Optimal breast-feeding is a cost-effective and important measure to improve mother and infant health. Breast-fed infants have a lower risk for diarrhoeal diseases( Reference Sankar, Sinha and Chowdhury 1 , 2 ), lower under-5 mortality rate( Reference Edmond, Zandoh and Quigley 3 ) and higher cognitive functioning( 2 ). Breast-feeding mothers have improved family planning and a reduced risk of breast and ovarian cancers( Reference Victora, Bahl and Barros 4 , Reference Horta, Bahl and Martines 5 ). Globally, efforts have been made to improve child health, including infant feeding. These initiatives include the International Code of Marketing of Breast-milk Substitutes (referred to hereafter as ‘the Code’); the Innocenti Declaration; the Baby Friendly Hospital Initiative (BFHI); the Millennium Development Goals; and, more recently, the Global Nutrition Targets 2025 and Sustainable Development Goals.
Despite these sustained efforts, improvements in breast-feeding practices have been slow and disproportionate worldwide( Reference Victora, Bahl and Barros 4 ). For example, exclusive breast-feeding (EBF) rates varied in Africa (35 %) and Asia (41 %) in 2010( Reference Cai, Wardlaw and Brown 6 ). Diarrhoea is a major public health issue in sub-Saharan African (SSA) countries, accounting for approximately 46 % of the global diarrhoea burden( 7 ). The impact of optimal breast-feeding practices on diarrhoea-related morbidity and mortality has been documented in many developing countries( Reference Ogbo, Page and Idoko 8 – Reference Hajeebhoy, Nguyen and Mannava 11 ). A recent population-based study from SSA countries with high diarrhoea mortality indicated that optimal breast-feeding practices were protective of diarrhoea in African children( Reference Ogbo, Agho and Ogeleka 12 ).
In many SSA countries with high diarrhoea mortality( Reference Boschi-Pinto, Velebit and Shibuya 13 ), there is limited up-to-date evidence on the risk factors for suboptimal breast-feeding practices at the national level to scale up and guide efforts at improving breast-feeding practices. Studies from regional areas and three national reports from SSA countries with high diarrhoea mortality have elucidated factors associated with suboptimal breast-feeding practices. These factors included: poverty, low maternal education, fewer health-service contacts( Reference Agho, Dibley and Odiase 14 , Reference Ogbo, Agho and Page 15 ), young maternal age, home delivery, cultural beliefs held for breast-feeding( Reference Ogbo, Agho and Page 15 – Reference Ogbo, Page and Agho 17 ) and limited family support( Reference Bezner, Laifolo and Shumba 18 ). The nationwide studies on breast-feeding from Nigeria( Reference Ogbo, Agho and Page 15 ) and Tanzania( Reference Victor, Baines and Agho 16 ) used older population-based data sets and only focused on EBF. Findings from these studies may be limited in providing up-to-date evidence on broader breast-feeding practices such as early initiation of breast-feeding, predominant breast-feeding or bottle-feeding for targeted interventions.
A study that is specific to the impact of sociodemographic and health service factors on breast-feeding across geographies in SSA countries with high diarrhoea mortality would be helpful for informing well-guided interventions. It is imperative that these approaches are evidence-based and directly connected to countries requiring those initiatives. Using the most recent nationally representative, consistent and reliable data, in the present study, we provide up-to-date information on risk factors for breast-feeding practices in SSA countries with high diarrhoea mortality. We take advantage of the standardised population-based breast-feeding data to better examine determinants of breast-feeding practices across SSA countries. Our study aimed to examine the impact of sociodemographic and health-service factors on breast-feeding in SSA countries with high diarrhoea mortality.
Methods
Data sources
The present study used the most recent and pooled Demographic and Health Survey (DHS) data for 50 975 children under 24 months of age (Burkina Faso (2010, N 5710); Demographic Republic of Congo (2013, N 6797); Ethiopia (2013, N 4193); Kenya (2014, N 7024); Mali (2013, N 3802); Niger (2013, N 4930); Nigeria (2013, N 11 712); Tanzania (2015, N 3894); and Uganda (2010, N 2913)). These SSA countries were selected for the study because of previously published reports which showed that diarrhoea mortality was highest among those countries in the African continent( Reference Boschi-Pinto, Velebit and Shibuya 13 , 19 ). The DHS project collects demographic, maternal and child health (including breast-feeding) information from a nationally representative sample of households. The data were collected by country-specific population commissions and departments of health in partnership with the Inner City Fund (ICF) International and Measure DHS, using standardised household questionnaires. A two-stage sampling strategy was used, where a country was divided into enumeration areas (clusters) based on the recent census frame for that country, and then households were randomly selected from each cluster. Household sociodemographic characteristics, maternal and child health data were obtained from eligible women aged 15–49 years in each household surveyed. The study used a total weighted sample of 50 975 maternal responses for children under 24 months of age (alive and living with the respondent), with response rate in the surveys ranging from 96 to 99 %. Many mothers in the selected SSA countries (except for Ethiopia and Niger) were in employment. Home delivery was prevalent in Ethiopia, Niger and Nigeria (Table 1). Additional information on the DHS and methodology for data collection has been described in country-specific DHS reports( 20 ).
Table 1 Characteristics of the study population by country; data from the most recent and pooled Demographic and Health Survey data setsFootnote *
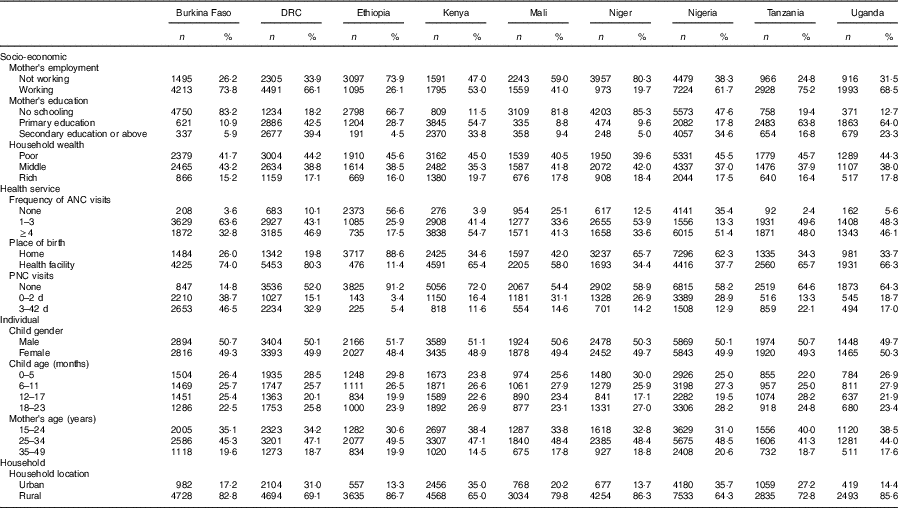
DRC, Demographic Republic of Congo; ANC, antenatal clinic; PNC, postnatal clinic.
* Burkina Faso (2010), N 5710; DRC (2013), N 6797; Ethiopia (2013), N 4193; Kenya (2014), N 7024; Mali (2013), N 3802; Niger (2013), N 4930; Nigeria (2013), N 11 712; Tanzania (2015), N 3894; and Uganda (2010), N 2913.
Outcome
The main outcomes were the breast-feeding indicators (early initiation of breast-feeding, exclusive breast-feeding (EBF), predominant breast-feeding and bottle-feeding), selected based on previous published studies( Reference Ogbo, Agho and Page 15 , Reference Ogbo, Page and Agho 17 , Reference Bahl, Frost and Kirkwood 21 ), which were assessed based on the following WHO definitions for infant and young child feeding practices( 22 ).
-
∙ Early (timely) initiation of breast-feeding: The proportion of children 0–23 months of age who were put to the breast within one hour of birth.
-
∙ Exclusive breast-feeding: The proportion of infants 0–5 months of age who received breast milk as the only source of nourishment, but allows oral rehydration solution, drops, or syrups of vitamins and medicines.
-
∙ Predominant breast-feeding: The proportion of infants 0–5 months of age who received breast milk as the main source of nourishment, but allows water, water-based drinks, fruit juice, oral rehydration solution, drops, or syrups of vitamins and medicines.
-
∙ Bottle-feeding: The proportion of infants 0–23 months of age who received any liquid (including breast milk) or semi-solid food from a bottle with a nipple/teat.
The WHO considered bottle-feeding an important breast-feeding measure because of its impact on optimal breast-feeding practices, and the association between bottle-feeding and increased diarrhoeal morbidity and mortality( 22 ).
Study factors
The independent variables included socio-economic, health service and individual variables selected based on previously published studies( Reference Agho, Dibley and Odiase 14 , Reference Victor, Baines and Agho 16 , Reference Agho, Ogeleka and Ogbo 23 ). Socio-economic characteristics included the mother’s educational level (categorised as no education, primary education or secondary education or above), employment status (categorised as not working or working) and household wealth index (categorised as poor, middle or rich). The household wealth index was calculated as a score of household assets, which was derived from a principal component analysis conducted by each in-country population commission or department of health in collaboration with ICF International. Health service factors included the number of antenatal clinic (ANC) visits (categorised as no ANC visit, one to three ANC visits, or four or more ANC visits), the place of delivery (categorised as home or health facility) and the timing of postnatal clinic (PNC) visits (categorised as no PNC visits, 0–2 d PNC visits or 3–42 d PNC visits). The individual factor was maternal age (categorised as 15–24 years, 25–34 years or 35–49 years).
Statistical analysis
Preliminary analyses involved calculations of prevalence estimates and frequency tabulations to describe data for each study factor (i.e. socio-economic, health service and individual variables). Prevalence (and corresponding 95 % CI) of breast-feeding indicators were estimated using the ‘svy’ function to adjust for sampling weights to ensure generalisability of the survey results at the national level.
Univariate and multivariate logistic regression models that expressed country as an ordinal variable and adjusted for maternal marital status, place of residence, child’s age and gender as confounders were used to investigate the association between study factors and breast-feeding in the nine SSA countries, using ‘xlogit’ command to estimate the OR. The models were restricted to the youngest living child aged less than 24 months living with the respondent (woman aged 15–49 years) to minimise recall bias, consistent with previous studies( Reference Ogbo, Page and Idoko 8 , Reference Ogbo, Page and Idoko 24 ). All statistical analyses were conducted using the statistical software package Stata version 13.0.
Ethics
Ethical approvals for data collection and subsequent analyses were obtained by Measure DHS project from country-specific research ethics committee before the surveys were conducted. These ethics committees included: Burkina Faso National Ethical Committee (Burkina Faso); Ethics Committee of the Demographic Republic of Congo Ministry of Planning (Demographic Republic of Congo); National Ethics Review Committee of the Ethiopia Science and Technology Commission (Ethiopia); Scientific and Ethical Review Committee of the Kenya Medical and Research Institute (Kenya); Ethical Committee of the Faculty of Medicine, Pharmacy and Odonto-stomatology, University of Bamako (Mali); National Consultative Ethics Committee of the Niger Ministry of Health (Niger); National Health Research Ethics Committee (Nigeria); National Health Research Ethical Committee (Tanzania); and Research and Ethics Committee, Uganda National Council for Science and Technology (Uganda). Permission to use the data was sought from Measure DHS/ICF International, and approval was granted.
Results
Prevalence of breast-feeding indicators
The overall prevalence of EBF in SSA with high diarrhoea mortality was 34·3 %; highest in Uganda at 63·4 %, Tanzania at 63·3 % and Ethiopia at 53·2 %, but lowest in Nigeria at 17·4 % and Niger at 23·4 % (Fig. 1). With the exception of Kenya at 6·5 %, Uganda at 7·6 % and Tanzania at 14·2 %, prevalence of predominant breast-feeding was higher for other countries, ranging from 23·3 % in Ethiopia to 68·7 % in Burkina Faso. Among the SSA countries, the prevalence of timely initiation of breast-feeding was highest in Mali (58·7 %), but lowest in Kenya and Nigeria (30·4 and 33·9 %, respectively). The prevalence of bottle-feeding was highest in Uganda (22·2 %), Nigeria (12·7 %) and Ethiopia (11·8 %), but lowest in Burkina Faso (1·7 %) and Niger (2·1 %).
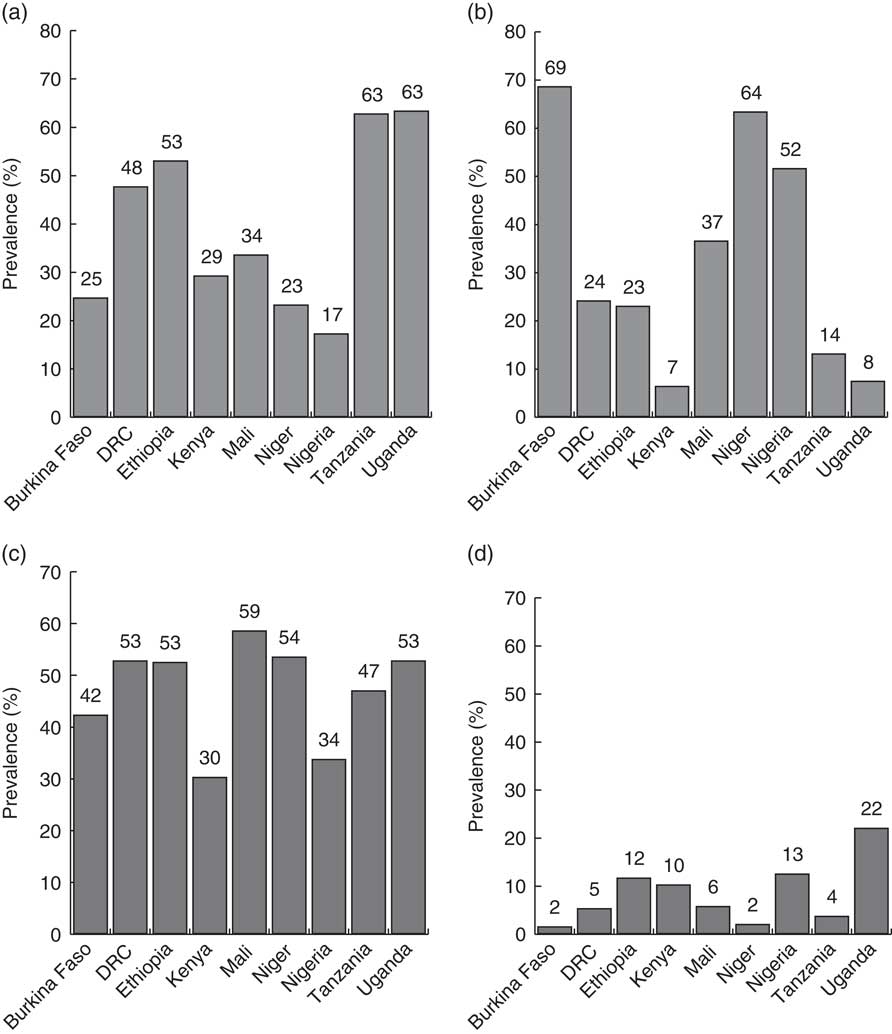
Fig. 1 Prevalence of breast-feeding practices in sub-Saharan African countries with high diarrhoea mortality: (a) exclusive breast-feeding; (b) predominant breast-feeding; (c) early initiation of breast-feeding; (d) bottle-feeding. Data from the most recent and pooled Demographic and Health Survey data sets for 50 975 children under 24 months of age: Burkina Faso (2010), N 5710; Demographic Republic of Congo (DRC; 2013), N 6797; Ethiopia (2013), N 4193; Kenya (2014), N 7024; Mali (2013), N 3802; Niger (2013), N 4930; Nigeria (2013), N 11 712; Tanzania (2015), N 3894; and Uganda (2010), N 2913. Early initiation of breast-feeding=the proportion of children 0–23 months of age who were put to the breast within one hour of birth; exclusive breast-feeding=the proportion of infants 0–5 months of age who received breast milk as the only source of nourishment, but allows oral rehydration solution, drops, or syrups of vitamins and medicines; predominant breast-feeding=the proportion of infants 0–5 months of age who received breast milk as the main source of nourishment, but allows water, water-based drinks, fruit juice, oral rehydration solution, drops, or syrups of vitamins and medicines; bottle-feeding=the proportion of infants 0–23 months of age who received any liquid (including breast milk) or semi-solid food from a bottle with a nipple/teat
Determinants of breast-feeding in sub-Saharan African countries with high diarrhoea mortality
Mothers with a secondary level of education or above were more likely to exclusively breast-feed their infants compared with mothers with no education (adjusted OR (AOR)=1·30; 95 % CI 1·14, 1·47; P<0·001; Table 2). Employed mothers were less likely to exclusively breast-feed compared with mothers not in employment (AOR=0·84; 95 % CI 0·77, 0·92; P<0·001). The odds for EBF were significantly higher in mothers who had frequent ANC visits (AOR=1·22; 95 % CI 1·07, 1·38; P=0·003 for 1–3 ANC visits and AOR=1·31; 95 % CI 1·14, 1·49; P<0·001 for ≥4 ANC visits). Delivery at a health facility and higher frequency of PNC visits were significantly associated with EBF (AOR=1·24; 95 % CI 1·11, 1·39; P<0·001 for health facility delivery and AOR=1·22; 95 % CI 1·10, 1·36; P<0·001 for 0–2 d PNC visits).
Table 2 Determinants of exclusive breast-feeding and predominant breast-feeding in nine sub-Saharan African countries with high burden of diarrhoea mortality; data from the most recent and pooled Demographic and Health Survey data sets for 50 975 children under 24 months of ageFootnote *
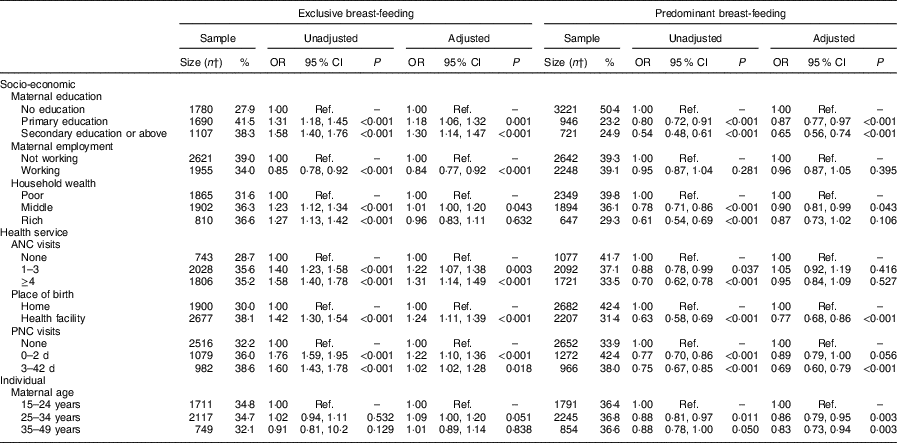
DRC, Demographic Republic of Congo; ANC, antenatal clinic; PNC, postnatal clinic; Ref., reference category.
Exclusive breast-feeding=infants 0–5 months of age who received breast milk as the only source of nourishment (but allows oral rehydration solution, drops, or syrups of vitamins and medicines); predominant breast-feeding=infants 0–5 months of age who received breast milk as the predominant source of nourishment (but allows water and water-based drinks fruit juice, ritual fluids, oral rehydration solution, syrups or drops of vitamins).
Multivariate models adjusted for the potential confounding factors of maternal marital status, place of residence, child’s age and gender.
* Burkina Faso (2010), N 5710; DRC (2013), N 6797; Ethiopia (2013), N 4193; Kenya (2014), N 7024; Mali (2013), N 3802; Niger (2013), N 4930; Nigeria (2013), N 11 712; Tanzania (2015), N 3894; and Uganda (2010), N 2913.
† Number of children aged 0–5 months who were exclusively or predominantly breast-fed.
The odds for predominant breast-feeding were significantly lower in educated mothers (AOR=0·87; 95 % CI 0·77, 0·97; P<0·001 for primary education and AOR=0·65; 95 % CI 0·56, 0·74; P<0·001 for secondary education or above) and mothers who delivered at a health facility (AOR=0·77; 95 % CI 0·68, 0·86; P<0·001; Table 2). Higher frequency of PNC visits and higher maternal age were significantly associated with lower likelihood for predominant breast-feeding (AOR=0·69; 95 % CI 0·60, 0·79; P<0·001 for 3–42 d PNC visits; AOR=0·86; 95 % CI 0·79, 0·95; P=0·003 for age 25–34 years and AOR=0·83; 95 % CI 0·73, 0·94; P<0·003 for age 35–49 years).
Mothers who delivered their babies at a health facility were significantly more likely to initiate breast-feeding within the first hour of birth compared to those who delivered at home (AOR=1·48; 95 % CI 1·40, 1·56; P<0·001). Improved household wealth (rich) and higher frequency of ANC visits were significantly associated with timely initiation of breast-feeding (Table 3).
Table 3 Determinants of early initiation of breast-feeding and bottle-feeding in nine sub-Saharan African countries with high burden of diarrhoea mortality; data from the most recent and pooled Demographic and Health Survey data sets for 50 975 children under 24 months of ageFootnote *
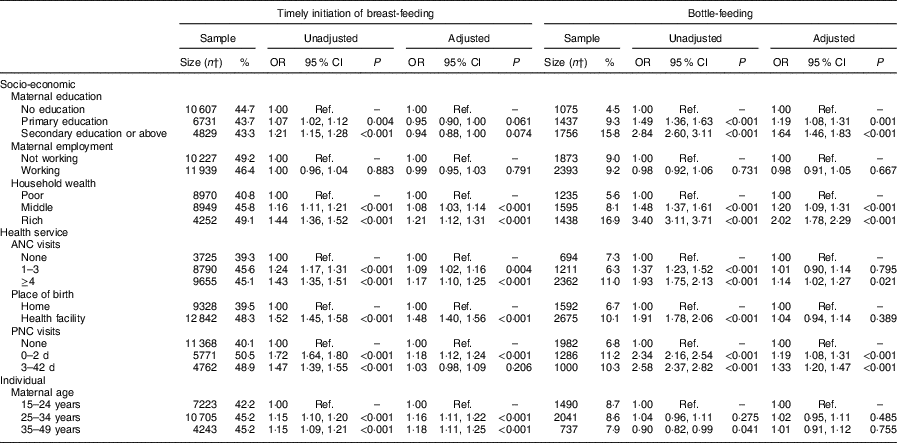
DRC, Demographic Republic of Congo; ANC, antenatal clinic; PNC, postnatal clinic; Ref., reference category.
Timely initiation of breast-feeding=children 0–23 months of age who were put to the breast within one hour of birth; bottle-feeding=children 0–23 months of age who received any liquid (including breast milk) or semi-solid food from a bottle with a nipple/teat.
Multivariate models adjusted for the potential confounding factors of maternal marital status, place of residence, child’s age and gender.
* Burkina Faso (2010), N 5710; DRC (2013), N 6797; Ethiopia (2013), N 4193; Kenya (2014), N 7024; Mali (2013), N 3802; Niger (2013), N 4930; Nigeria (2013), N 11 712; Tanzania (2015), N 3894; and Uganda (2010), N 2913.
† Number of children aged 0–23 months who were timely initiated to breast milk or bottle-fed.
Educated mothers were more likely to bottle-feed their babies compared with mothers with no education (AOR=1·64; 95 % CI 1·46, 1·83; P<0·001 for secondary education or above; Table 3). Improved household wealth (middle and rich) was significantly associated with higher likelihood for bottle-feeding (AOR=1·20; 95 % CI 1·09, 1·31; P<0·001 for middle household wealth and AOR=2·02; 95 % CI 1·78, 2·29; P<0·001 for rich household wealth). Higher frequency of PNC visits was associated with bottle-feeding (AOR=1·33; 95 % CI 1·20, 1·47; P<0·001 for 3–42 d visits).
Discussion
In SSA countries with high diarrhoea mortality, EBF prevalence was higher in Uganda, Ethiopia and Tanzania, but lower in Nigeria and Niger. Predominant breast-feeding was lower in Kenya, Uganda and Tanzania, but higher in Burkina Faso. Prevalence of timely initiation of breast-feeding was highest in Mali, while bottle-feeding rate was highest in Uganda, Nigeria, Ethiopia and Kenya. Higher educational attainment and frequent health-service contacts of mothers (i.e. ANC visits, PNC visits and delivery at a health facility) were associated with EBF. Frequent ANC visits, higher household wealth and delivery at a health facility were associated with timely initiation of breast-feeding. Mothers with education and those from wealthier households were more likely to bottle-feed.
Epidemiology of breast-feeding in sub-Saharan African countries with high diarrhoea mortality
Previous population-based studies conducted in SSA countries reported that the overall prevalence of EBF was 36 %( Reference Yalçin, Berde and Yalçin 25 ) and that the proportion of mothers who continued to breast-feed at 12 months was among the highest globally( Reference Victora, Horta and de Mola 26 ). The authors found that EBF prevalence was highest in Rwanda, Malawi, Burundi, Ghana and Zambia, but lowest in Guinea, Nigeria, Côte d’Ivoire, Sierra Leone and Gabon( Reference Yalçin, Berde and Yalçin 25 ). Consistent with a previous study( Reference Yalçin, Berde and Yalçin 25 ), our study – which was specific to countries with high diarrhoea mortality – found that EBF rates were higher in Tanzania and Ethiopia, but lower in Nigeria and Niger. The previous study did not provide estimates for Uganda, which had the highest EBF prevalence in our analysis. A study from Tanzania found that 46 % of mothers initiated breast-feeding within the first hour of birth and that 16 % predominantly breast-fed( Reference Victor, Baines and Agho 16 ), which is consistent with our findings.
Nationally representative epidemiological studies that focus on key breast-feeding practices are limited in many SSA countries, including Niger, Mali, Uganda and Democratic Republic of Congo, compared with Ethiopia, Kenya, Nigeria and Tanzania. The present findings were consistent with studies from SSA which showed that lower maternal education, maternal employment, young maternal age, fewer visits to health facilities and lower household wealth were associated with suboptimal breast-feeding( Reference Victor, Baines and Agho 16 , Reference Ogbo, Page and Agho 17 , Reference Yalçin, Berde and Yalçin 25 , Reference Kimani-Murage, Madise and Fotso 27 ). Other contextual factors that have been shown to negatively impact optimal breast-feeding in SSA with high diarrhoea mortality include: a cultural belief system that seldom supports optimal breast-feeding( Reference Agho, Ogeleka and Ogbo 23 ); parents’ preference for a male child; limited family and political support; poor financial investment; limited enforcement of the Code; and fragmented health systems with poor support for the BFHI( Reference Rollins, Bhandari and Hajeebhoy 28 , Reference Ogbo, Page and Idoko 29 ). Determinants of breast-feeding practices in SSA with high diarrhoea mortality are multifaceted; however, government measures, facility- and community-based initiatives that are comprehensive, measurable and culturally appropriate can be employed to ensure improvements in breast-feeding practices in SSA countries with high diarrhoea mortality( Reference Rollins, Bhandari and Hajeebhoy 28 ).
Policy and pragmatic implications of the findings
Our study found that countries such as Uganda (63 %), Tanzania (63 %) and Ethiopia (53 %) currently have higher EBF rates than Global Nutrition Target 5 (which is to increase the EBF rate to at least 50 % by 2025)( 30 ). Despite these in-country achievements, sustained efforts are still needed to ensure that EBF rates remain on an upward trajectory, particularly given the higher rates of bottle-feeding in Uganda and Ethiopia. A multicentre large-scale community-based initiative conducted in Bolivia, Ghana and Madagascar, with appropriate funding from an international donor, resulted in improvements in breast-feeding practices( Reference Quinn, Guyon and Schubert 31 ). Evaluation of breast-feeding initiatives in countries such as Nigeria, Niger, Burkina Faso and Kenya (with low EBF rates), and the full implementation of context-specific and cost-effective community-based interventions that seek to improve breast-feeding practices, are warranted in SSA countries with high diarrhoea mortality. Improvements in breast-feeding practices will not only result in the achievement of Global Nutrition Target 5( 30 ), but also are a key aspect to attain Sustainable Development Goal 3 of ending preventable deaths among under-5s( 32 ).
Evidence from Sri Lanka revealed that improvements in breast-feeding practices were observed over a 10-year period as a result of interventions at national and sub-national levels. The initiatives included high-level political support for breast-feeding programmes, effective and clear transmission of breast-feeding messages through multiple communication networks, and a culture supportive of breast-feeding( 33 ). National-level policy, programme and coordination for breast-feeding interventions are present in many SSA countries, including Tanzania( 34 ), Nigeria( 35 ), Uganda( 36 ), Kenya( 37 ) and Ethiopia( 38 ). However, limited resources, a dependence on donor funding and a lack of sub-national breast-feeding committees have been flagged as challenges to improving breast-feeding outcomes in those countries. Scaling up efforts to achieving the Global Nutrition Targets in SSA countries with high diarrhoea mortality would require the creation and strengthening of sub-national breast-feeding committees and increased public health financing, galvanised by strong political resolve at all levels of government( Reference Rollins, Bhandari and Hajeebhoy 28 , 33 ). Integrated networks of both national and sub-national breast-feeding committees that coordinate and advocate for breast-feeding at the health system and community levels would also maximise impacts.
The present study found that maternal employment and lower household wealth were associated with suboptimal breast-feeding practices. In many SSA countries, employed mothers are entitled to 3 months of fully paid maternity leave, but this time period is inadequate for mothers to appropriately engage in optimal EBF. Evidence from Canada( Reference Baker and Milligan 39 ) and Scotland( Reference Skafida 40 ) has shown that the expansion of maternity leave and appropriate employer-driven support for female employees post-birth were associated with optimal breast-feeding behaviours. Legislative changes to employment regulations to allow employed mothers to appropriately breast-feed their infants are continuing in SSA countries. For instance, a regional Nigerian government recently increased paid maternity leave from 3 to 6 months for female public servants and initiated a 10 d paid paternity leave for male public workers, with a view to promote optimal infant feeding and improve household income and productivity( Reference Ogbo, Page and Agho 17 ). Similarly, efforts are ongoing in Kenya to extend the maternity leave from 3 to 6 months, with the promotion of breast-feeding as part of the rationale for the proposed policy( Reference Igadwah 41 ). In Tanzania, labour regulations were recently amended to allow female employees to breast-feed for 2 h during working hours for at least six consecutive months, after 3 months of fully paid maternity leave( Reference Velma Law 42 ). These workplace changes in the form of legislation are needed at the national level in SSA countries with high diarrhoea mortality to improve breast-feeding practices.
In addition, a cohort study from the UK revealed that incentives for employers to support breast-feeding mothers promulgate employer-driven support for optimal breast-feeding( Reference Hawkins, Griffiths and Dezateux 43 ). In SSA countries with high diarrhoea mortality, economic incentives (such as tax rebates) to private employers and self-employed mothers may be required to provide environments conducive for breast-feeding (such as provision of crèches or breast-feeding breaks). The enforcement of the Labour Act to maternity protection (particularly among private employers) and establishment of measures to monitor compliance are also proposed as adjuncts to improve breast-feeding behaviours of employed mothers( Reference Rollins, Bhandari and Hajeebhoy 28 ).
Our study showed that bottle-feeding rates were highest in Nigeria, Ethiopia, Kenya and Uganda (≥10 %). This was despite Uganda’s high rate of EBF at 63 %. The Code seeks to protect, promote and support breast-feeding at the facility and community levels and has been adopted and streamlined as legislation in all SSA countries with high diarrhoea mortality, with the exception of Kenya, where compliance to the Code remains voluntary( 44 ). For example, in Nigeria, the Code was ratified as the National Agency for Food and Drug Administration and Control Act (as amended)( 35 ), and as the Food and Drugs Act of 1997 in Uganda( 44 ). Reports of violation of the Code are numerous in many developing countries( 35 , 45 – Reference Sokol, Aguayo and Clark 48 ), including Burkina Faso, Nigeria and Uganda, with subsequent increase in infant formula sales and impact on bottle-feeding( Reference Piwoz and Huffman 49 ). The Code violation has been largely attributed to a lack of enforcement and training for officers( Reference Taylor 50 ), as well as the marketing strategies of infant food manufacturers( Reference Brady 51 ). Capacity development for enforcement officials, health workers and key stakeholders, as well as proper enforcement of the Code and adequate funding for enforcement institutions, are also needed as strategies to reduce the increasing trends in utilisation of infant formula in SSA countries with high diarrhoea mortality( Reference Rollins, Bhandari and Hajeebhoy 28 , Reference Piwoz and Huffman 49 ).
Previous studies from developing countries (including Kenya, Nigeria and Uganda) have indicated that the BFHI has been influential in improving breast-feeding behaviours( Reference Braun, Giugliani and Soares 52 – Reference Pérez-Escamilla 56 ). The present study found that fewer contacts with the health facility (i.e. limited ANC and PNC visits or home birthing) were associated with suboptimal breast-feeding practices. Strengthening the BFHI in the form of continued training for health professionals and traditional birth attendants, as well as the incorporation of the Baby Friendly Community Initiative (BFCI) in scale-up efforts would maximise breast-feeding outcomes in SSA countries, particularly in communities with a higher proportion of home birthing and poor health-service use. Monitoring and evaluation strategies of BFHI-certified facilities as set out in the establishment of the BFHI will also have a positive impact on breast-feeding rates.
The WHO and UNICEF developed the BFCI to promote and support optimal breast-feeding at the community level( Reference Cattaneo, Bettinelli and Chapin 57 ). In SSA countries with high diarrhoea mortality, family members have an influence on new mothers and provide necessary support to mothers post-birth, but their advice may reflect cultural beliefs which may not promote optimal breast-feeding( Reference Grassley and Eschiti 58 ). Studies have indicated that community-based interventions that involved a close family member and postnatal home visits resulted in improvement in breast-feeding behaviours( 33 , Reference de Oliveira, Camacho and Tedstone 59 ). Our study showed that most mothers gave birth at home in Ethiopia, Niger and Nigeria. Aspects of the BFCI may increase utilisation of health services among mothers, with subsequent impact on breast-feeding rates. Community-based breast-feeding initiatives (e.g. BFCI) should be incorporated into infant nutrition programmes in high-priority areas and should be tailored to the specific sociocultural and socio-economic environment in which mothers live to maximise health outcomes.
Strengths and limitations
The present study has specific limitations. First, the breast-feeding indicators were collected as self-reported measures, and this may have resulted in a recall and/or measurement bias. These measures may have underestimated and overestimated the association between study factors and breast-feeding practices. Second, unmeasured confounding factors (e.g. culture, partner support or maternal health status following childbirth) may have affected the study results. Finally, the establishment of a temporal association between study factors and breast-feeding indicators is challenging given that we employed cross-sectional data. Despite these limitations, the study findings are nationally representative for each country studied. We believe that selection bias is unlikely to have affected the study findings because of the high response rates in the surveys (96–99 %). Our study provided evidence on key breast-feeding practices in nine SSA countries with high diarrhoea mortality, using reliable and comparable data that were collected with a standardised questionnaire across geographical regions.
Conclusion
The present study found that EBF prevalence was low and varied across SSA countries with high diarrhoea mortality; higher in Uganda, Ethiopia and Tanzania, but lower in Nigeria and Niger. Determinants of suboptimal breast-feeding included no maternal education, low household wealth and limited contact with health services. Mothers from wealthier households and those with higher educational attainment engaged in bottle-feeding. To improve breast-feeding practices in SSA countries with high diarrhoea mortality, infant feeding initiatives should target all mothers, particularly women from poor households and those with limited access to health services. In addition, targeting mothers from high-income households will also increase breast-feeding rates.
Acknowledgements
Acknowledgements: The authors are grateful to Measure DHS/ICF International for providing the data for the analysis. Financial support: This study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: The authors declare that they have no competing interests. Authorship: F.A.O. conceptualised the study, obtained the data, analysed and interpreted the data, drafted the initial manuscript and critically revised the manuscript. K.E.A., A.P. and J.E. contributed to the conceptualisation of the study, analyses and interpretation of the data, and critically revised the manuscript. All other authors contributed to acquisition and interpretation of data, and critically revised the manuscript. All authors read and approved the final manuscript as submitted. Ethics of human subject participation: Ethical approvals for data collection and subsequent analyses were obtained by Measure DHS project from each country-specific research ethics committee before the surveys were conducted. Measure DHS/ICF International granted permission to use the data.







