate of the association between inmate characteristics and S. aureus colonization.
A second limitation is the cross-sectional design; it was not possible to ensure that the exposures (inmate characteristics) preceded the outcome (colonization with MSSA and/or MRSA). False negative results may have occurred in the assessment of the role of some risk factors which were less common and our statistical power was limited. Last, detection of transient MSSA or MRSA carriers could have been missed despite sampling of the nares and oropharynx. Although these body sites are generally accepted as appropriate areas to obtain samples, evidence is mounting to suggest the importance of other body sites, especially for spa type 002 (USA300), to obtain an accurate colonization profile. Reservoirs for MSSA or MRSA colonization may be located in other areas of the body such as the groin and axilla [Reference Peters27–Reference Miko30].
Detection and prevention strategies to reduce infections caused by S. aureus may be improved by targeting those at highest risk of colonization. The overall prevalence of MRSA colonization in inmates in this study (8·2%) was nearly 10 times greater than in the general population [Reference Graham, Lin and Larson21]. Furthermore, risk factors for S. aureus colonization upon entry into prison were found to vary by gender. Control of epidemic S. aureus in prisons will have to take into account the constant introduction of these strains by newly entering inmates. Future studies will need to evaluate the transmission dynamics of S. aureus within the correctional setting because of the high prevalence of S. aureus colonization in inmates entering prison.
ACKNOWLEDGEMENTS
We thank the NYS DOCCS. For DOCCS general support we thank: Commissioner Brian Fischer, Assistant Commissioner for Health Services Elizabeth Ritter, Superintendents Philip Heath and Sabina Kaplan, Deputy Superintendents Marjorie Byrnes, Kevin Winship, and Michael Capra, Margret Franklin, Randi Uszak, Jon Hansen, Francis Akinyombo, Brian Lane, Paul Korotkin, Drs Lori Goldstein, Denise Sepe, and Bani Choudry. For Columbia University general support we thank, Dr Nowai Keleekai. We also express our gratitude to the inmates for their participation. This work was supported by National Institute of Allergy and Infectious Diseases, National Institutes of Health (R01AI82 536).
DECLARATION OF INTEREST
None.







