Introduction
In critically ill patients, the intestine is susceptible to injury, and gastrointestinal (GI) injury is common(Reference Reintam, Parm and Kitus1). Evidence suggests that an estimated 50 % of patients have enterocyte damage at admission to the intensive care unit (ICU), and GI symptoms occur in approximately 62 % of patients in the ICU(Reference Piton, Belon and Cypriani2). The Working Group on Abdominal Problems (WGAP) of the European Society of Intensive Care Medicine (ESICM) proposed a set of definitions and grading system for GI dysfunction. They defined acute GI injury (AGI) as malfunctioning of the GI tract in critically ill patients due to their acute illness(Reference Reintam Blaser, Malbrain and Starkopf3). Studies have shown that critically ill patients with AGI experience higher mortality rates as compared with patients without AGI(Reference Reintam, Parm and Kitus4–Reference Li, Zhang and Wang6).
As one of the GI tract functions is to ingest, digest and absorb nutrients from food and water, AGI can manifest as feeding intolerance (defined as intolerance to enteral nutrition (EN) wherein intake of ≥20 kcal (≥84 kJ)/kg body weight per d cannot be achieved within 72 h of feeding attempts via the enteral route)(Reference Reintam Blaser, Malbrain and Starkopf3). However, clinical practice guidelines of the ESICM recommend initiation of early EN (EEN) within 24–48 h of ICU admission, advancing towards optimal nutritional goals over the next 48–72 h(Reference Blaser, Starkopf and Alhazzani7). Therefore, some questions remain regarding the use of nutritional support in critically ill patients: (1) Is EN safe in critically ill patients with AGI? (2) Will these patients benefit from using the injured organ? (3) Does the GI tract of critically ill patients require ‘rest’ for the recovery of GI function? The present review seeks to explore answers to these questions based on the contemporary body of evidence.
Gastrointestinal dysfunction in the acute phase of critical illness
The acute phase of critical illness lasts for at least 7 d(Reference Stahel, Flierl and Moore8), and has been variously defined in clinical trials as the first few days after onset of illness, or the first 5–7 d after admission to the ICU(Reference Casaer, Mesotten and Hermans9–11). The acute phase is usually caused by injury (shock, trauma or infection)(Reference Stahel, Flierl and Moore8), which imposes stress on the body due to compensatory pathophysiological changes in an attempt to restore stability within the internal environment(Reference Stratakis and Chrousos12). The adaptive response to acute critical illness includes the metabolic response to stress(Reference Preiser, van Zanten and Berger13), which involves neuroendocrine, inflammatory and immune mechanisms that cause rapid catabolism and induce resistance to anabolic signals, including insulin. This prioritises the delivery of glucose to organs that cannot use other substrates as energy(Reference Lena, Kalfon and Preiser14, Reference Preiser, Ichai and Orban15). These adaptive changes are complex and sequential and have largely been a barrier to the successful development of targeted interventions to modulate the metabolic response to critical illness(Reference Preiser, Ichai and Orban15).
In addition, excessive or inadequate adaptive responses evoked by intensive stress(Reference Stratakis and Chrousos12) can have an impact on the GI tract, including negative effect on gut motility(Reference Gué, Peeters and Depoortere16, Reference Chrousos17), mucosal blood flow(Reference Richardson, Norton and Sales18) and increased mucosal permeability(Reference Wallon, Yang and Keita19) through neuroendocrine regulation(Reference Konturek, Brzozowski and Konturek20). In particular, the adaptive response to acute stress also includes an adrenergic response with the consequent increase in catecholamine levels which may cause constriction of GI vessels and decrease intestinal blood flow(Reference Halter, Pflug and Porte21). In addition to endogenous regulation, exogenous catecholamines administered to treat shock may also divert blood flow away from the mesenteric circulation and decrease microvascular perfusion in the gut(Reference Krejci, Hiltebrand and Sigurdsson22). GI blood flow is reduced in some critically ill patients despite treatment with fluid replacement and interventions to normalise blood pressure and cardiac function(Reference Chapman, Fraser and Vozzo23). Moreover, intense vasoconstriction and hypoperfusion of the intestines have been shown to persist during early sepsis even in patients with normal blood pressure(Reference Hinshaw24). Decreased GI blood flow induces up-regulation of apoptosis and decreases proliferation of small bowel mucosal cells, which leads to thinning of the gut mucosa and decrease in absorptive surface of the small bowel(Reference Chung, Evers and Townsend25). With continued disease progression, tissue oedema, endothelial injury(26) and capillary leaking occur in the gut(Reference Verburgh, Reintam-Blaser and Kirkpatrick27). These injuries result in improper digestion and absorption(Reference Reintam Blaser, Malbrain and Starkopf3), impairment of the intestinal barrier(Reference Li, Chen and Huo28) and dysregulation of the intestinal microbiota(Reference Lankelma, van Vught and Belzer29).
The symptom profile of improper digestion and absorption is characterised by temporary self-limiting GI symptoms, which progress to the feeding intolerance syndrome as GI dysfunction becomes more severe(Reference Reintam, Parm and Kitus1, Reference Reintam Blaser, Malbrain and Starkopf3). Small-intestinal mucosal integrity may also be compromised in critically ill patients, leading to increased intestinal permeability, especially in patients intolerant to EN(Reference Burgstad, Besanko and Deane30). Furthermore, critical illness alters the gut microbiota; the gut microbiota of critically ill patients is characterised by low diversity, low abundance of key commensal genera and overgrowth of a single bacterial genus(Reference Lankelma, van Vught and Belzer29) (Fig. 1). Dysbiosis of the gut microbiota may result in organ dysfunction(Reference Jacobs, Haak and Hugenholtz31), and should be part of the evaluation of GI function in the future. The gut microbiome has large potential as a future therapeutic or diagnostic target. However, current evidence on microbiota-targeted therapies in critical illness remains unclear(Reference Jacobs, Haak and Hugenholtz31).
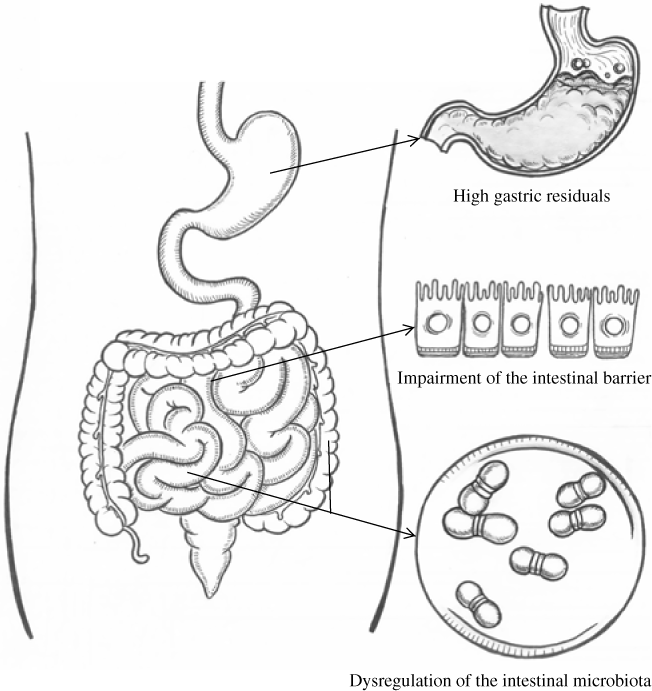
Fig. 1. Acute gastrointestinal injury in critically ill patients. Acute gastrointestinal injury manifests as improper digestion and absorption(Reference Reintam Blaser, Malbrain and Starkopf3), impairment of the intestinal barrier(Reference Li, Chen and Huo28) and dysregulation of the intestinal microbiota(Reference Lankelma, van Vught and Belzer29). Improper digestion and absorption: enteral feeding is impacted whatever the clinical reason (vomiting, high gastric residuals, diarrhoea, gastrointestinal bleeding or presence of entero-cutaneous fistulas)(Reference Reintam Blaser, Malbrain and Starkopf3). Impairment of the intestinal barrier means increased intestinal permeability(Reference Li, Chen and Huo28). Dysregulation of the intestinal microbiota is characterised by low diversity, low abundance of key commensal genera and overgrowth of one bacterial genus. In the figure, high gastric residuals indicate improper digestion and absorption; impairment of the intestinal barrier and dysregulation of the intestinal microbiota are not identified well due to the shortage of evaluation tools(Reference Reintam Blaser, Malbrain and Starkopf3).
The ESICM WGAP classification of AGI in critical illness is mainly based on the degree of impairment of digestion and absorption functions(Reference Reintam Blaser, Malbrain and Starkopf3, Reference Verburgh, Reintam-Blaser and Kirkpatrick27). AGI grade I refers to development of new GI symptoms (such as vomiting, gastric residual volume, diarrhoea, GI bleeding, paralysis of lower GI tract, or abnormal bowel sounds), which are related to a known cause and perceived as transient (risk of developing GI dysfunction or failure). Lack of improvement in these symptoms and no changes in the general condition are graded as AGI grade II (GI dysfunction). AGI grade II is an indication for intervention (for example, prokinetics, postpyloric feeding) to restore GI function. Persistence of GI symptoms or worsening of multiple organ dysfunction syndrome and lack of improvement in enteral feeding are classified as AGI grade III (GI failure); this connotes a stage wherein interventions cannot restore GI function. Lastly, presence of acute GI problems that are directly life-threatening is graded as AGI grade IV (GI failure with a severe impact on distant organ function)(Reference Reintam Blaser, Malbrain and Starkopf3) (Table 1). However, identification of an appropriate method to implement EN in patients with AGI is the key to problems.
Table 1. Classification of acute gastrointestinal injury (AGI)(Reference Reintam Blaser, Malbrain and Starkopf3)

GI, gastrointestinal; IAH, intra-abdominal hypertension; IAP, intra-abdominal pressure; BW, body weight; APP, abdominal perfusion pressure; ACS, abdominal compartment syndrome.
Enteral nutrition in the acute phase of critical illness
Sufficient and appropriate nutrition is essential to sustain the body’s metabolism; therefore, malnutrition results in high morbidity and mortality in the ICU setting(Reference Giner, Laviano and Meguid32). Some studies have suggested a favourable impact of EEN on outcomes in critically ill patients such as reduced disease severity, lower incidence of infectious complications and shorter length of stay in the ICU(Reference Heyland, Stephens and Day33–Reference Khalid, Doshi and Digiovine35). In contrast, in one randomised controlled trial (RCT), EN within 24 h of admission in ICU patients was not associated with a reduction in hospital discharge mortality, duration of hospitalisation or ICU length of stay(Reference Doig, Simpson and Finfer36); another RCT demonstrated no difference in 30 d mortality or rates of adverse events in patients who received parenteral nutrition (PN) or EN within 36 h of an unplanned admission to the ICU(Reference Harvey, Parrott and Harrison10). Furthermore, in a single-centre, prospective, controlled clinical trial, aggressive EEN (initiating mechanical ventilation (MV) on day 1 v. day 5) in mechanically ventilated patients was associated with greater infectious complications and prolonged length of hospital stay(Reference Ibrahim, Mehringer and Prentice37). In another recent study, early isoenergetic EN did not reduce the mortality or the risk of secondary infection in critically ill patients with shock; however, it was associated with an increased risk of digestive complications compared with early isoenergetic PN(Reference Reignier, Boisramé-Helms and Brisard38). However, 2016 Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines as well as clinical practice guidelines from ESICM recommend the use of EN within 24–48 h in critically ill patients who require nutritional support instead of delaying EN or using early PN because of decreased incidence of infectious complications(Reference Blaser, Starkopf and Alhazzani7, Reference McClave, Taylor and Martindale39), and greater economic benefit and convenience of the EN route. However, the decrease in infectious complications may be attributable to improvements in current management of vascular access(Reference Bion, Richardson and Hibbert40), prevention of ventilator-associated pneumonia(Reference Li Bassi, Senussi and Aguilera Xiol41), as well as developments in feeding technology, but not to EN per se (Reference Harvey, Parrott and Harrison10, Reference Bakker, van Brunschot and van Santvoort42). Collectively, these findings indicate that there are not enough data to determine the superiority of EEN v. delayed EN or to assess the effect of EEN in reducing mortality rates among critically ill patients.
Considerations for implementation of EN other than the timing of initial EN and dose of EN should be important points. One RCT of critically ill patients has shown that permissive underfeeding in the first 7 d of an ICU stay may be associated with lower hospital mortality rates than that achieved with target feeding(Reference Arabi, Tamim and Dhar43). Subsequently, a large multicentre RCT was performed to evaluate the effect of 2 weeks of permissive underfeeding (defined as 40–60 % of the calculated energy requirement) v. that of standard EN (defined as 70–100 % of the calculated energy requirement) on the mortality of critically ill patients admitted to the ICU within 48 h after surgery, medical treatment or trauma(Reference Arabi, Aldawood and Haddad44). Protein intake was similar in the two groups, but the permissive underfeeding group received less non-protein energy than the standard EN group(Reference Arabi, Aldawood and Haddad44). The results(Reference Arabi, Tamim and Dhar43, Reference Arabi, Aldawood and Haddad44) showed that administration of less non-protein energy in the permissive underfeeding group was not associated with lower mortality compared with that associated with administration of a full amount of non-protein energy in the standard EN group; however, blood glucose levels, insulin requirements, need for renal-replacement therapy, and daily fluid balance were lower in the permissive underfeeding group(Reference Arabi, Aldawood and Haddad44). In addition, two RCT of lower energy feeding: initial trophic feeding (defined as 10–20 kcal/h (42–84 kJ/h) or up to 500 kcal/d (2090 kJ/d)) v. full EN of approximately 1300 kcal/d (5440 kJ/d) for 6 d of MV in patients with acute lung injury (ALI) or acute respiratory failure showed similar clinical outcomes; however, fewer episodes of GI intolerance were observed with initial trophic feeding(11, Reference Rice, Mogan and Hays45). Furthermore, overfeeding of critically ill patients may be associated with hypercapnia, increased risk of infection, metabolic disturbances such as hyperglycaemia, liver dysfunction and extended time on MV(Reference Preiser, van Zanten and Berger13, Reference Klein, Stanek and Wiles46). In addition, early energy overfeeding was shown to be associated with high mortality in non-septic patients with critical illness(Reference Weijs, Looijaard and Beishuizen47). Most results showed that restrictive EN (permissive underfeeding in critically ill patient or trophic feeding in patients with ALI) is superior or not inferior to standard EEN or full EEN. These findings suggest that a high dose of EN may cause harm in the acute phase of critical illness.
In the acute phase after a severe insult, aggressive nutritional therapy (for example, by providing exogenous energy support according to energy expenditure) may not have beneficial effects. In fact, this approach is potentially detrimental as it may cause a metabolic overload and/or suppress the ubiquitin–proteasome pathway and the related autophagy pathway, which are potentially important for cellular repair and organ recovery(Reference Hartl and Jauch48, Reference Schetz, Casaer and Van den Berghe49).
Based on the currently available evidence, early overfeeding may be appropriate in the acute phase of critical illness. Although we think that overuse of the gut may adversely affect the prognosis in the acute phase of critical illness, no studies have compared early trophic feeding with no EN.
Acute gastrointestinal injury and enteral nutrition
Studies conducted on dogs have shown that enteral nutrients can increase blood flow to the GI tract during the ‘postprandial hyperaemic response’. This may preserve gut integrity and prevent gut-derived complications(Reference Kazamias, Kotzampassi and Koufogiannis50, Reference Purcell, Davis and Branson51). Other evidence suggests that trophic effects of EN help maintain intestinal physiology, prevent atrophy of gut villi, reduce intestinal permeability, protect against ischaemia–reperfusion injury by stimulating intestinal perfusion, and preserve gut immunity by affecting gut-associated lymphoid tissue(Reference Schmidt and Martindale52).
However, EN may lead to GI complications(Reference Hsu, Sun and Lin53) (vomiting, diarrhoea, GI bleeding, aspiration-related pneumonia, refeeding syndrome, or gut ischaemia)(Reference Boullata, Carrera and Harvey54). One study found a high frequency of EN-related GI complications in critically ill patients, of which high gastric residuals was the most common; in addition, GI intolerance to EN seemed to prolong the ICU stay and increase mortality(Reference Montejo55). Other studies have found an association of early nutrition or EN with increased incidence of ventilator-associated pneumonia in patients with invasive MV and shock(Reference Reignier, Darmon and Sonneville56), and EN resulted in upper digestive intolerance, which was associated with nosocomial pneumonia, prolonged ICU stay and a high ICU mortality in critically ill patients(Reference Mentec, Dupont and Bocchetti57). However, improvement of feeding protocols may help decrease or prevent the complications of EN(Reference Boullata, Carrera and Harvey54). However, even if EN is safe in patients with AGI, identification of patients who are likely to benefit from EN in the acute phase of critical illness is a key imperative.
There is a paucity of studies that have investigated the influence of AGI on the prognosis of critically ill patients who received EN. Therefore, the effect of EN in critically ill patients with AGI is not well characterised. In addition, there are few guidelines for provision of EN to patients with AGI. The ESICM WGAP recommends initiation of minimal EN (20 ml/h), the dose of which is actually greater than that of trophic feeding, with subsequent increase in EN to 100 % of the calculated energy requirement in patients with AGI grade I; in patients with AGI grade II or III, it recommends initiation or trial of minimal EN (20 ml/h) along with other therapy based on the symptoms (for example, prokinetics) (EN is not indicated for patients with AGI grade IV, who typically do not tolerate EN)(Reference Reintam Blaser, Malbrain and Starkopf3). The aim of trial EN in the ESICM WGAP recommendations is to improve GI symptoms and to subsequently increase EN to 100 % of the calculated energy requirement(Reference Reintam Blaser, Malbrain and Starkopf3). The 2016 SCCM/ASPEN guidelines recommend evaluation of GI function and contractility before initiation of EN(Reference Heidegger, Berger and Graf34); however, the guidelines do not address the methodology for evaluation of GI function and the modalities for initiation of EN. In addition, these recommendations(Reference Reintam Blaser, Malbrain and Starkopf3, Reference McClave, Taylor and Martindale39) are not entirely evidence-based, especially with respect to administration of EN in patients with AGI.
Theoretically, it seems reasonable to suggest that excessive use of an injured GI tract may have deleterious effects. Although there is a paucity of evidence pertaining to the effect of EN in patients with AGI, studies on EEN in patients with acute pancreatitis (AP)(Reference Bakker, van Brunschot and van Santvoort42), which is often associated with AGI, have provided indirect evidence. Although in this multicentre RCT, early nasoenteric tube feeding (<24 h) was not associated with increased incidence of complications as compared with that with an oral diet after 72 h, it did not reduce the rate of infection or death(Reference Bakker, van Brunschot and van Santvoort42). In another recent RCT, early nasojejunal feeding (<24 h) in patients with AP did not improve persistent organ failure or mortality compared with no nutritional support(Reference Stimac, Poropat and Hauser58).
In addition, SCCM/ASPEN guidelines recommend assessment of patients at the time of admission to the ICU for nutrition risk and high nutrition risk (Nutrition Risk Screening 2002 (NRS 2002) >3 or Nutrition Risk in the Critically Ill (NUTRIC) score ≥5) in order to identify patients who are most likely to benefit from EEN therapy based on expert consensus(Reference McClave, Taylor and Martindale39); however, these nutrition risk scores were designed to guide nutrition therapy including EN and/or PN(Reference Heyland, Dhaliwal and Jiang59, Reference Jie, Jiang and Nolan60), and high risk scores may be an indication for implementing nutrition therapy, but not necessarily EN. Considering the latest evidence that shows that PN is not inferior to EN(Reference Reignier, Boisramé-Helms and Brisard38), and may serve as a substitute for EN, GI function still should be the main consideration for implementing EN in patients with both high nutrition risk and AGI.
All in all, EN may increase GI complications which themselves are manifestations of AGI. Although EEN may be safely implemented through improvement of feeding protocols in critically ill patients (even those with AP, which usually manifests as AGI), studies of EEN in patients with AP have shown no indications of its benefit in patients with AGI(Reference Bakker, van Brunschot and van Santvoort42, Reference Stimac, Poropat and Hauser58). It is possible that early enteral feeding may not be as effective as we anticipated(Reference Bakker, van Brunschot and van Santvoort42).
Gut rest strategy in critically ill patients
In critically ill patients with severe injury, therapeutic approaches focus on the pathology (for example, trauma, or necrotic or infected tissue), often allowing survival from a potentially lethal condition. While the adaptive metabolic response to minor trauma is beneficial, an exaggerated metabolic response may cause secondary metabolic damage in patients who survive severe, potentially lethal conditions due to advances in modern medicine(Reference Hartl and Jauch48). Effective treatment must prevent this secondary metabolic damage; however, these interventions must not cause progressive harm. In critical care, evidence suggests that the excessive use of an injured organ is associated with a poor prognosis and restrictive therapy might protect the injured organ from progressive harm, for example, low tidal volume(Reference Ney and Kuebler61) and restrictive fluid management(62) in acute respiratory distress syndrome (ARDS)/ALI, restrictive strategy of erythrocyte transfusion in anaemia(Reference Hébert, Wells and Blajchman63), and restrictive usage of diuretics in acute renal failure(Reference Mehta, Pascual and Soroko64, Reference Bagshaw, Gibney and Kruger65). Studies conducted on patients with AP did not find a beneficial effect of EEN on prognosis when compared with gut rest (72 h), which underlines the case for gut rest strategy (Table 2).
Table 2. Aggressive or restrictive therapy in critically ill patients*

ARDS, acute respiratory distress syndrome; AKI, acute kidney injury; AGI, acute gastrointestinal injury; EN, enteral nutrition; MV, mechanical ventilation; ALI, acute lung injury.
* Using a lower tidal volume with MV can reduce mortality and help reduce the duration of MV compared with use of normal or physiological tidal volume in patients with ARDS/ALI(Reference Ney and Kuebler61). A conservative (as against aggressive) approach to fluid management improves lung function and shortens the duration of MV in patients with ALI(62). A restrictive strategy of erythrocyte transfusion has been shown to be equally effective and potentially superior to a liberal transfusion strategy in critically ill patients(Reference Hébert, Wells and Blajchman63). Use of diuretics, which causes overuse of residual kidney function, has been shown to be associated with an increased risk of death and non-recovery of renal function in critically ill patients with acute renal failure(Reference Mehta, Pascual and Soroko64, Reference Bagshaw, Gibney and Kruger65).
Theoretically, ‘organ rest’ strategy relies on complete rest of the organ, such as lung rest during extracorporeal membrane oxygenation in animal experiments(Reference Frattallone, Fuhrman and Kochanek66). However, in clinical settings, lung rest strategy implies maintaining a small work load, for example, with low inspiratory pressure, low oxygen concentration, and moderate positive end expiratory pressure in patients with ARDS/ALI on MV(Reference Peek, Mugford and Tiruvoipati67); in other words, this represents a partial lung rest strategy. Similarly, trophic feeding(Reference Wallon, Yang and Keita19) entails a small volume of EN not intended to meet energy requirements but rather to maintain the structural and functional integrity of the GI tract; this may be referred to as a partial gut rest strategy. However, it is necessary to perform more trials to demonstrate the superiority of partial gut rest strategy in critically ill patients with AGI. In addition, comparison of trophic feeding with gut rest strategy should also be performed. Patients with AGI should be enrolled in RCT on EN in future.
The present review might provide a new approach for research on EN in critically ill patients. We propose that delayed trophic feeding (after 72 h from intensive stress) is the optimal choice for critically ill patients with AGI. As a protective strategy, trophic feeding may reduce the gut burden and help maintain intestinal physiology, sufficient to prevent mucosal atrophy and maintain gut integrity in critically ill patients(Reference McClave, Taylor and Martindale39); therefore, it may be applicable to critically ill patients with AGI. To the best of our knowledge, there are no trials that have proven this hypothesis; therefore, large-scale studies are warranted to investigate this approach.
Conclusions
Injury to the GI tract manifests as improper digestion and absorption, impairment of the intestinal barrier and dysregulation of the intestinal microbiota. EN is believed to improve GI function in critically ill patients. However, we propose that delayed trophic feeding may be the optimal strategy in the acute phase of critical illness considering the adaptive metabolic response and AGI. Trophic feeding may be an organ-protective strategy in critically ill patients, similar to ventilation with a low tidal volume, restrictive fluid resuscitation and restrictive blood transfusion.
Author ORCIDs
Hongxiang Li 0000-0002-1399-8039
Acknowledgements
There was no financial support for this study.
D. Z. and Y. L. produced the first draft of the manuscript. L. Q. made the figure. H. L. critically revised the manuscript. All authors saw and approved the final draft of the manuscript.
There are no conflicts of interest.





