In his presentation, Prof. Delange critically reviewed the scientific data on iodine requirements during pregnancy, lactation and the neonatal periodReference Delange1. In addition, he summarised the current worldwide experience of using measurements of the concentration of thyroid stimulating hormone (TSH) in blood from neonates as a tool to evaluate and control iodine deficiency disordersReference Delange1. My response addresses these two main topics.
Iodine requirements during pregnancy, lactation and the neonatal period
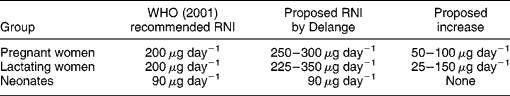
Table 1 compares the current WHO recommended nutrient intake (RNI) of iodine during pregnancy, lactation and the neonatal period2, and Prof. Delange's proposed new iodine intakes. Since no change is proposed in the RNI for neonates, I will not address this group.
Table 1 The current World Health Organization (WHO) recommended nutrient intake (RNI) of iodine2 and the new amounts proposed by DelangeReference Delange1.
There are two main ways to judge the intake of iodine by pregnant and lactating women: measuring the excretion of iodine in urine, and by estimating physiological needs.
Estimating the RNI from data on urinary iodine excretion
As Prof. Delange points out, the data in Table 1 of his paperReference Delange1 reveal a wide range of iodine intakes by pregnant women (as estimated by the urinary iodine concentration) both in countries where the population is deemed to be iodine sufficient (145–786 μg day− 1) and in populations of iodine deficient countries (24–255 μg day− 1). It is interesting that where comparisons can be made, the average iodine intake of pregnant women is greater by approximately 35–45 μg day− 1 compared with the general population. This may be because a proportion of these women take iodine in a prenatal micronutrient supplement during their pregnancy. However, I agree that these ranges are too wide to allow any conclusions to be made about the RNI during pregnancy. An examination of the data Delange presents in Table 2Reference Delange1 leads to the same conclusion regarding lactation.
Estimates of the RNI based on physiology
Several studies have confirmed an increase in maternal thyroxine production during pregnancy, which necessitates an increase in maternal iodine intakeReference Glinoer3. The increased production of thyroxine results from several factors including: oestrogen stimulation of thyroxine binding globulin (TBG) leading to an increase in the reservoir of maternal thyroxine (T4); the transfer of thyroxine to the foetus; and the activity of 5-deiodinase III in the placenta, which is likely to result in the transfer of iodine to the foetus. A study has shown that athyreotic women need on average a 50% increase in their dose of levothyroxine (l-T4) during pregnancyReference Mandel, Larson, Seely and Brent4. This study also provided evidence of a 50–75 μg day− 1 increase in l-T4 production, equivalent to a need for an extra 30–50 μg day− 1 of iodine by a pregnant woman.
In a similar manner, there is an increase in the maternal iodine requirement during lactation. Using a breast milk iodine content of 150–180 μg l− 1 and an estimated daily consumption of 0.5 l of milk by a neonate and 1.5 l by an infant, a lactating mother would need an increase of approximately 0.5 l × 150 μg l− 1 = 75 μg day− 1 for a neonate and 1.5 l × 150 μg l− 1 = 225 μg day− 1 for an infant. If one adds these figures to the RNI for an adult of 150 μg day− 1 recommended by the WHO, a lactating woman would need a total of 225–375 μg iodine day− 1. These calculations are very close to Prof. Delange's estimated need for an extra 25–150 μg day− 1 of iodine, giving an RNI of 225–350 μg day− 1 of iodine during lactation (Table 1).
The safety of the recommended higher dose
Is there any risk of adverse effects of the recommended intake of 250–300 μg iodine day− 1 during pregnancy? A Danish study, which examined the thyroid function of babies born to women in a population with an estimated iodine intake of 75 μg day− 1 was compared with babies born to women who were supplemented with 150 μg day− 1 of iodine to give an estimated total iodine intake of 225 μg day− 1 . The study found a higher cord blood TSH in the supplemented group (9.00 vs. 7.07 mIU l− 1, P < 0.05)Reference Nohr and Laurberg5. The investigators took this to be evidence that ‘the foetal thyroid is sensitive to the inhibitory effects of iodine’. However, the concentration of free T4 in cord blood was higher in neonates born to supplemented compared with unsupplemented mothers (12.5 vs. 11.7 pmol l− 1, P < 0.05), the concentrations of total T4 and T3 were similar, and the T4 thyroglobulin concentration was lower (34.3 vs. 56.7 μg dl− 1, P < 0.001)Reference Nohr and Laurberg5. These appear to be positive effects on the babies born to iodine supplemented mothers. The 1988–1994 National Health and Nutrition Examination Survey (NHANES III) in the USA reported a high serum TSH concentration in persons with a urinary iodine concentration >500 μg g− 1 creatinineReference Hollowell, Staehling and Flanders6. A concentration of urinary iodine >1000 μg g− 1 creatinine was associated with a serum TSH concentration >4.5 mIU per l Reference Hollowell, Staehling and Flanders6.
Conclusions
I evaluate that the amount of iodine required by pregnant-women is 50 μg day− 1 greater than the current WHO recommendation of 200 μg day− 1, which is at the lower end of the additional 50–100 μg day− 1 estimated by Prof DelangeReference Delange1. A total intake of 250–300 μg day− 1 iodine during pregnancy appears safe. For lactating-women, I estimate the requirement is for an extra 25 μg iodine day− 1 when breastfeeding neonates, and up to an additional 175 μg iodine day− 1 when breastfeeding infants. These estimates are nearly identical with Prof Delange's estimate of an increase of 25–150 μg iodine day− 1.
Screening neonatal TSH concentration to monitor iodine deficiency and sufficiency
Prof. Delange makes the important point that the neonatal TSH concentration is a reflection of the sufficiency of brain thyroid hormones, and indirectly of iodine intake during pregnancy. He summarises the evidence that the cord blood TSH concentration correlates with other measures of iodine sufficiency, such as urinary iodine concentration, and concludes that the frequency of neonates who have a TSH concentration >5 mIU l− 1 in whole blood is < 3% in iodine sufficient populations. I think that the following issues need to be considered:
Most screening programmes for newborn babies collect heel prick blood and not cord blood.
The number of days after birth at which the blood sample is taken will greatly affect the percentage of babies with a TSH concentration >5 mIU l− 1. There is a surge in TSH concentration to 60–80 mIU l− 1 shortly after birth, and the TSH concentration generally does not fall to < 5 mIU l− 1 until 3–5 days of ageReference Fisher, Brown, Braverman and Utiger7.
The gestational age and/or the birth weight of the neonate affect the surge in TSH concentration; the postnatal TSH concentration is lower in preterm and low-birth weight babiesReference Murphy, Hume, van Toor, Matthews, Ogston, Wu, Visser and Williams8.
The use of topical iodine on the mother during pregnancy or on the neonate increases the TSH concentration in the neonateReference Chanoine, Boulvain, Bourdoux, Pardou, Van Thi, Ermans and Delange9.
In programmes that measure the T4 concentration in the blood of neonates as a primary screening test and if it is below a specified threshold, such as 10th percentile, measure the TSH concentration, then the percentage of neonates with a TSH concentration >5 mIU l− 1 is likely to be higher than if the TSH concentration was the primary screening tool measured on the entire population.
Different TSH assays show a variation in results of up to 15%10.
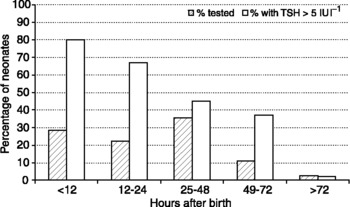
Figure 1 shows the percentage of neonates with a TSH concentration of >5 mIU l− 1 depending on how soon after birth the sample of blood was collected using data from the newborn screening programme in Oregon in the USA, an area considered to be iodine sufficient, with a mean urinary iodine concentration estimated at 210 μg per dayReference LaFranchi11. As in many hospitals in the USA, infants are discharged from hospital soon after birth, which explains the low percentage who were tested more than 2 days after birth; 85% of specimens were collected by 48 h of age, a time when the majority neonates have a TSH concentration >5 mIU l− 1. After 72 h of age, the average TSH concentration was 1.8 mIU l− 1. A similar study in Sydney, Australia, reported that in the subgroup of infants, whose specimens were collected when they were >72 h of age, 6.3% had a TSH concentration >5 mIU per lReference McElduff, McElduff, Gunton, Hams, Wiley and Wilcken12. Although the population of Sydney is considered to be iodine sufficient by current WHO standards, with an average intake of 160 μg day− 1, the population might be of borderline sufficiency.
Fig. 1 Data from the Oregon Newborn Screen Programme showing the percentage of neonates from whom heel prick blood was collected according to the time after delivery, and the percentage with a concentration of TSH in whole blood of >5 IU l− 1. The percentage with a high TSH concentration declines within the first 72 h after birth, but 86% of neonates are tested before they are 48-h-old because of the tendency to discharge mothers as soon after delivery as possible.
A study in Atlanta, USA, where the estimated daily intake of iodine is 155 μg day− 1, illustrates the effect of topical iodine. This population, in which 98% of mothers and/or neonates were reported to have been exposed to topical iodine, 82.3% of cord blood TSH concentrations were >5 mIU l− 1, when compared with 42.9% in whole blood taken at 3 days of ageReference Copeland, Sullivan, Houston, May, Mendoza, Salamatullah, Solomons, Nordenberg and Maberly13.
The Danish study, in which some mothers took prenatal iodine supplements, 68% of cord blood TSH concentrations were >5 mIU l− 1 in babies born to unsupplemented mothers, while 88% were >5 mIU l− 1 in babies born to mothers who received an extra 150 μg day− 1 of iodineReference Nohr and Laurberg5. The investigators speculated that iodine supplements taken by women, whose intake was borderline, may have an inhibitory effect on foetal TSH productionReference Nohr and Laurberg5.
Finally, an internet search using the US National Library of Medicine PubMed literature search facility disclosed that 57 of 194 countries have some form of a newborn screening program for hypothyroidism.
Conclusions
The concentration of TSH in cord blood gives more consistent results than values measured in heel prick blood, which vary considerably depending on how soon after birth they are taken, and on the gestational age and birth weight of the neonate. The majority of screening programmes, however, obtain heel prick blood specimens after birth. The determination of the TSH concentration in blood specimens collected >72 h of age are most informative, particularly if values can be correlated with the mother's urinary iodine concentration. Even without this correlation, relative changes in the percentage of values of neonatal TSH concentration >5 mIU l− 1 before and after an intervention, such as improved salt iodisation, will provide useful information.
The countries that most need monitoring have not yet established newborn screening programmes. In these countries, it might be possible to collect a representative sample of cord blood specimens (~200) to measure the TSH concentration, with the following caveats:
i. Specimens should be taken from full-term, normal birth weight babies.
ii. Neither mother nor neonate should have been exposed to excessive iodine.
iii. The TSH concentration should be measured by a central laboratory using a standard assay.
iv. Consideration should be given to measuring the concentration of thyroglobulin, which may be a better marker of iodine sufficiency than TSH.
Finally, more data are needed from countries, whose population have a borderline intake of iodine, on the concentration of TSH in cord blood or blood collected from neonates born to woman who received iodine supplements during pregnancy.
In his presentation, Prof. Delange critically reviewed the scientific data on iodine requirements during pregnancy, lactation and the neonatal periodReference Delange1. In addition, he summarised the current worldwide experience of using measurements of the concentration of thyroid stimulating hormone (TSH) in blood from neonates as a tool to evaluate and control iodine deficiency disordersReference Delange1. My response addresses these two main topics.
Iodine requirements during pregnancy, lactation and the neonatal period
Table 1 compares the current WHO recommended nutrient intake (RNI) of iodine during pregnancy, lactation and the neonatal period2, and Prof. Delange's proposed new iodine intakes. Since no change is proposed in the RNI for neonates, I will not address this group.
Table 1 The current World Health Organization (WHO) recommended nutrient intake (RNI) of iodine2 and the new amounts proposed by DelangeReference Delange1.
There are two main ways to judge the intake of iodine by pregnant and lactating women: measuring the excretion of iodine in urine, and by estimating physiological needs.
Estimating the RNI from data on urinary iodine excretion
As Prof. Delange points out, the data in Table 1 of his paperReference Delange1 reveal a wide range of iodine intakes by pregnant women (as estimated by the urinary iodine concentration) both in countries where the population is deemed to be iodine sufficient (145–786 μg day− 1) and in populations of iodine deficient countries (24–255 μg day− 1). It is interesting that where comparisons can be made, the average iodine intake of pregnant women is greater by approximately 35–45 μg day− 1 compared with the general population. This may be because a proportion of these women take iodine in a prenatal micronutrient supplement during their pregnancy. However, I agree that these ranges are too wide to allow any conclusions to be made about the RNI during pregnancy. An examination of the data Delange presents in Table 2Reference Delange1 leads to the same conclusion regarding lactation.
Estimates of the RNI based on physiology
Several studies have confirmed an increase in maternal thyroxine production during pregnancy, which necessitates an increase in maternal iodine intakeReference Glinoer3. The increased production of thyroxine results from several factors including: oestrogen stimulation of thyroxine binding globulin (TBG) leading to an increase in the reservoir of maternal thyroxine (T4); the transfer of thyroxine to the foetus; and the activity of 5-deiodinase III in the placenta, which is likely to result in the transfer of iodine to the foetus. A study has shown that athyreotic women need on average a 50% increase in their dose of levothyroxine (l-T4) during pregnancyReference Mandel, Larson, Seely and Brent4. This study also provided evidence of a 50–75 μg day− 1 increase in l-T4 production, equivalent to a need for an extra 30–50 μg day− 1 of iodine by a pregnant woman.
In a similar manner, there is an increase in the maternal iodine requirement during lactation. Using a breast milk iodine content of 150–180 μg l− 1 and an estimated daily consumption of 0.5 l of milk by a neonate and 1.5 l by an infant, a lactating mother would need an increase of approximately 0.5 l × 150 μg l− 1 = 75 μg day− 1 for a neonate and 1.5 l × 150 μg l− 1 = 225 μg day− 1 for an infant. If one adds these figures to the RNI for an adult of 150 μg day− 1 recommended by the WHO, a lactating woman would need a total of 225–375 μg iodine day− 1. These calculations are very close to Prof. Delange's estimated need for an extra 25–150 μg day− 1 of iodine, giving an RNI of 225–350 μg day− 1 of iodine during lactation (Table 1).
The safety of the recommended higher dose
Is there any risk of adverse effects of the recommended intake of 250–300 μg iodine day− 1 during pregnancy? A Danish study, which examined the thyroid function of babies born to women in a population with an estimated iodine intake of 75 μg day− 1 was compared with babies born to women who were supplemented with 150 μg day− 1 of iodine to give an estimated total iodine intake of 225 μg day− 1 . The study found a higher cord blood TSH in the supplemented group (9.00 vs. 7.07 mIU l− 1, P < 0.05)Reference Nohr and Laurberg5. The investigators took this to be evidence that ‘the foetal thyroid is sensitive to the inhibitory effects of iodine’. However, the concentration of free T4 in cord blood was higher in neonates born to supplemented compared with unsupplemented mothers (12.5 vs. 11.7 pmol l− 1, P < 0.05), the concentrations of total T4 and T3 were similar, and the T4 thyroglobulin concentration was lower (34.3 vs. 56.7 μg dl− 1, P < 0.001)Reference Nohr and Laurberg5. These appear to be positive effects on the babies born to iodine supplemented mothers. The 1988–1994 National Health and Nutrition Examination Survey (NHANES III) in the USA reported a high serum TSH concentration in persons with a urinary iodine concentration >500 μg g− 1 creatinineReference Hollowell, Staehling and Flanders6. A concentration of urinary iodine >1000 μg g− 1 creatinine was associated with a serum TSH concentration >4.5 mIU per l Reference Hollowell, Staehling and Flanders6.
Conclusions
I evaluate that the amount of iodine required by pregnant-women is 50 μg day− 1 greater than the current WHO recommendation of 200 μg day− 1, which is at the lower end of the additional 50–100 μg day− 1 estimated by Prof DelangeReference Delange1. A total intake of 250–300 μg day− 1 iodine during pregnancy appears safe. For lactating-women, I estimate the requirement is for an extra 25 μg iodine day− 1 when breastfeeding neonates, and up to an additional 175 μg iodine day− 1 when breastfeeding infants. These estimates are nearly identical with Prof Delange's estimate of an increase of 25–150 μg iodine day− 1.
Screening neonatal TSH concentration to monitor iodine deficiency and sufficiency
Prof. Delange makes the important point that the neonatal TSH concentration is a reflection of the sufficiency of brain thyroid hormones, and indirectly of iodine intake during pregnancy. He summarises the evidence that the cord blood TSH concentration correlates with other measures of iodine sufficiency, such as urinary iodine concentration, and concludes that the frequency of neonates who have a TSH concentration >5 mIU l− 1 in whole blood is < 3% in iodine sufficient populations. I think that the following issues need to be considered:
Most screening programmes for newborn babies collect heel prick blood and not cord blood.
The number of days after birth at which the blood sample is taken will greatly affect the percentage of babies with a TSH concentration >5 mIU l− 1. There is a surge in TSH concentration to 60–80 mIU l− 1 shortly after birth, and the TSH concentration generally does not fall to < 5 mIU l− 1 until 3–5 days of ageReference Fisher, Brown, Braverman and Utiger7.
The gestational age and/or the birth weight of the neonate affect the surge in TSH concentration; the postnatal TSH concentration is lower in preterm and low-birth weight babiesReference Murphy, Hume, van Toor, Matthews, Ogston, Wu, Visser and Williams8.
The use of topical iodine on the mother during pregnancy or on the neonate increases the TSH concentration in the neonateReference Chanoine, Boulvain, Bourdoux, Pardou, Van Thi, Ermans and Delange9.
In programmes that measure the T4 concentration in the blood of neonates as a primary screening test and if it is below a specified threshold, such as 10th percentile, measure the TSH concentration, then the percentage of neonates with a TSH concentration >5 mIU l− 1 is likely to be higher than if the TSH concentration was the primary screening tool measured on the entire population.
Different TSH assays show a variation in results of up to 15%10.
Figure 1 shows the percentage of neonates with a TSH concentration of >5 mIU l− 1 depending on how soon after birth the sample of blood was collected using data from the newborn screening programme in Oregon in the USA, an area considered to be iodine sufficient, with a mean urinary iodine concentration estimated at 210 μg per dayReference LaFranchi11. As in many hospitals in the USA, infants are discharged from hospital soon after birth, which explains the low percentage who were tested more than 2 days after birth; 85% of specimens were collected by 48 h of age, a time when the majority neonates have a TSH concentration >5 mIU l− 1. After 72 h of age, the average TSH concentration was 1.8 mIU l− 1. A similar study in Sydney, Australia, reported that in the subgroup of infants, whose specimens were collected when they were >72 h of age, 6.3% had a TSH concentration >5 mIU per lReference McElduff, McElduff, Gunton, Hams, Wiley and Wilcken12. Although the population of Sydney is considered to be iodine sufficient by current WHO standards, with an average intake of 160 μg day− 1, the population might be of borderline sufficiency.
Fig. 1 Data from the Oregon Newborn Screen Programme showing the percentage of neonates from whom heel prick blood was collected according to the time after delivery, and the percentage with a concentration of TSH in whole blood of >5 IU l− 1. The percentage with a high TSH concentration declines within the first 72 h after birth, but 86% of neonates are tested before they are 48-h-old because of the tendency to discharge mothers as soon after delivery as possible.
A study in Atlanta, USA, where the estimated daily intake of iodine is 155 μg day− 1, illustrates the effect of topical iodine. This population, in which 98% of mothers and/or neonates were reported to have been exposed to topical iodine, 82.3% of cord blood TSH concentrations were >5 mIU l− 1, when compared with 42.9% in whole blood taken at 3 days of ageReference Copeland, Sullivan, Houston, May, Mendoza, Salamatullah, Solomons, Nordenberg and Maberly13.
The Danish study, in which some mothers took prenatal iodine supplements, 68% of cord blood TSH concentrations were >5 mIU l− 1 in babies born to unsupplemented mothers, while 88% were >5 mIU l− 1 in babies born to mothers who received an extra 150 μg day− 1 of iodineReference Nohr and Laurberg5. The investigators speculated that iodine supplements taken by women, whose intake was borderline, may have an inhibitory effect on foetal TSH productionReference Nohr and Laurberg5.
Finally, an internet search using the US National Library of Medicine PubMed literature search facility disclosed that 57 of 194 countries have some form of a newborn screening program for hypothyroidism.
Conclusions
The concentration of TSH in cord blood gives more consistent results than values measured in heel prick blood, which vary considerably depending on how soon after birth they are taken, and on the gestational age and birth weight of the neonate. The majority of screening programmes, however, obtain heel prick blood specimens after birth. The determination of the TSH concentration in blood specimens collected >72 h of age are most informative, particularly if values can be correlated with the mother's urinary iodine concentration. Even without this correlation, relative changes in the percentage of values of neonatal TSH concentration >5 mIU l− 1 before and after an intervention, such as improved salt iodisation, will provide useful information.
The countries that most need monitoring have not yet established newborn screening programmes. In these countries, it might be possible to collect a representative sample of cord blood specimens (~200) to measure the TSH concentration, with the following caveats:
i. Specimens should be taken from full-term, normal birth weight babies.
ii. Neither mother nor neonate should have been exposed to excessive iodine.
iii. The TSH concentration should be measured by a central laboratory using a standard assay.
iv. Consideration should be given to measuring the concentration of thyroglobulin, which may be a better marker of iodine sufficiency than TSH.
Finally, more data are needed from countries, whose population have a borderline intake of iodine, on the concentration of TSH in cord blood or blood collected from neonates born to woman who received iodine supplements during pregnancy.