Tea (Camellia sinensis, Theaceae) is the second most popular beverage in the world, next to water. The potential health benefits associated with green tea consumption have been partially attributed to the antioxidative properties of polyphenols, particularly of flavanols (also known as catechins)( Reference Balentine, Wiseman and Bouwens 1 ). Previous studies explored the potential preventive effects of catechins against chronic diseases, such as neurodegenerative diseases and CVD and cancer( Reference Sae-tan, Grove and Lambert 2 – Reference Yang, Maliakal and Meng 4 ). Epigallocatechin-3-gallate (EGCG), the most abundant catechin present in green tea( Reference Bose, Lambert and Ju 5 ), is considered as an important bioactive molecule that may contribute to many of the health influences of green tea, including anti-obesity and anti-diabetic properties( Reference Hursel, Viechtbauer and Westerterp-Plantenga 6 , Reference Rains, Agarwal and Maki 7 ).
On the basis of its potential anti-obesity effect, green tea has been marketed during recent years as an herbal supplement for the control of body weight. However, its efficacy has not been consistently proved, and the potential beneficial effects of EGCG on body-weight control and cardiometabolic disease risk factors remain controversial( Reference Sae-tan, Grove and Lambert 2 , Reference Hsu, Tsai and Kao 8 , Reference Frank, George and Lodge 9 ).
Obesity and its co-morbidities are significant public health issues. Low-energy diets are a cornerstone of the treatment of obese patients. However, weight loss is difficult to achieve and maintain( Reference Elfhag and Rossner 10 ). Loss of body weight with energy restriction consistently results in a decrease in resting energy expenditure( Reference Martin, Heilbronn and de Jonge 11 ). Several reports have shown that green tea can enhance energy expenditure and fat oxidation and, thereby, improve body-weight loss and later maintenance( Reference Rains, Agarwal and Maki 7 ). However, whether reductions in RMR with weight loss are similar when a hypoenergetic diet is supplemented with EGCG is controversial, because studies with green tea extract have been potentially confounded by the concomitant presence of caffeine( Reference Hursel, Viechtbauer and Westerterp-Plantenga 6 , Reference Rains, Agarwal and Maki 7 , Reference Westerterp-Plantenga 12 ).
Conversely, concern has been raised on the safety of the intake of high doses of catechins, which has led to the publication of systematic reviews on the safety of the consumption of green tea polyphenols( Reference Mazzanti, Menniti-Ippolito and Moro 13 , Reference Yellapu, Mittal and Grewal 14 ), including the one by the US Pharmacopeia( Reference Sarma, Barrett and Chavez 15 ). Hence, some reports of adverse hepatic effects associated with the consumption of green tea preparations have been published. Likewise, an extract containing high levels of EGCG and marketed as a weight-loss supplement (Exolise, www.afssaps.sante.fr) was withdrawn from the French and Spanish markets owing to the incidence of several cases of hepatotoxicity after its consumption. Despite the withdrawal of this extract from the market, other green tea-based herbal supplements remain in the market.
The knowledge of a potential modifying influence of EGCG on changes in body composition and cardiometabolic risk factors, as well as in energy and substrate metabolism in obese women achieved by a low-energy diet is limited. Therefore, the purposes of the present study were (1) to examine the effects of dietary supplementation with EGCG on changes in body weight and composition, as well as in cardiometabolic risk factors (insulin resistance, blood lipids and C-reactive protein (CRP)) after a 12-week energy-restricted diet in obese women, (2) to investigate the influence of EGCG consumption on changes in energy and substrate metabolism and (3) to test whether the daily consumption of 300 mg/d of EGCG for a period of 12 weeks would affect liver function in a sample of obese, pre-menopausal, non-diabetic Spanish women.
Experimental methods
Design
The present study was a randomised, double-blind and parallel design with groups matched for BMI and age. The study population was randomly assigned to receive either EGCG (300 mg EGCG/d) or placebo (300 mg lactose/d) dietary supplement, which was consumed three times daily with breakfast, lunch and dinner for a period of 12 weeks. A commercial green tea extract (TEAVIGOTM; DSM Nutritional Products) comprising >97 % of pure EGCG was used in the study.
Women were recruited in 2006 from September to December. The trial was conducted in 2007 from January to December at the Clinical Trials Unit of TECNALIA (Txagorritxu Hospital, Vitoria-Gasteiz). Participants visited the study site before (visit 1), every week during the intervention period and after the 12-week energy-restricted diet (visit 12) with the last EGCG dose consumed the evening before visit 12. They were instructed not to consume any other beverage containing catechins or caffeine during the intervention programme. On visits 1 and 12 of the intervention period, subjects completed a schedule of anthropometric measurements, body composition measurements, calorimetry assessment, blood draws and questionnaires, as described below.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Ethical Committee in Hospital of Txagorritxu (Vitoria-Gasteiz). All women received verbal and written information about the nature and purpose of the study, and all of them gave written consent for participation in the study.
Randomisation and blindness
Participants were stratified by age (18 to < 34 or ≥ 34 to 49 years) and BMI category ( ≥ 30 to < 35 or ≥ 35 to 40 kg/m2), and randomised to either the control group or the EGCG group using a stratified block design. The random allocation sequence was generated by an independent statistician. A medical doctor enrolled and assigned the participants to the intervention groups following the allocation sequence. Supplement packs were prepared and labelled by a third party who had no further involvement in any aspect of the study in order to conceal treatment allocation from the study staff (Department of New Products Food, LEIA Foundation, Vitoria, Spain). The participants, dietitians and scientists in charge of measurements and determinations were blind to the treatments throughout the trial. The primary study outcome was the reduction in body weight. Sample size calculations were done using power analysis based on previous obesity study reports. More than 80 % power at a two-tailed α level of 0·05 was provided to test the significance of weight reduction (effect size 5 %) over placebo with a minimum of thirty-one subjects per group.
Participants
All women had a stable weight (body-weight changes < 3 kg in the last 3 months), had no history of renal or liver illness, CVD or diabetes, were not pregnant, and had total cholesterol (TC) levels ≤ 7·758 mmol/l, TAG levels ≤ 3·387 mmol/l and blood pressure levels ≤ 140/90 mmHg. Women were also free of medication (except oral contraceptives) for hypertension, hyperlipidaemia, hyperuricaemia or other illness. As polyphenols can reduce the bioavailability of non-haem Fe( Reference Temme and Van Hoydonck 16 ), Fe deficiency and Fe-deficiency anaemia, as assessed by blood Hb, ferritin and Fe levels were considered additional criteria for exclusion.
Women were recruited through a newspaper advertisement. Of the 118 women assessed for eligibility, a total of eighty-eight pre-menopausal (age 19–49 years) obese women (BMI inclusion criteria 30·0–39·9 kg/m2) from Vitoria (North Spain) met the inclusion criteria, initially volunteered to participate in the study and underwent a comprehensive medical examination (Fig. 1). All the women assigned to the EGCG group were enrolled to participate, but two women who were randomly assigned to the control group refused to participate. Another two participants, one from the EGCG group at week 8 and one from the control group at week 9, left the study due to the inability to follow the research protocol (vacations), and one participant dropped from the EGCG group due to pregnancy (dropout rate 3 %).

Fig. 1 Flow of participants. EGCG, epigallocatechin-3-gallate.
Diet
Body-weight reduction was induced by a low-energy mixed (55 % carbohydrates, 30 % lipids and 15 % proteins) diet providing 2510 kJ (600 kcal) of energy less than the individually estimated energy requirements based on measured RMR and multiplied by a factor of 1·3, corresponding to a low physical activity level in both groups. Energy content and macronutrient composition of diets were designed to achieve weight losses of 0·5 to 1 kg per week( Reference Bantle, Wylie-Rosett and Albright 17 , Reference Davis, Emerenini and Wylie-Rosett 18 ), and are considered as a low-risk intervention( Reference Davis, Emerenini and Wylie-Rosett 18 , Reference Wylie-Rosett, Albright and Apovian 19 ). To optimise compliance, dietary instructions were reinforced weekly by a dietitian, and participants received their supplement packs containing the treatment or placebo for 1 week during each visit. The consultation included both nutritional assessment (including 24 h recall) and weighing. Women were advised not to change their physical activity habits during the energy restriction programme.
Anthropometry
Body weight ( ± 10 g) was measured after voiding using a digital integrating scale (SECA 760). Height was measured to the nearest 5 mm using a stadiometer (Seca 220; Seca) at the start of the study. BMI was calculated as weight (kg)/(height)2 (m2). Waist and hip circumferences were measured to the nearest 0·1 cm in triplicate with an anthropometric non-elastic tape (Seca 200; Seca), and the waist:hip ratio was calculated.
Body composition
A dual-energy X-ray absorptiometry scanner 140 (QDR 4500 W; Hologic) with QDR software for Windows version 12.4 was used to estimate fat mass and bone-free lean mass tissue.
Cardiometabolic risk factors
Fasting ( ≥ 12 h overnight fast) blood samples were taken from an antecubital vein after gas exchange measurements. The samples were processed after collection and stored at − 80°C for later analysis. Fasting plasma glucose (mmol/l), TC (mmol/l), HDL-cholesterol (mmol/l) and TAG levels (mmol/l) were measured by an enzymatic spectrophotometric technique with an autoanalyser (COBAS FARA; Roche Diagnostics). Insulin (pmol/l) was measured by ELISA kits (LINCO Research). Insulin sensitivity was assessed by the homeostasis assessment model for insulin resistance (HOMA-IR)( Reference Wallace, Levy and Matthews 20 ). Serum fasting high-sensitivity CRP concentrations were measured by enzyme immunoassay (IBL International GMBH). All the samples were prepared according to the manufacturer's recommendation, measured in duplicate and the mean scored. Blood biochemical data were available from seventy-eight women (94 %) at follow-up (thirty-nine from the EGCG group and thirty-nine from the control group).
Energy metabolism
Respiratory exchange measurements were made by indirect calorimetry (Vmax; Sensormedics) to estimate fasting RMR and non-protein respiratory quotient (NPRQ), following the recommended measurement conditions( Reference Compher, Frankenfield and Keim 21 ) and as described elsewhere( Reference Labayen, Forga and Martinez 22 ). RMR was expressed as kJ/kg body weight per d. Fasting urine samples were collected (between approximately 21.00–22.00 hours of the day before and 08.00–09.00 hours of the examination day) to determine N output, and then lipid oxidation rates were calculated( Reference Labayen, Diez and Parra 23 ). Gas exchange measurement data were available from seventy-eight women (94 %) at follow-up (forty-one from the EGCG group and thirty-seven from the control group).
Liver function
Plasma concentrations of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, γ-glutamyltransferase, bilirubin and urea were measured by enzymatic tests in an automated analyser (Hitachi Modular P800; Roche). All samples were measured in duplicate and the mean scored.
The 2-keto[1-13C]isocaproate oxidation measurement is a helpful tool to assess in vivo liver mitochondrial function( Reference Grattagliano, Lauterburg and Palasciano 24 ). We performed the breath test as described previously( Reference Parra, Gonzalez and Martinez 25 , Reference Ruiz, Lasa and Simon 26 ). Briefly, after an overnight fast, the women received 6·5 μmol/kg of 2-keto[1-13C]isocaproate sodium salt (Euriso-Top) dissolved in 200 ml orange juice. Breath samples were obtained by exhaling through a straw into a tube (Labco) before and at 10 min intervals during 2 h after ingestion of 2-keto[1-13C]isocaproate. Enrichment of 13CO2 in the breath was measured by isotope ratio MS on a BreathMAT plus spectrometer (Finnigan). Results are expressed as the percentage of 2-keto[1-13C]isocaproate oxidised over the two test hours. Measurements of 2-keto[1-13C]isocaproate oxidation were available from thirty-seven women.
Statistical analyses
Data are presented as means and standard deviations. Analyses were performed using SPSS version 20.0 (SPSS, Inc.). Variables with a skewed distribution were logarithmically transformed to obtain a more symmetrical distribution. Group differences at baseline were assessed by a non-paired Student's t test. Baseline v. post changes in the study measurements were assessed using paired t tests. We used ANCOVA for repeated measures to test for the existence of an interaction effect (time × group) between treatment (EGCG) and changes in body weight and composition, energy and substrate metabolism (RMR, NPRQ and lipid oxidation rates), cardiometabolic risk factors (i.e. HOMA-IR, TC, HDL-cholesterol and LDL-cholesterol, TAG and CRP) and liver function estimates (hepatic enzymes, bilirubin, urea and 2-keto[1-13C]isocaproate oxidation), and to calculate differences in change and 95 % CI between the EGCG and control groups at the end of the intervention period. Only data from the women who finished the 12-week diet intervention programme and whose body composition data were available (n 83) were included in the analyses. The final sample did not differ in key characteristics (i.e. age, body weight, BMI or body fat mass) from the original sample (all P>0·1). However, sensitivity analyses were also performed using the last-observation (baseline data)-carried-forward imputation method for the women who did not provide post-test data (n 3, 3·5 %).
Results
Characteristics of the study sample
At baseline, all the body composition measurements (P>0·4; Table 1), cardiometabolic risk factors (P≥ 0·06; Table 2) and liver function estimates (P>0·3; Table 3) were similar in the EGCG and control groups. The intervention in both groups resulted in a significant decrease in body weight, BMI, fat mass, lean mass and waist circumference (P< 0·001). We did not observe any statistically significant difference in RMR and NPRQ (P>0·1), whereas the lipid oxidation rate was substantially reduced after the intervention in both groups (P< 0·001).
Table 1 Effects of dietary supplementation with epigallocatechin-3-gallate (EGCG) on body weight and composition and energy metabolism after a 12-week energy-restricted diet intervention (post) in obese Spanish women (Mean values and standard deviations; differences in change and 95 % confidence intervals (n 83))
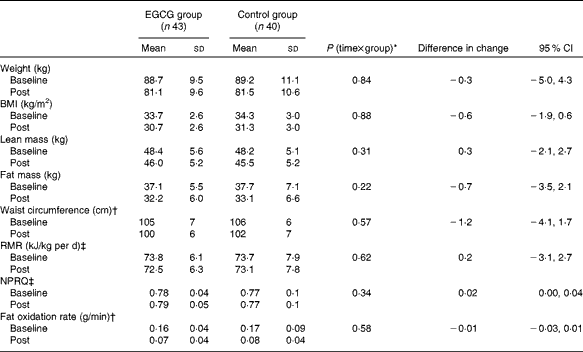
NPRQ, non-protein respiratory quotient.
* Interaction effect between time and treatment group (EGCG or control).
† Analyses were performed with logarithmically transformed data.
‡ RMR and NPRQ data were available from seventy-eight women: forty-one from the EGCG group and thirty-seven from the control group.
Table 2 Effects of dietary supplementation with epigallocatechin-3-gallate (EGCG) on cardiometabolic risk factors after a 12-week energy-restricted diet intervention (post) in obese Spanish women (Mean values and standard deviations; differences in change and 95 % confidence intervals (n 78))
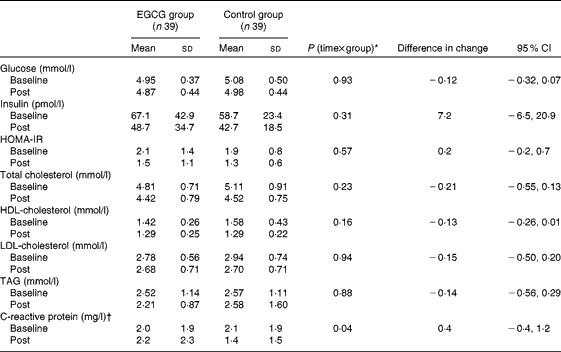
HOMA-IR: homeostasis model assessment for insulin resistance.
* Interaction effect between time and group (EGCG or control).
† Analyses were performed with logarithmically transformed data.
Table 3 Effects of dietary supplementation with epigallocatechin-3-gallate (EGCG) on liver function after a 12-week energy-restricted diet intervention (post) in obese Spanish women (Mean values and standard deviations; differences in change and 95 % confidence intervals (n 78))
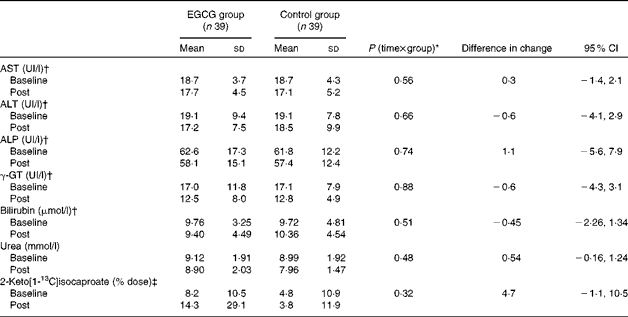
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γ-GT, γ-glutamyltransferase.
* Interaction effect between time and treatment group (EGCG or control).
† Analyses were performed with logarithmically transformed data.
‡ The 2-keto[1-13C]isocaproate breath test was available from thirty-seven women: twenty-one from the EGCG group and sixteen from the control group.
The levels of insulin, HOMA-IR, TC, HDL-cholesterol and LDL-cholesterol were also decreased after the intervention in both groups (P< 0·05), but we did not find any statistically significant change in TAG levels (P>0·2). CRP and glucose levels did not significantly change in the EGCG group (P>0·2), but significantly decreased in the control group after the 12-week intervention (P< 0·05).
The levels of aspartate aminotransferase (P< 0·05), alkaline phosphatase and γ-glutamyltransferase (P< 0·001) significantly decreased in both groups (Table 3). There were no statistically significant changes in alanine aminotransferase, bilirubin and urea levels and in the 2-keto[1-13C]isocaproate oxidation rates either in the EGCG group or the control group (Table 3).
Comparison between the groups for changes in body weight and composition and changes in RMR and substrate metabolism
There were no significant interaction effects between time and treatment group (EGCG or control) on body composition and energy and substrate metabolism outcomes (Table 1). Likewise, we did not observe any significant difference in changes achieved in body weight, BMI, fat mass, lean mass and waist circumference between the EGCG and control groups (P>0·3; Table 1). Similarly, the changes in RMR, NPRQ and whole-body fat oxidation did not significantly differ between the EGCG and control groups (Table 1). The results did not differ when the analyses were repeated using the last-observation (baseline data)-carried-forward imputation method for the women who did not provide post-test data (data not shown).
Comparison between the groups for changes in cardiometabolic risk factors
The results showed no significant interaction effect between time and treatment group (EGCG or control) on cardiometabolic risk factors (Table 2). There were no significant differences in the changes in the cardiometabolic risk factors considered (i.e. glucose, insulin, HOMA-IR, TC, HDL-cholesterol and LDL-cholesterol, and CRP levels) between the EGCG and control groups after the 12-week intervention (Table 2). The results did not differ when the analyses were repeated using the last-observation (baseline data)-carried-forward imputation method for the women who did not provide post-test data (data not shown).
Tolerability and adverse effects
None of the subjects withdrew from the study because of discomfort or adverse effects associated with the treatment. Hepatic function parameters (i.e. alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, γ-glutamyltransferase and urea) were within the normal range both at the beginning and at the end of the intervention trial, and there were no statistically significant differences in the changes observed after the 12-week intervention between the EGCG and control groups (Table 3). Furthermore, there were no significant differences in the 2-keto[1-13C]isocaproate oxidation rates between the EGCG and control groups. The results did not differ when the analyses were repeated using the last-observation (baseline data)-carried-forward imputation method for the women who did not provide post-test data (data not shown).
Discussion
The results of the present study indicate that the intake of 300 mg/d of green tea EGCG for 12 weeks does not enhance the energy-restricted diet-induced changes in body composition, energy and substrate metabolism and cardiometabolic risk factors in obese women. Likewise, we did not observe any significant difference in the reductions of body weight, total and central adiposity, RMR, NPRQ, lipid oxidation rates, insulin, HOMA-IR, TC, HDL-cholesterol and LDL-cholesterol, TAG and CRP levels between the group receiving 300 mg/d of EGCG and the control group after a 12-week energy-restricted diet intervention. The results also show that the intake of 300 mg/d of EGCG during the 12 weeks of intervention had no adverse effect on liver function enzymes in obese women. It should be noted that the results of the present study are based on a well-characterised group of non-morbid obese, pre-menopausal, Caucasian women; however, we do not know whether these findings apply to other populations or to obese men.
In the present study, we observed no additional effect of EGCG on the changes in body weight and total and central adiposity after 12 weeks of a low-energy diet treatment in obese women, which concurs with the conclusions of a meta-analysis( Reference Phung, Baker and Matthews 27 ). Thus, Phung et al. ( Reference Phung, Baker and Matthews 27 ) concluded that the ingestion of a mixture of green tea catechins and caffeine for approximately12 weeks had a small and not clinically relevant effect on BMI and body weight and composition. From the small number of studies in which catechins were tested alone, Phung et al. ( Reference Phung, Baker and Matthews 27 ) also highlighted that the results do not suggest any positive influence on anthropometric measurements. Several trials have examined the effect of catechins/green tea extracts on body-weight and adiposity loss for 12 weeks in normal-weight to obese subjects. However, the majority of the studies reporting that regular consumption of green tea may influence energy metabolism, body weight and composition were conducted in Asian or Asian-descendent subjects( Reference Hsu, Tsai and Kao 8 , Reference Wang, Wen and Du 28 – Reference Nagao, Hase and Tokimitsu 30 ). A meta-analysis has shown that the effect of green tea catechins on body-weight loss was larger for Asian v. Caucasian participants, suggesting a moderating effect of ethnicity, due to differences in allele frequencies of adenosine A2A receptor and catechol-O-methyltransferase polymorphisms, on the thermogenic effect of green tea( Reference Hursel, Viechtbauer and Westerterp-Plantenga 6 ). Likewise, Nagao et al. ( Reference Nagao, Hase and Tokimitsu 30 ) examined the effects of 12 weeks of supplementation with different doses of green tea catechins on adiposity reductions in a sample of normal-weight to obese Japanese participants, and they observed larger reductions in body weight and adiposity with the intake of 690 mg of catechins, containing 135 mg EGCG compared with a control dose of approximately 3 mg EGCG. Similarly, Suliburska et al. ( Reference Suliburska, Bogdanski and Szulinska 31 ) observed the beneficial effects of 3 months of green tea extract supplementation on BMI in a sample of obese patients. However, both studies were performed during the weight maintenance period( Reference Nagao, Hase and Tokimitsu 30 ) or following a normoenergetic diet( Reference Suliburska, Bogdanski and Szulinska 31 ). The present results are in agreement with the study by Diepvens et al. ( Reference Diepvens, Kovacs and Nijs 32 ), in which forty-six overweight, Caucasian, pre- and postmenopausal women followed a low-energy diet supplied with a green tea extract. They observed that the green tea extract had no added benefits for any measurements of body weight or body composition. In the same line, Kovacs et al. ( Reference Kovacs, Lejeune and Nijs 33 ) did not find any significant effect of green tea on body-weight maintenance in 104 male and female overweight participants. Furthermore, much of the benefits of green tea catechins have been attributed to EGCG specifically, but the trial that compared the effects of 300 mg/d of EGCG supplementation combined with a programme of regular aerobic exercise with placebo in a sample of overweight to obese postmenopausal women has shown non-significant changes in body-weight and adiposity loss( Reference Wang, Wen and Du 28 ). As far as we are aware, the present study is the first to examine the effect of EGCG, without the concomitant presence of caffeine, during a low-energy diet intervention programme in obese Caucasian women.
We did not find any significant beneficial effect of EGCG on changes in post-absorptive RMR and fat oxidation rates after the dietary intervention programme. Previous studies have partly attributed the beneficial influence of green tea on energy and substrate metabolism outcomes to the concomitant presence of caffeine on green tea extracts( Reference Rains, Agarwal and Maki 7 ). Caffeine, naturally present in green tea, increases energy expenditure and whole-body fat oxidation( Reference Westerterp-Plantenga 12 ). It has been suggested that the green tea catechins plus caffeine combination is necessary to elicit an effect on energy expenditure and substrate metabolism( Reference Gregersen, Bitz and Krog-Mikkelsen 34 ). Rudelle et al. ( Reference Rudelle, Ferruzzi and Cristiani 35 ) observed an approximately 4 % increase in 24 h energy expenditure in subjects who consumed 540 mg/d of catechins (50 % of EGCG), though with 300 mg/d of added caffeine. Bérubé-Parent et al. ( Reference Bérubé-Parent, Pelletier and Doré 36 ) reported no additional dose–response effect on energy expenditure with a varying daily dosing size of EGCG from 270 to 1200 mg/d in the presence of 600 mg caffeine. Gregersen et al. ( Reference Gregersen, Bitz and Krog-Mikkelsen 34 ) did not find any significant evidence of an acute effect on energy expenditure and substrate oxidation rates when green tea extracts were consumed alone. Thielecke et al. ( Reference Thielecke, Rahn and Bohnke 37 ) using the same amount of EGCG (300 mg/d) as in the present study did not observe any significant effect on energy expenditure. They have also reported that fasting fat oxidation was only increased by caffeine intake, with or without EGCG. The study by Dulloo et al. ( Reference Dulloo, Duret and Rohrer 38 ) is the only report showing significant additional effects of green tea on energy expenditure and fat oxidation. Differences in study sample, design and treatments could explain this discrepancy between study results. In the study by Dulloo et al. ( Reference Dulloo, Duret and Rohrer 38 ), ten normal-weight men were examined during 24 h in a respiratory chamber after the intake of three randomly assigned dietary treatments (50 mg caffeine+90 mg EGCG, 50 mg caffeine and placebo), whereas in the present study, we examined a larger sample of obese pre-menopausal women during a 12-week energy-restricted diet intervention.
Green tea and green tea catechins have been shown to influence plasma levels of lipids and insulin resistance in the majority of animal studies. However, the results of human studies have been heterogeneous. The lack of a specific effect of EGCG on insulin resistance and blood lipid levels observed in the present study concurs with previous double-blind trials in which different doses of green tea catechins have been investigated in obese and/or type 2 diabetic patients( Reference Nagao, Hase and Tokimitsu 30 , Reference Brown, Lane and Coverly 39 – Reference Ryu, Lee and Lee 42 ). Similar to the present findings, consumption of green tea had no effect on insulin resistance, as determined by the measurements of HOMA-IR or fasted serum insulin( Reference Brown, Lane and Coverly 39 – Reference Ryu, Lee and Lee 42 ). Furthermore, Nagao et al. ( Reference Nagao, Hase and Tokimitsu 30 ), Frank et al. ( Reference Frank, George and Lodge 9 ) and Brown et al. ( Reference Brown, Lane and Coverly 39 ) showed that the intake of green tea extract for 8–12 weeks had no significant effect on serum lipid levels between groups in human studies. Differences in the results between animal and human studies may be due to the higher doses tested in animals.
A daily consumption of 300 mg EGCG for 12 weeks did not affect liver function biomarkers in the present study. Indeed, we observed that hepatic enzyme levels decreased at the end of the energy-restricted diet intervention programme in either the EGCG or control group. These results are in agreement with previous studies in which similar (267 mg/d for 3 weeks)( Reference Yang, Yang and Chao 29 ) or higher doses (from 714 to 1500 mg/d for 3 or 16 weeks, respectively)( Reference Frank, George and Lodge 9 , Reference Hsu, Liao and Lin 43 ) of catechins were tested.
We acknowledge the limitations of the present study. First, previous reports have proposed that the mechanism by which catechins could reduce adiposity may be related to the possible enhancing effect of EGCG on diet-induced thermogenesis( Reference Gregersen, Bitz and Krog-Mikkelsen 34 , Reference Rudelle, Ferruzzi and Cristiani 35 , Reference Dulloo, Duret and Rohrer 38 , Reference Hursel and Westerterp-Plantenga 44 ); however, the design of the present study did not allow us to explore this possibility here. Second, the present study had a similar sample size to previous randomised controlled trials; however, replication of the present findings in larger samples may be necessary to confirm the findings. Third, one potential problem to detect the effect of EGCG on changes in energy and substrate metabolism in obese subjects could be the presence of metabolic disturbance such as low RMR or high respiratory quotient at baseline. However, in the present study, there were no statistically significant differences between the measured RMR at baseline (6538 (sd 720) kJ/d) and the predicted RMR (6540 (sd 588) kJ/d) using the equation of Mifflin et al. ( Reference Ruiz, Ortega and Rodriguez 45 ). Moreover, we observed that women whose respiratory quotient was above 0·86 were similarly distributed between the groups: three women in the control group and two women in the EGCG group. The homogeneity of our well-characterised study sample due to the strict inclusion criteria and to the highly controlled intervention is the strength of the present study. Participants were Spanish Caucasian, non-diabetic and non-morbid obese pre-menopausal women who followed an energy-restricted diet with a similar macronutrient composition based on Mediterranean dietary habits whose energy content was estimated from measured RMR.
In conclusion, the present study provides evidence indicating that supplementation with 300 mg/d of green tea EGCG in obese women for 12 weeks did not enhance the energy-restricted diet-induced body-weight and adiposity reductions, and did not improve weight-loss-induced changes in cardiometabolic risk factors. Furthermore, there did not seem to be an effect of the tested EGCG dose on changes in energy and substrate metabolism at the end of the intervention programme in obese Caucasian women. The intake of 300 mg/d of EGCG for 12 weeks did not cause any adverse effect on liver function biomarkers.
Acknowledgements
The authors thank the women for their participation in the study, and Raquel Ares, Silvia Francisco, Izaskun Felipe and Emilio Sanz for their contribution to the participants' recruitment and medical supervision of the study. They acknowledge DSM Nutritional Products, Barcelona, Spain for the provision of the study supplements.
The present study was supported by the Department of Industry of the Government of the Basque Country (S-PE06LE04). The Department of Industry of the Government of the Basque Country had no role in the design, analyses or writing of this article.
The authors' contributions are as follows: I. L. contributed to the study concept and design, performed the statistical analysis, supervised the study and drafted the manuscript; I. L. and E. L. obtained funding; I. L., J. M.-A., E. L. and J. M. analysed and interpreted the data; J. M.-A., L. B., P. A., E. L., J. M. and I. L. critically revised the manuscript for important intellectual content.
None of the authors had any conflict of interest.






