Introduction
During the past decades, the optimal amount of nutrition and route of feeding in critically ill patients have been debated controversially in the literature(Reference Preiser, van Zanten and Berger1). It is currently unclear what the optimal protein energy targets should be and exactly when they should be reached(Reference Elke, van Zanten and Lemieux2). Current international nutrition guidelines recommend the initiation of medical nutrition therapy in the form of enteral nutrition (EN) within 24–48 h in the critically ill patient who is unable to maintain sufficient oral intake(Reference McClave, Taylor and Martindale3–Reference Reintam Blaser, Starkopf and Alhazzani6). However, EN alone is often insufficient to achieve energy and protein targets particularly in the early phase of critical illness due to frequent interruptions for procedures and metabolic or gastrointestinal (GI) intolerance(Reference Heyland, Schroter-Noppe and Drover7).
Parenteral nutrition (PN) provides advantages in achieving target nutrition goals earlier, which might be particularly relevant in patients at high nutrition risk. In fact, the combined use of EN and PN may reduce large nutrition deficits in critically ill patients and might be attractive in those patients who cannot achieve their energy and protein goals during their intensive care unit (ICU) stay from EN alone(Reference Bost, Tjan and van Zanten8). One strategy to optimise protein intake is to combine EN and PN (EN+PN) early after admission to the ICU to reach nutrition targets in patients at nutritional risk as soon as possible. Another approach would be the early initiation of EN with the addition of supplemental PN if the nutritional targets cannot be reached by EN alone (SPN) after several days.
For critically ill patients, achieving the protein goal is perhaps more important than achieving the energy goal, as several large-scale randomised controlled trials (RCT) have not been able to demonstrate any benefit from near-goal energy delivery(Reference Rice, Mogan and Hays9–Reference Allingstrup, Kondrup and Wiis11). The few RCT evaluating protein targets will be discussed in this paper, but clear evidence is still lacking. In fact, determining the optimal protein dose and timing for critically ill patients is a high-priority research question(Reference Arabi, Casaer and Chapman12). Even with a combined EN and PN approach, it may remain challenging to reach the currently recommended protein goals with available nutrition products.
The EFFORT trial investigates the influence of higher prescription of protein (≥2·2 g/kg per d) v. usual protein prescription (≤1·2 g/kg per d) on the outcome of nutritionally high-risk critically ill patients(Reference Heyland, Patel and Bear13). One of the biggest challenges in this trial will be continuously achieving adequate amounts of protein in the higher-dose group(Reference Heyland, Weijs and Coss-Bu14,Reference van Zanten, Petit and De Waele15) . Since this might be more consistently achieved through an early combination of EN+PN, we plan to conduct a substudy in the EFFORT trial wherein patients randomised to the higher-dose group automatically receive combined EN+PN v. EN alone in the usual-care group, known as the EFFORTcombo trial. The purpose of this paper is therefore to critically review the current evidence, to generate hypotheses and, thus, to provide the scientific rationale for the concept of combining EN+PN applied in the early phase of critical illness in nutritionally high-risk critically ill patients and to present the details of trial methods.
Current evidence and discussions about enteral and parenteral nutrition
EN is the most common route of feeding in the ICU(Reference Ridley, Peake and Jarvis16) and is uniformly recommended in current international nutrition guidelines(Reference McClave, Taylor and Martindale3–Reference Reintam Blaser, Starkopf and Alhazzani6). However, recent data have demonstrated that EN is still often withheld or started with significant delay after admission to the ICU in the clinical routine(Reference Heyland, Schroter-Noppe and Drover7,Reference Cahill, Dhaliwal and Day17) . The progression of EN into a full feed is highly subjective to the clinician(Reference Heyland, Schroter-Noppe and Drover7,Reference Cahill, Dhaliwal and Day17) and often takes several days due to feeding intolerance and common interruptions of EN(Reference Heyland, Cook and Winder18–Reference McClave, Sexton and Spain20). Thus, EN may lead to protein–energy deficiency with a possible negative impact on patient outcome – especially in the patient’s first ICU week(Reference Berger and Chiolero21–Reference Villet, Chiolero and Bollmann23).
For years, PN was thought to be associated with neutral or even harmful effects, as older studies suggested that the risk:benefit ratio for use of PN in the ICU setting may be much narrower than that for use of EN(Reference Casaer, Mesotten and Hermans24,Reference Kelly, Tappenden and Winkler25) . Few studies have indicated that the use of PN was associated with more infectious complications, most probably related to hyperalimentation and hyperglycaemia, as consistently shown in earlier meta-analyses(Reference Braunschweig, Levy and Sheean26–Reference Peter, Moran and Phillips-Hughes29). The Early Parenteral Nutrition Completing Enteral Nutrition in Adult Critically Ill Patients (EPaNIC) study by Casaer et al. (Reference Casaer, Mesotten and Hermans24,Reference De Vlieger, Ingels and Wouters30–Reference Casaer, Langouche and Coudyzer33) demonstrated some potentially harmful effects of early PN in critically ill patients. In this study, patients were randomised to early supplementation of insufficient EN with PN v. withholding PN for 1 week(Reference Casaer, Mesotten and Hermans24). Patients in the early PN group received intravenous glucose under conditions of intensive insulin therapy for the first 3 d, when EN was still insufficient, and then, if the patient was still in the ICU, PN was started on day 3. In the late PN group, PN was only initiated at day 8. The major findings demonstrated that early PN led to a prolonged dependency on intensive care treatments and increased infection rates. In contrast, withholding PN improved clinical outcomes, which was associated with relevant cost-saving effects. Importantly, in the large subgroup with a contraindication for EN upon admission, harm by early PN was even more pronounced, whereas the authors suggested a suppression of the physiological response mechanism autophagy by feeding in the PN group as reason for the observed negative effects. Yet, there are several limitations that limit the validity and generalisability of the findings. For example, the application of glucose instead of PN under conditions of tight glycaemic control within the first few days is rather rare at other ICU. As evidenced by the primary publication, the harm signal was evident in the early group even before PN started on day 3, so the harm cannot be attributed to the introduction of PN on day 3. Furthermore, the majority of patients underwent surgery (90 %) and within these 60 % cardiac surgery, resulting in an overall short ICU-stay (3–4 d) with a rather low mortality. Enrolled patients were thus at very low nutritional risk and would not have received any artificial nutrition in many ICU around the world. Thus, the results of the EPaNIC trial cannot be expanded to nutritionally high-risk patients in other settings.
Nevertheless, based on the EPaNIC findings and because EN was thought to be cheaper, safer and more physiological, international nutrition guidelines recommend that the enteral route should be preferably used in critically ill patients without a contraindication to EN(Reference McClave, Taylor and Martindale3,Reference Dellinger, Levy and Rhodes34–Reference Singer, Berger and Van den Berghe36) and did not support the routine use of PN in the early phase of critical illness(Reference Heyland, Dhaliwal and Drover37). However, the more recent evidence from randomised studies about the safety and efficacy of PN might make physicians more comfortable with prescribing PN earlier(Reference Harvey, Parrott and Harrison38,Reference Reignier, Boisrame-Helms and Brisard39) .
The CALORIES trial by Harvey et al. (Reference Harvey, Parrott and Harrison38) involved 2388 critically ill patients receiving exclusive PN or EN as soon as possible within 36 h after admission. No significant differences were found in adverse events, mortality or in the infectious complications, demonstrating the equivalence of EN and PN. However, this study included less severely ill patients(Reference Harvey, Parrott and Harrison38). More recently, Reignier et al. (Reference Reignier, Boisrame-Helms and Brisard39) investigated the effects of EN v. PN in the NUTRIREA-2 trial including 2410 patients receiving invasive mechanical ventilation and vasopressor support for shock. In this isoenergetic trial, early EN did not reduce mortality or the risk of secondary infections, but was associated with an increased risk of digestive complications such as vomiting, diarrhoea and bowel ischaemia when compared with early PN(Reference Reignier, Boisrame-Helms and Brisard39). Both the NUTRIREA-2 and CALORIES studies contrasted previously mentioned safety concerns about PN and overall challenged the paradigm that EN is superior to PN with respect to clinical outcomes in critical illness. The rather low amount of delivered protein in the EN and PN group, as well as the short duration of these studies may represent the main reasons why no clinical advantages could be detected either in the EN or in the PN group.
Given the fact that GI dysfunction is commonly observed in severely ill patients, and that PN was demonstrated to be safe in the more recent trials, early high-protein PN may help to securely and rapidly achieve the recommended nutrition goals during feeding intolerance and GI symptoms. The described concerns about EN safety and EN progression illuminate a promising opportunity for PN as alternative nutrition strategy to bridge the gap between the nutritional goals and delivered energy/proteins, whenever EN is withheld or reduced, at any time point during the ICU stay.
Experience in combining enteral and parenteral nutrition
Pichard and colleagues (Heidegger et al. (Reference Heidegger, Romand and Treggiari40)) systemically investigate the concept of EN and PN in the ICU to reduce overall nutrition deficiency(Reference Heidegger, Romand and Treggiari40). The pragmatic concept was introduced with the idea to start PN in patients with proven intolerance to EN and defined as supplemental PN (SPN). In an RCT, patients who were EN-intolerant, and therefore were unable to reach their nutritional target by day 3, were randomised to the control group (EN alone) or SPN. Nutritional targets were measured by indirect calorimetry. Only patients receiving less than 60 % of their target during the first 3 d were enrolled, therefore leading to a considerable protein–energy debt in all enrolled patients. In this trial, increased nutritional adequacy and a reduced number of nosocomial infections were observed in the SPN group(Reference Heidegger, Berger and Graf41).
In a different but related concept, the effect of a combined EN+PN strategy was tested in the recent TOP-UP pilot trial, where PN was started immediately after randomisation without testing for EN intolerance to achieve the prescribed nutrition goals, referred to as combined EN+PN(Reference Wischmeyer, Hasselmann and Kummerlen42). The energy targets were calculated in a pragmatic approach based on the actual body weight, with the overall goal to reach the full energy target at day 1 post-randomisation. The proposed nutrition strategy was feasible and effective regarding the separation of protein–energy intake between the two groups. Considering the clinical relevance, no overall benefit could be demonstrated in this small pilot study; however, the results revealed some encouraging trends of improved functional outcomes in the combined EN+PN group, which needs to be evaluated in following confirmatory studies.
The most recent EAT-ICU trial tested the effects of early goal-directed nutrition v. standard nutritional care in adult critically ill patients(Reference Allingstrup, Kondrup and Wiis11). In the early goal-directed nutrition group, the nutritional requirements were estimated by indirect calorimetry and 24 h urinary urea. This group received an intense EN+PN therapy to cover 100 % of the calculated target. Patients randomised to the control group received standard care, providing 25 kcal/kg per d (104·6 kJ/kg per d) by EN alone. While the feasibility of this strategy was demonstrated by a significant separation of both treatment groups with respect to energy and protein uptake, no significant effect was detected regarding clinical relevance. However, frequent hyperglycaemia despite extraordinarily high dosages of administered insulin demonstrated rather poor metabolic control, which overall might have influenced the evaluated physical outcome assessment as the primary endpoint.
Table 1 gives a short summary of the characteristics of the above-mentioned trials.
Table 1. Comparison of recent trials combining enteral nutrition (EN) and parenteral nutrition (PN)

SPN, supplemental parenteral nutrition; EN+PN, combined enteral and parenteral nutrition; EGDN, early goal-directed nutrition; APACHE II, Acute Physiology and Chronic Health Evaluation II; SAPS II, Simplified Acute Physiology II; SOFA, Sequential Organ Failure Assessment; IBW, ideal body weight; BW, body weight.
What can we learn from recent trials?
Focus on the right patients
One of the reasons why recent trials aiming at high amounts of energy or protein in the ICU setting have failed to demonstrate a positive outcome might be inappropriate patient populations. For example, well-nourished patients following elective surgery, with a short ICU length of stay, such as those studied in the EPaNIC trial are unlikely to benefit from augmented feeding approaches (or requiring artificial feeding at all). Critically ill patients are a heterogeneous group of patients with respect to the extent to which they will benefit from artificial nutrition therapy.
The patients’ previous nutritional state is of paramount importance as it determines the availability of self-defence mechanisms such as endogenous antioxidant mechanisms(Reference Chow43,Reference Khare, Mohanty and Das44) . On the other hand, patients who are either previously malnourished or at risk of malnutrition (either under- or overweight), or with expected prolonged ICU stay will most likely benefit from an intense nutrition therapy(Reference Heyland, Dhaliwal and Jiang45–Reference Khalid, Doshi and DiGiovine49).
In extension to the assessment of nutritional risk, increasing attention is paid to the presence of sarcopenia, frailty and the associated impaired physical functioning, as they have been demonstrated to be important predictors of a longer ICU and hospital length of stay, post-discharge mortality, quality of life and lower likelihood to return to home, as summarised in greater detail in recent reviews(Reference Bear, Wandrag and Merriweather50–Reference Jolley, Bunnell and Hough52). Notably, sarcopenic patients might benefit from an intense nutritional therapy, as recently demonstrated by Koga et al. (Reference Koga, Fujita and Yagi53) in a retrospective analysis, where sarcopenic patients supplied with early EN showed a reduced hospital mortality compared with those who did not receive early EN, while that effect was not visible in non-sarcopenic patients.
Focus on protein
The influence of protein on the outcome of critically ill patients has been discussed, controversially(Reference Heyland, Patel and Bear13,Reference Heyland, Stapleton and Compher54) , but the above-displayed evidence leads to the conclusion that nutrition interventions targeting only energy adequacy did not show statistically significant improvements in many studies. Increased protein intake, however, was associated with improved long-term physical recovery and lower mortality in observational trials(Reference Alberda, Gramlich and Jones47,Reference Heyland, Stephens and Day55–Reference Ferrie, Allman-Farinelli and Daley57) and did not influence duration of renal dysfunction(Reference Doig, Simpson and Bellomo58).
One systematic review performed by Davies et al. (Reference Davies, Chapple and Chapman59) showed no relationship between protein delivery and mortality whereas both the low and high protein groups in this review were protein-malnourished (0·67 g/kg per d and 1·02 g/kg per d). However, even in nutrition trials targeting the adequate provision of protein, EN failed to provide more than 1·5 g/kg per d(Reference van Zanten, Petit and De Waele15), highlighting the need for high-protein nutrition products or effective strategies to reach the protein goals. Heyland et al. (Reference Heyland, Patel and Bear13) recently performed a meta-analysis assessing the effect of higher v. lower protein intake but the effect could not be analysed in detail due to high heterogeneity of the existing trials and incomplete datasets. The authors were only able to aggregate the effect of higher protein dosing on mortality (risk ratio 0·89; 95 % CI 0·66, 1·19; P=0·42)(Reference Heyland, Patel and Bear13). Despite the current lack of evidence and controversial discussion, current guidelines recommend the daily provision of 1·2–2·5 g/kg protein(Reference McClave, Taylor and Martindale3,Reference Singer, Blaser and Berger5,Reference Elke, Hartl and Kreymann60) .
Focus on functional outcomes
Outcome measures should be patient-centred, reliable, accurate and simple to measure in ways that minimise bias. The majority of large RCT are measuring ‘hard’ outcomes, because they are objective, comparatively easy to obtain and clearly observable by researchers. Major outcome parameters, such as mortality, have been used in nutrition trials despite observed decreasing overall mortality rates and therefore many nutrition trials have remained non-significant. Although these parameters are undoubtedly important, they do not adequately capture the patients’ perspective after discharge from hospital and might not be sensitive enough for nutrition interventions(Reference Poolman, Swiontkowski and Fairbank61). With the paradigm ‘add life to years, not years to life’, more and more interventions aim to increase the quality of life after critical illness(Reference Dowdy, Eid and Sedrakyan62–Reference Dinglas, Faraone and Needham65). In this connection, the evaluation of mid- and long-term survival by functional outcomes is increasingly considered, because these outcomes evaluate muscle mass, muscle function and physical function closely connected to the patient’s quality of life in the longer term(Reference Parry, Huang and Needham66). Furthermore, functional outcomes reflect the overall state of the patient and are affected by a variety of treatments, not only nutrition and mobilisation.
More recent nutrition studies have used physical outcome assessment, or surrogate parameters and some have revealed trends of improved functional outcomes in intense nutrition therapy groups(Reference Allingstrup, Kondrup and Wiis11,Reference Ridley, Peake and Jarvis16,Reference Wischmeyer, Hasselmann and Kummerlen42,Reference Chen67) . In addition, Wu et al. (Reference Wu, Zhong and Zhu68) observed unchanged ‘classic’ parameters such as hospital length of stay, postoperative morbidity rates, and standard blood biochemistry profiles, in a patient cohort after oesophagectomy. However, these patients had better physical functioning and less fatigue(Reference Wu, Zhong and Zhu68).
On the other hand, physical outcome assessment is complex, and its performance requires adequate teaching of study sites to receive reliable data for a rigorous knowledge transfer. Poor metabolic control, for example reflected by hyperglycaemia and a low number of patients, might have confounded the physical outcome assessment as the primary endpoint in the EAT-ICU trial(Reference Allingstrup, Kondrup and Wiis11). Additionally, the primary endpoint in this study showed some weakness as: (a) little evidence exists about its use, as it has rarely been used before; (b) the assessment at 6 months after ICU discharge bears the risk that the effects may be influenced by other relevant aspects than the ICU treatment itself; and (c) the physical outcome showed a large variance in the assessment, emphasising the need for strict adherence to standardised operation protocols. Based on these findings received from rather smaller clinical studies, a well-timed physical outcome assessment matching the study intervention is encouraged to be evaluated in following confirmatory studies(Reference Devlin, Skrobik and Gélinas69).
Conclusion
Based on the evidence gathered from recent trials the authors conclude as follows:
-
(1) Targeting energy adequacy only might not be enough to improve the outcome of critically ill patients. Increasing attention should be paid on effective supplementation strategies to achieve recommended protein goals.
-
(2) In isoenergetic trials, the route of administration might not influence ‘standard’ outcome parameters such as mortality and hospital length of stay.
-
(3) PN, as well as EN+PN seem to be safe, feasible and effective to achieve the prescribed nutritional targets in critically ill patients.
-
(4) Without consideration of metabolic tolerance, early aggressive EN+PN may not be effective in improving patient outcomes in unselected patients.
-
(5) In nutritionally high-risk patients, combined EN+PN may improve functional and other patient-reported outcomes.
From the EFFORT trial to the EFFORTcombo trial
Based on our review of the current evidence, we hypothesise that a combination of EN+high-protein PN v. EN alone in nutritionally high-risk patients can improve the functional outcomes. To test this hypothesis, we plan the nested substudy ‘EFFORTcombo’ in the context of the EFFORT trial.
The EFFORT trial (ClinicalTrials.gov/NCT03160547) was developed as a multi-centre pragmatic volunteer-driven, registry-based RCT in which 4000 patients will be randomly assigned to either a higher prescribed dose of protein (≥2·2 g/kg per d) or usual protein prescription (≤1·2 g/kg per d)(Reference Heyland, Patel and Bear13). However, the EFFORT trial does not specify how these determined protein dosages can be achieved. As protein delivery has been challenging in the past and only 55 % of prescribed protein (equal to 0·7 g/kg per d) is actually delivered as reported in the International Nutrition Survey (INS)(Reference Heyland, Weijs and Coss-Bu14), we propose that the addition of high-protein PN to EN, compared with EN alone, represents a promising nutrition strategy to increase nutritional adequacy to achieve the goals set in the original EFFORT trial. In comparison with the EFFORT trial, in the proposed multicentre EFFORTcombo (ClinicalTrials.gov/NCT04012333) substudy: (a) patients randomised to the high protein dosage will receive a combination of high-protein PN and EN; and (b) the main outcome for this substudy is short-term physical function as assessed by the 6-min walk test.
In addition, we will use a high-protein PN product and thus expect to reach the nutrition goals faster and more securely through this combination as shown in Fig. 1. We hypothesise that the augmented protein delivery to these nutritionally high-risk patients will translate into improved functional and patient-reported outcomes. Written informed consent will obtained from all patients or their legal representatives before enrolment. The ethics committee of the RWTH Aachen University approved the study (EK339/19) and local jurisdictional approval will be obtained for each centre.
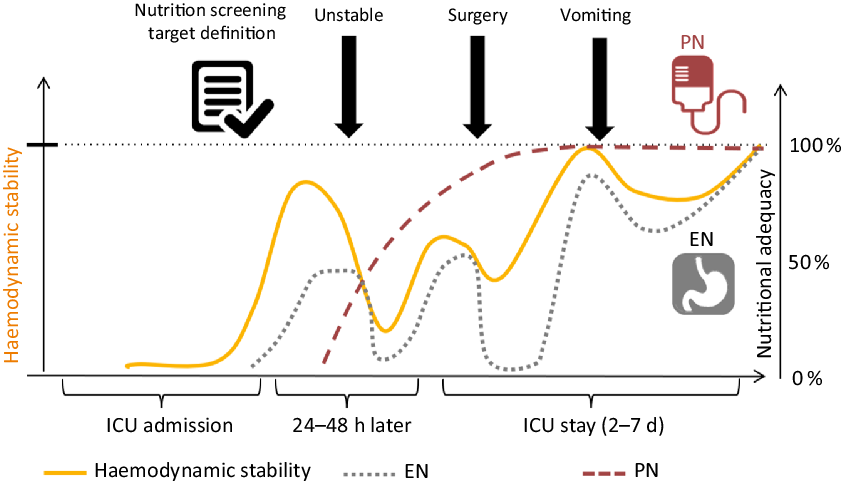
Fig. 1. The concept of nutrition support for critically ill patients. PN, parenteral nutrition; EN, enteral nutrition; ICU, intensive care unit.
Inclusion and exclusion criteria
As a nested substudy within the EFFORT trial, the EFFORTcombo study includes mechanically ventilated critically ill adult patients (≥18 years), who are at high nutritional risk as defined in detail in our published EFFORT protocol(Reference Heyland, Patel and Bear13). Table 2 illustrates in detail all inclusion and exclusion criteria.
Table 2. Inclusion and exclusion criteria: comparison between the EFFORT and EFFORTcombo trials, modified from Heyland et al. (Reference Heyland, Patel and Bear13)
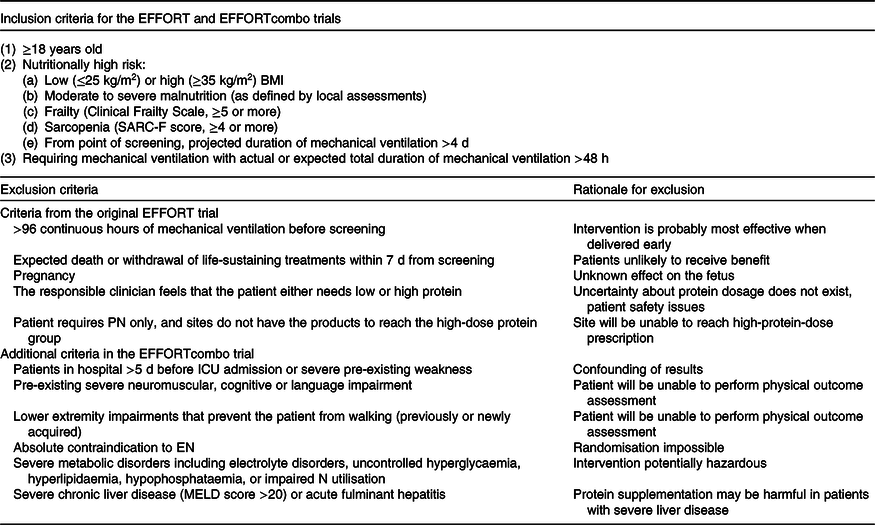
PN, parenteral nutrition; ICU, intensive care unit; EN, enteral nutrition; MELD, Model for End-Stage Liver Disease.
Investigational high-protein product
To provide high-protein PN in patients randomised to the EN+PN group, we will use Olimel® N12 with electrolytes provided by Baxter® International Inc. Olimel is a 3-in-1 parenteral admixture solution containing the following drug substances: dextrose solution, amino acid solution with electrolytes (Na, K, Mg, phosphate) and lipid emulsion with an olive oil:soyabean oil ratio of 80:20 and 12 g N/l. This product will be similar in energy density to the standard EN solutions (1–1·4 kcal/ml; 4·18–5·86 kJ/ml). Olimel® N12 will be administered via central venous line until the daily target of ≥2·2 g protein/kg per d is reached.
Peri-Olimel is a PN product that can be used either peripherally or centrally and will be used whenever a central venous line for PN is not available. Both products are indicated for PN in adults.
Nutrition protocol
As soon as the patient is haemodynamically stable and there is a nasogastric tube or feeding tube in place, EN will be started within 24–48 h after admission to ICU, as per local standards. If the patient has not been started on EN but there is an indication and intention to start on EN in the first 7 d, the patient will still be considered eligible for this study. The type of enteral formula should be of similar energy density (1–1·5 kcal/ml; 4·18–6·28 kJ/ml), but otherwise used in accordance with local standards. In both groups, targets will be set using pre-ICU known weight (for example, dry actual weight). For patients with BMI >30 kg/m2, ideal body weight based on a BMI of 25 kg/m2 will be used. As per current guidelines, we recommend monitoring for metabolic and GI tolerance as well as the provision of usual nutritional therapy by accredited clinicians with expertise in directing the feeding of critically ill patients. If equipoise regarding the nutritional regimen or protein dosage is not given in the clinician’s prescription for an individual patient, the patient will not be included in the trial. Metabolic and feeding tolerance will be assessed by blood glucose, insulin dose, glucose infusion rates, phosphate, urea, TAG and electrolytes, which will be monitored frequently, as clinically indicated and consideration of recent guidelines for monitoring of nutrition therapy will be endorsed(Reference Berger, Reintam-Blaser and Calder70).
Those patients randomised into the high protein group will receive EN+PN, with PN added as soon as possible following randomisation. While the identification and randomisation of appropriate patients will take 24–48 h, the PN should be started within 48–96 h. The study PN solution will be started at 25 ml/h and increased if tolerated (for example, the infusion rate can be increased by 25 ml every 4–6 h) so that >80 % of protein nutrition goals will be reached within 48–96 h of starting PN. We aim to avoid overfeeding energy and if the protein target cannot be met by combined EN+PN, protein supplements (enteral protein supplements or intravenous amino acids) should be added as per local standards to reach the goal of ≥2·2 g/kg per d. The PN rate will be adjusted in a compensatory fashion to ensure that patients receive >80 % of their target goal rate on a continuous basis, for example if EN infusion rates change due to GI intolerance or interruption. Therefore, PN should be continued for a minimum of 7 d even at a minimal rate (10 ml/h).
Both EN and PN will be continued for a minimum of 7 d post-randomisation and be continued on the ward. PN should be continued at a minimum of 10 ml/h until day 7 to enable easy compensations of the fluctuation in oral nutrition and/or EN rates as well on the normal ward. The EN rate will be always adjusted to the individual patients, while considering the minimum PN rate of 10 ml/h. At 7 d post-randomisation, if the patient is still in the ICU, and PN is clinically indicated to achieve high-protein goals, Olimel® N12E will be used in the high-dose group. In the low-dose group, if a patient develops a contraindication to EN, after day 7, PN can be used with product selection and duration determined by local standards but protein goals should not be above 1·2 g/kg per d. In either group, after the end of the 7 d post-randomisation study period, if the patient has been discharged from the ICU and PN is clinically indicated, standard PN solutions can be used. Olimel® N12E will be discontinued at ICU discharge (unless it occurs before day 7 as explained below), day 28 (maximum of PN treatment if the patients are still on ICU), or until death, whichever comes first.
The primary endpoint – functional outcome assessment
The primary objective of this substudy is to demonstrate improved short-term physical function by a 6-min walk test at hospital discharge. We also will assess in-hospital secondary outcomes and patient-reported 6-month outcomes similar to the NEXIS trial (ClinicalTrials.gov/NCT03021902). These secondary outcomes include the overall strength of upper and lower extremity (Medical Research Council sum score), quadriceps and handgrip strength (dynamometry), body composition (ultrasound and available CT scans), overall physical function (Short Physical Performance Battery and Functional Status Score for the ICU), which will be assessed longitudinally while the patient is still in the hospital. The physical functioning (Katz activities of daily living and Lawton’s instrumental activities of daily living) as well as health-related quality of life (Short Form-36 and EQ-5D-5L (5-level EQ-5D version)) will be assessed while the patient is in the hospital and 6 months after discharge. All outcome assessments will be performed by trained outcome assessors strictly following detailed standard operating protocols. All assessors will be blinded to the treatment group.
Summary
Taken together, international observational studies revealed considerable practice variations, and the existing clinical trial data, albeit weak and outdated, did not always support the routine use of PN in the early phase of critical illness. Importantly, the more recent evidence about the safety and efficacy of PN might make physicians more comfortable with prescribing PN earlier to bridge the gap between nutrition goals and actual delivery of energy and protein. This might be especially for patients at high nutritional risk, or patients with an increased risk for prolonged ICU stay. In this context, we are proposing the EFFORTcombo trial that evaluates the effects of an early combined EN + high-protein PN nutrition strategy to decrease the nutritional deficiencies in the critically ill patients at nutritional risk. We hypothesise that this nutritional strategy will improve the functional outcomes of these nutritionally high-risk patients.
Acknowledgements
The present review is endorsed by TIFOnet (Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin DGAI). We appreciate Elena Laaf, Jennifer Kistermann and Sebastian Wendt organising the educational meeting. We thank Laura Schmidt and Joao Batista for taking part as trainers in physical outcome assessment. We thank Katharina Seidenspinner, Christina Neubauer, Jennifer Corol, Daniel Tschopp, Isabel Maushagen and Gaby Oberson for participating in this meeting and sharing their expertise.
This is an investigator-initiated study. We thank Baxter Healthcare Corporation for providing financial support to organise an associated international and multi-professional expert meeting to debate current evidence, which is discussed in this paper, to teach best clinical nutrition practice and to help to draft a research agenda for future studies about combined EN+PN in critically ill patients. Baxter Healthcare Corporation had no influence on the design, analysis or writing of this article, or on the here-presented study protocol.
A. H., C. S. and D. K. H. contributed equally to the conception and design of the research together with S. J. S., R. S., C. H., C. L., G. E. and P. M.; A. H. and C. S. drafted the manuscript together with D. K. H.; figures were provided by A. H. and C. S.; A. H., C. S., K. C. C., J. N., U. S., D. E. B., S. L. and T. L. contributed to the acquisition of data and to the study selection. All authors contributed to analysis and interpretation of the reviewed data, critically revised the manuscript, agreed to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Several authors have conflicts of interest to declare:
G. E. has received lecture fees and travel expenses by Baxter Healthcare Corporation, Fresenius Kabi and consulting fees from Fresenius Kabi and Nutricia.
C. S. has received lecture fees and travel expenses by Fresenius Kabi and consulting fees from Fresenius Kabi and biosyn. C. S. received a co-funding grant from Baxter Healthcare Corporation to realise this investigator-initiated trial and Baxter Healthcare Corporation provides the investigational product for the here-presented EFFORTcombo study.
S. J. S. received research support from MSD (Haar, Germany) not related to this paper. He holds stocks in Rhoen-Klinikum, Bayer AG and Siemens AG and held stocks in the recent past from GE Healthcare, Merck & Co Inc., and Fresenius SE.
D. E. B. reports receiving advisory board fees, speaker fees or conference attendance support from Nutricia, Nestlé Nutrition, BBraun, Baxter Healthcare Corporation, Fresenius Kabi, Abbott Nutrition, Cardinal Health and Avanos.
U. S. reports on receiving personal fees from Fresenius-Kabi and on having received lecture fees as well as refunds of travel expenses from BA.Akademie, whereas all these revenues are not related to the submitted work.
The other authors declare no conflicts of interest that may be perceived as inappropriately influencing the representation or interpretation of the reported research results.
Registration
The EFFORT trial is registered with ClinicalTrials.gov as NCT03160547. The EFFORTcombo trial is registered as NCT04012333.





