Neural tube defects (NTD) are a group of congenital neurodevelopmental abnormalities including spina bifida and anencephaly, which have been shown in two landmark studies to be potentially preventable by periconceptional folic acid (FA) supplementation in two-thirds of cases( 1 , Reference Czeizel and Dudas 2 ). National and international guidelines recommend that all women planning a pregnancy should take 400 µg FA each day as an oral supplement, starting periconceptionally prior to the closure of the neural tube which occurs at 3–4 weeks after conception( 3 – 5 ). Scientific evidence suggests that the risk of NTD is substantially attenuated at red-blood-cell (RBC) folate concentrations of 906–1000 nmol/l( Reference Daly, Kirke and Molloy 6 , Reference Crider, Devine and Hao 7 ). In recent kinetic studies, it has been shown that it takes 12 weeks of daily supplementation with 400 µg FA to achieve these levels of RBC folate at which NTD prevention is optimised( Reference Lamers, Prinz-Langenohl and Bramswig 8 – Reference Hurthouse, Gray and Miller 10 ).
Irish studies suggest that 19–44 % of women take FA preconceptionally( Reference McKeating, Farren and Cawley 11 – Reference Cawley, Mullaney and McKeating 14 ). One recent longitudinal study conducted at our centre has shown that periconceptional FA supplementation has declined from 45 % of women in 2009 to 43 % of women in 2013( Reference McKeating, Farren and Cawley 11 ). While many Irish studies have reported on the use of FA supplements, only two Irish studies have commented on the duration of preconceptional FA use( Reference Tarrant, Younger and Sheridan-Pereir 12 , Reference Cawley, Mullaney and McKeating 14 ). These studies reported that only one in four women was taking FA for 3 months or more preconceptionally.
International data suggest that between 10 and 55 % of women use FA preconceptionally( Reference Brough, Rees and Crawford 15 – Reference Kim, Han and Cho 17 ). Cross-sectional studies from Korea and East London report the lowest rates of preconceptional FA use( Reference Brough, Rees and Crawford 15 , Reference Kim, Han and Cho 17 ). Ethnic minority groups have been shown to have lower rates of preconceptional FA use( Reference Howell, Barnett and Underwood 18 ). There is a dearth of studies however investigating duration of preconceptional FA use. Of those which have been published, data from the Norwegian Mother and Baby Study, a longitudinal study, showed that 11·7 % of women took FA for 2 months or more preconceptionally, while data from a cross-sectional study conducted in Quebec, Canada indicated of those women who took FA preconceptionally, over two-thirds took for ≥12 weeks preconceptionally( Reference Nilsen, Vollset and Gjessing 19 , Reference Morin, De Wals and St-Cyr-Tribble 20 ). While a limited number of international studies have investigated the timing at which FA supplementation commences during pregnancy, this remains an area which is largely under-investigated. Overall, findings from published studies in this area are variable and suggest that of those women who begin taking FA postconceptionally, 9 to 37 % commence taking FA between 4 and 6 weeks postconception( Reference Tarrant, Younger and Sheridan-Pereir 12 , Reference Brough, Rees and Crawford 15 , Reference Nilsen, Vollset and Gjessing 19 ). The longitudinal Norwegian Mother and Baby Study showed that among women who commenced FA postconceptionally, only 24 % began supplementing in the first month postconception; with one-third commencing FA in the second month postconception and just under half commencing FA after this point( Reference Nilsen, Vollset and Gjessing 19 ). A cross-sectional study conducted in an ethnically diverse population (50 % non-Caucasian) in East London asked pregnant women about their FA supplementation practices at that point in their pregnancy. It showed that of those women who commenced FA postconceptionally, 37 % started at less than 6 weeks’ gestation while 63 % started after this point( Reference Brough, Rees and Crawford 15 ). The main Irish study conducted in this area was a cross-sectional investigation among 214 women. It revealed that of those who started FA postconceptionally, only 9 % (n 19) commenced supplementation within the first month after conception( Reference Tarrant, Younger and Sheridan-Pereir 12 ).
Given the limited Irish data describing the duration of periconceptional FA supplementation and the rising incidence of NTD in Ireland, examination of the duration of periconceptional FA supplementation is important. Kinetic studies have shown that those who consumed 400 µg FA/d reached an RBC folate concentration associated with optimally reduced risk of NTD after 3 to 6 months( Reference Crider, Devine and Hao 7 – Reference Norsworthy, Skeaff and Adank 9 ). Therefore, it is important to establish whether or not women who take FA preconceptionally are doing so for the required duration to optimise NTD prevention. For those who do not take FA preconceptionally, it is important to examine the stage of pregnancy at which these women commence FA supplementation given that the neural tube has already closed by day 28 postconception.
Aside from these timing and duration issues, it is also important to determine the dose of FA used by women attending their first antenatal visit, to estimate the duration of FA supplementation required to optimise folate levels for NTD prevention.
The objective of the present study was to provide accurate estimates of the commencement time, duration and dosage of FA supplementation taken by Irish women in the periconceptional period. The study also aimed to establish the factors associated with optimal FA supplementation practices.
Methods
The present study was cross-sectional and observational, conducted between January 2014 and April 2016. A total of 856 women were recruited from the antenatal booking clinic of the Coombe Women and Infants University Hospital. This hospital is one of the largest maternity hospitals in the European Union and cares for women from all socio-economic groups and from across the urban–rural divide. The sociodemographic characteristics of our sample population are closely representative of the broader population attending the hospital for antenatal care( 21 ) and also reflect the major sociodemographic indicators of the national obstetric population( 22 ). These findings support the suitability of this sample population in undertaking an investigation of FA supplementation among pregnant women in Ireland( 21 , 22 ).
Data collection
Women’s clinical and sociodemographic details were computerised routinely at the first antenatal visit and updated immediately after delivery. The inclusion criteria for the current study were attendance for antenatal care after ultrasound examination confirmed an ongoing singleton pregnancy in the first trimester. The exclusion criteria were multiple pregnancies, age less than 18 years and an inability to understand English.
A convenience sample of women was recruited by a trained research dietitian at their first antenatal visit. If the woman agreed to participate in the study, she was provided with the relevant questionnaires. These questionnaires were completed with the research dietitian, taking approximately 20 min. Table 1 details the sociodemographic and other variables collected to characterise the sample population, along with the instruments used to collect these data. Table 2 describes the sub-categories for each of the categorical variables collected.
Table 1 Variables examined and data collection protocols

Table 2 Characteristics of the study population (n 856) presenting for antenatal care at a large university maternity hospital, Republic of Ireland, January 2014–April 2016
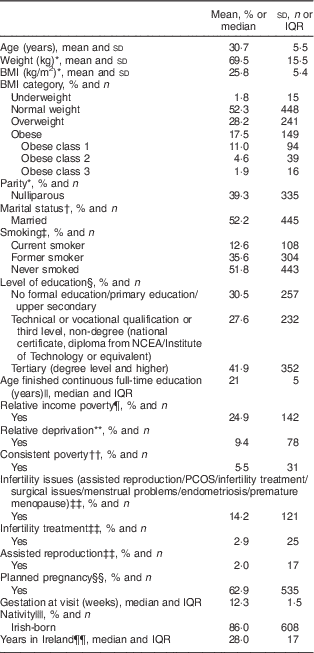
IQR, interquartile range; NCEA, National Council for Educational Awards; PCOS, polycystic ovary syndrome.
* Data for 853 women.
† Data for 852 women.
‡ Data for 855 women.
§ Data for 841 women.
|| Data for 490 women.
¶ Data for 570 women.
** Data for 833 women.
†† Data for 565 women.
‡‡ Data for 854 women.
§§ Data for 851 women.
|||| Data for 707 women.
¶¶ Data for 838 women.
Written informed consent was obtained from all study participants. The study was approved by the Hospital’s Research Ethics Committee (study reference number: 27-2013).
Folic acid questionnaire
The FA questionnaire included questions about the use of FA both pre- and periconceptionally. Data were also collected describing the stage of pregnancy at which FA supplementation was commenced and the number of weeks (both preconceptionally and postconceptionally) for which the woman took FA. The questionnaire also included questions about the dose and brand name of FA used and compliance with the established minimum duration of supplementation for optimal NTD prevention (i.e. 12 weeks of preconceptional FA( Reference Lamers, Prinz-Langenohl and Bramswig 8 – Reference Hurthouse, Gray and Miller 10 )).
Anthropometric data
Weight was measured digitally using a Tanita Body Composition Analyser model MC-180MA III (Tanita Corp., Tokyo, Japan). Height was measured to the nearest centimetre using a Seca wall-mounted digital metre stick with the woman standing in her bare feet (Seca, Birmingham, UK). BMI was calculated.
Sociodemographic questionnaire
Maternal characteristics such as socio-economic status, highest level of formal education, smoking status and number of years spent living in Ireland were obtained using the sociodemographic questionnaire. Information on socio-economic status was derived using questions from the Survey on Income and Living Conditions( 23 , 24 ). Material indices of disadvantage including relative income poverty and relative deprivation status were assessed, while consistent poverty status was also calculated. Relative income poverty status was determined by comparing equivalised household income against the 60 % median income threshold. Relative deprivation was assessed by determining whether respondents had experienced the enforced absence (due to financial constraint) of two or more basic necessities from a list of eleven over the past year. Consistent poverty was identified if a respondent reported being in relative income poverty, in addition to experiencing the enforced absence of two or more of the eleven basic markers of deprivation over the previous year( 24 ). The detailed algorithmic methods used for the calculation of these indicators are available in a document produced by the European Commission( 23 ), and this methodology has been adopted by the Irish Central Statistics Office( 24 ).
Statistical analysis
Data from the participant questionnaires were anonymised and coded on a Microsoft Excel© spreadsheet. Where appropriate, continuous variables were collapsed into categorical variables. The distribution of continuous data was assessed for normality by assessing the kurtosis and skewness of their distribution, the associated Kolmogorov–Smirnov statistics, and by a visual inspection of the distribution histogram and boxplot.
Descriptive statistics were first used to describe the characteristics of the study cohort. Inferential cross-tabulation with χ 2 tests for independence were then used to analyse differences in categorical variables (e.g. compliance with FA supplementation for ≥12 weeks preconceptionally) between different population groups. Finally, binary logistic regression analyses were performed to assess the independent association of a number of putative predictor variables with the likelihood of using FA for ≥12 weeks preconceptionally. Factors were included in the multivariate model based on their significant association with the use of FA for ≥12 weeks preconceptionally upon univariate analysis (P<0·05). In total, nine variables were included in the model: age, parity, marital status, smoking status, use of assisted reproduction, whether the pregnancy was planned or unplanned, years of full-time education completed, relative income poverty status and consistent poverty status.
Results
Table 2 shows the clinical and sociodemographic characteristics of the study population. This study population is largely representative of the hospital and national obstetric populations( 21 , 22 ).
Table 3 shows the duration and dosage of FA supplementation before the confirmation of pregnancy at the first antenatal visit. While almost all women (97 %) were taking FA at this stage of their pregnancy, only one in four women took FA for at least 12 weeks preconceptionally (24 %; 208/850).
Table 3 Folic acid (FA) supplementation practices among women (n 856) presenting for antenatal care at a large university maternity hospital, Republic of Ireland, January 2014–April 2016
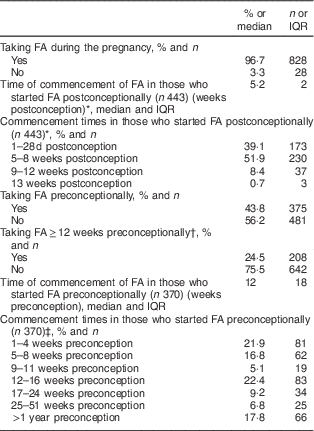
* Of the 481 women who did not take FA preconceptionally, 453 took FA postconceptionally and twenty-eight did not take FA at any point in pregnancy; data on duration of postconceptional FA usage for 443/453.
† Data missing for six women.
‡ Data on timing of commencement for 370 of the 375 women who reported taking FA before the pregnancy.
Of the women who took FA for any duration before pregnancy (44 %; n 375), 370/375 reported the number of weeks preconceptionally for which they had used FA. Of these 370, 56 % (208/370) took FA for ≥12 weeks preconceptionally.
Of the women who took FA only after becoming pregnant (n 453), 443 (98 %) reported the stage of pregnancy at which they started taking FA. Of these 443 women who took FA only after becoming pregnant, the median stage of gestation at which FA supplementation commenced was 5·2 weeks (36 d post conception), and 61 % (270/443) started taking FA more than 28 d postconception (i.e. after the closure of the neural tube). Of the 828 women who reported taking FA during their pregnancy, data describing compliance with supplement use existed for 822. Of these women, 97 % (796/822) reported daily supplement use.
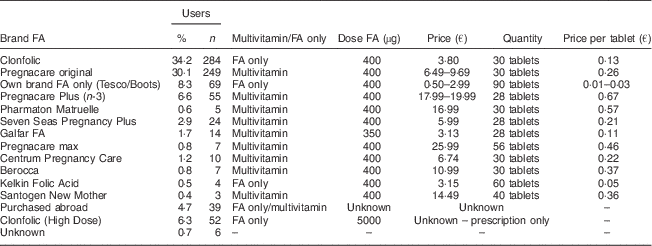
The women used a wide range of FA only and multivitamin supplements (Table 4). No woman used a generic multivitamin which contained 200 µg FA; the majority used either a prenatal multivitamin (400 µg FA) or an isolated FA only supplement (400 µg FA). Fourteen women (1·69 %; 14/828) reported taking Galfar FA.
Table 4 Brand and retail price of folic acid (FA) supplements taken by women (n 828) presenting for antenatal care at a large university maternity hospital, Republic of Ireland, January 2014–April 2016
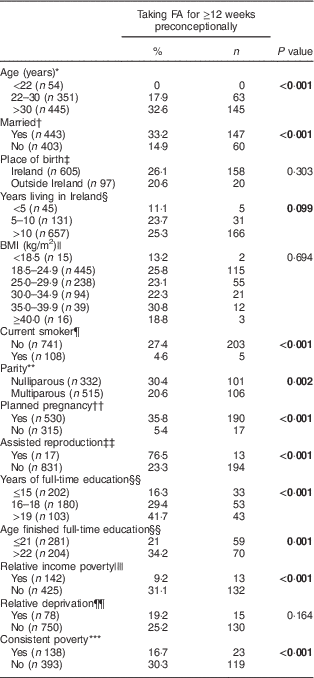
On further univariate analyses, the factors positively associated with FA supplementation for ≥12 weeks preconceptionally were increasing maternal age, being married, nulliparity, planned pregnancy, assisted reproduction and increasing level of education. Smoking, relative income poverty and consistent poverty were negatively associated with taking FA for ≥12 weeks preconceptionally (Table 5).
Table 5 Univariate analysis of factors associated with folic acid (FA) supplementation for at least 12 weeks preconceptionally among women (n 856) presenting for antenatal care at a large university maternity hospital, Republic of Ireland, January 2014–April 2016
Significant P values are indicated in bold.
* Data for 850 women.
† Data for 846 women.
‡ Data for 702 women.
§ Data for 833 women.
|| Data for 847 women.
¶ Data for 849 women.
** Data for 847 women.
†† Data for 845 women.
‡‡ Data for 848 women.
§§ Data for 485 women.
|||| Data for 567 women.
¶¶ Data for 828 women.
*** Data for 531 women.
When binary logistic regression analysis was applied to assess the factors associated with FA supplement use for ≥12 weeks preconceptionally, only planned pregnancy (P<0·001) and nulliparity (P=0·002) remained positively associated with this optimal duration of supplementation (Table 6). Fewer than half of the women in the total cohort were included in this model, as data describing years of full-time education were collected on only 485 of the 856 women. Another factor which contributed to the smaller sample size for the multivariate model was the inclusion of consistent poverty as an independent variable in the model. The calculation of consistent poverty requires the presence of both relative income poverty and deprivation status data. Previous studies have shown that many people are reluctant to answer questions regarding their income, and also that they may not remember or know exactly what their income is, particularly if it is expressed on a monthly basis( Reference Moore, Stinson and Welniak 25 , Reference Moore and Loomis 26 ). In the current study, data for consistent poverty could be derived for only 531 of the 856 study participants.
Table 6 Multivariate analysis of factors associated with folic acid (FA) supplementation for at least 12 weeks preconceptionally among women presenting for antenatal care at a large university maternity hospital, Republic of Ireland, January 2014–April 2016Footnote *
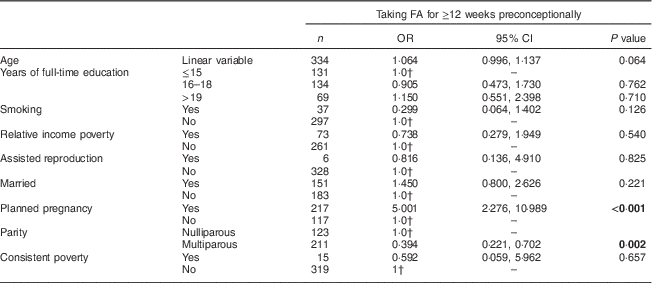
Significant P values are indicated in bold.
* Data for 334 women for whom all variables were available.
† 1·0 denotes the reference category.
Discussion
The current cross-sectional observational study of 856 women presenting for antenatal care found that almost all women (97 %) reported taking FA during their pregnancy. However, only one in four women had taken FA for at least 12 weeks before conception. Of those who started taking FA only after becoming pregnant, almost two-thirds reported starting FA after day 28 of gestation; that is, after the neural tube had closed (Table 3). On multivariate analysis only planned pregnancy and nulliparity remained significantly associated with taking FA for at least 12 weeks preconceptionally. This contemporary information is important for the design and dissemination of forthcoming public health campaigns intended to improve maternal FA supplementation practices and folate levels before and after conception.
Previous studies in Ireland have shown that 84–96 % of women use FA at any time during their pregnancy( Reference Tarrant, Younger and Sheridan-Pereir 12 – Reference Cawley, Mullaney and McKeating 14 , Reference McNulty, Pentieva and Marshall 27 ). Irish studies also showed that 19–44 % of women start FA supplementation before pregnancy( Reference Tarrant, Younger and Sheridan-Pereir 12 – Reference Cawley, Mullaney and McKeating 14 , Reference McNulty, Pentieva and Marshall 27 , Reference Burton, Wilson and Gillies 28 ). These findings concur with data from the current study, which show that almost all women were taking FA once they found out that they were pregnant and that 44 % were taking FA for some duration before conception.
Previous Irish studies investigating the timing and duration of FA supplementation preconceptionally showed that just one in four women takes FA for the optimal ≥12 weeks prior to conception( Reference Tarrant, Younger and Sheridan-Pereir 12 , Reference Cawley, Mullaney and McKeating 14 ). These data compare favourably with findings from East London which showed that only 12 % of women were taking FA at any point preconceptionally( Reference Brough, Rees and Crawford 15 ). These low rates of preconceptional FA use in East London may have been influenced by the high percentage of ethnic minority groups in the sample population; a factor which has previously been associated with suboptimal supplementation in other studies( Reference Howell, Barnett and Underwood 18 ). In a separate Canadian investigation, of those women who took FA preconceptionally, over two-thirds took for ≥12 weeks preconceptionally( Reference Morin, De Wals and St-Cyr-Tribble 20 ), a pattern consistent with current Canadian guidelines which recommend that FA be taken for 3 months preconceptionally( 29 ). European guidelines regarding FA supplementation do not currently feature an explicit recommendation concerning the required duration of FA supplementation preconceptionally. This suggests that there is a need to update our national and European FA guidelines to include a recommendation on the ideal timing of FA preconceptionally, in view of the recent kinetic studies in this area( Reference Crider, Devine and Hao 7 – Reference Hurthouse, Gray and Miller 10 ). This message should be incorporated into renewed public health campaigns in this area to enhance the clinical effectiveness of periconceptional FA supplementation.
This is important because recent kinetic studies suggest that it takes at least 12 weeks to achieve the 1000 nmol/l level of RBC folate which is associated with reduced risk of NTD( Reference Crider, Devine and Hao 7 ). Our study shows that one in four women reported taking FA for 12 weeks or more before becoming pregnant. However, of the women who reported taking FA before their pregnancy and who reported their duration of preconceptional FA use (370/375), only 56·2 % (208/370) reported taking FA for 12 weeks or more preconceptionally as is required to achieve these optimal RBC folate levels for NTD prevention. This suggests that even among those who are taking FA preconceptionally, duration of FA supplementation is suboptimal.
There is also a paucity of data examining the timing of FA commencement postconceptionally. In our study, of the women who started taking FA only after becoming pregnant (n 453), 443 reported the point at which they commenced FA supplementation. The median time for commencement of supplementation among these women was 5·2 (interquartile range 2) weeks (36 d) postconception. Of the 443 women who supplemented only postconceptionally, 39·1 % (173/443) reported starting FA between days 1 and 28 of their pregnancy, and 51·9 % (230/443) reported starting supplementation between weeks 5 and 8 of their pregnancy. This is important as the gross anatomy of the fetal brain and spine develop fully in the first few weeks of pregnancy (i.e. at 3–4 weeks’ gestation)( Reference Ray, Singh and Burrows 30 ). Studies from Ireland and internationally also suggest that of those women who start FA postconceptionally, the majority commence supplementation after the first month of gestation( Reference Tarrant, Younger and Sheridan-Pereir 12 , Reference Brough, Rees and Crawford 15 , Reference Nilsen, Vollset and Gjessing 19 ). It is possible that most women begin FA at week 5 postconception because this is when they first realise that they are pregnant. However, at this stage of the pregnancy, they have surpassed the window of opportunity to achieve optimal levels of RBC folate for NTD prevention( Reference Crider, Devine and Hao 7 – Reference Hurthouse, Gray and Miller 10 ).
There are limited Irish data investigating the brand of FA supplements being taken by women. A previous study( Reference Tarrant, Younger and Sheridan-Pereir 12 ) showed that the most popular brand was Clonfolic, a 400 μg FA/d mono-supplement, and this finding mirrors that of the current study which found that 34 % of pregnant women who supplemented took Clonfolic as their FA supplement of choice. Our study showed that 6·3 % of women were taking high-dose FA supplements. The majority of women supplementing with FA in the current study took a 400 μg/d dose. Fourteen women (1·69 %; 14/828) reported taking Galfar FA which contains 350 μg FA, with none reporting a lower dose of 200 μg/d which would be more typical of generic multivitamin supplements. A higher-dose FA supplement is recommended for women considered to be at higher risk of an NTD (e.g. those with a previous NTD-affected pregnancy, those with diabetes)( 5 ).
On multivariate analysis only planned pregnancy and nulliparity were associated with taking FA for at least 12 weeks preconceptionally, echoing the findings of our previous study( Reference McGuire, Cleary and Sahm 13 ) and suggesting that these factors are important determinants not only of the decision to take FA preconceptionally, but also of the duration for which women take FA preconceptionally. Planned pregnancy has been consistently positively associated with periconceptional FA use( Reference Tarrant, Younger and Sheridan-Pereir 12 , Reference Cawley, Mullaney and McKeating 14 , Reference McNally and Bourke 31 ); while lower parity has also been previously shown to predict FA supplementation in the preconceptional period( Reference Tarrant, Younger and Sheridan-Pereir 12 , Reference Cawley, Mullaney and McKeating 14 , Reference McNally and Bourke 31 , Reference Stockley and Lund 32 ). Unlike previous studies in the literature( Reference Farah, Kennedy and Turner 33 ), BMI was not associated with less favourable FA supplementation practices at any time preconceptionally.
The present study has strengths. First, the information on FA supplementation was recorded using a supervised questionnaire completed at a personal consultation with a single, trained dietetic researcher at the first antenatal visit. This reduced the risks of respondent error, recall bias or inter-observer variation. This supervised approach has previously been shown to result in lower levels of recall bias compared with self-administered questionnaires( Reference Bowling 34 ). Maternal socio-economic, sociodemographic and anthropometric data, including measured weight, height and BMI, were also recorded accurately in a standardised way, enhancing the integrity of those data( Reference Fattah, Farah and O’Toole 35 ). The study sample is also representative of the broader national obstetric population from a socio-economic and sociodemographic perspective( 22 ), increasing the applicability and relevance of our findings in the broader public health context. A potential weakness of the single-centre, convenience sampling method employed in the present study is that the women recruited may differ from the wider population. However, the hospital does accept women from all socio-economic groups, and from across the urban–rural divide, with roughly one in eight of all births in the Republic of Ireland being delivered at this hospital in 2013( 21 ). The substantially increased time and personnel costs associated with a consecutive sampling protocol precluded the use of such recruitment techniques in our study. However, by inviting all women attending the hospital to take part in the study, we aimed to recruit a sample population which was largely representative of the national obstetric population; and our post hoc analyses confirmed that this objective had been met. A further challenge with all observational studies is the potential for confounding when evaluating the association between individual factors and the outcome variable. However, this was addressed by using multivariate statistical analyses.
Conclusion
In conclusion, the present study shows that even among the 44 % of women who are supplementing with FA preconceptionally, 44 % (162/370) report taking FA for less than the required 12 weeks to achieve optimal RBC folate levels for NTD prevention. Among those who take FA only postconceptionally, the majority start supplementing after day 28 of gestation, at which point the neural tube has already closed. Given our finding that planned pregnancy and nulliparity are the factors most strongly associated with taking FA for at least 12 weeks preconceptionally, we suggest that future public health campaigns should encourage all women at risk of becoming pregnant, even those not planning a pregnancy, to take FA prophylactically. The low rates of preconceptional FA supplementation observed in the present study, and the suboptimal duration of preconceptional FA supplementation reported among even those who do take FA prior to conception, further emphasise the need for enhanced health messaging in this area. The delayed stage at which postconceptional FA supplementation is typically initiated, often at a time after the neural tube has already closed, constitutes a further strong rationale for the promotion of prophylactic FA supplementation in future public health campaigns. In the future, we are confident that we will be able to contribute to the knowledge gap which currently exists between FA intake levels and RBC folate.
Acknowledgements
Acknowledgements: The authors acknowledge the pregnant women who participated in this study. Financial support: This work was supported by Safefood and the University College Dublin Centre for Human Reproduction at the Coombe Women and Infants University Hospital, Dublin. Conflict of interest: None. Authorship: S.C. was involved in recruitment of the patients, collection and processing of the participant data, analysis of the data and drafting the manuscript. L.M. was involved in the analysis of the results and the drafting of the manuscript. R.K. was involved in the analysis of the results and drafting of the manuscript. M.F. was involved in the drafting of the manuscript. D.M. was involved in formulating the research question, designing the study and drafting the manuscript. M.J.T. was involved in formulating the research question, designing the study and drafting the manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki. All procedures involving human subjects/patients were approved by the Coombe Women and Infants University Hospital Research Ethics Committee. Written informed consent was obtained from all of the participating women.









