- CEBQ
child eating-behaviour questionnaire
Obesity is increasingly a cross-generational problem, with recent data revealing that 17% of US children and adolescents exceed the 95th percentile for BMI(Reference Ogden, Carroll, Curtin, McDowell, Tabak and Flegal 1 ), and rates in other developed countries are fast catching up(Reference Wang and Lobstein 2 ). Understanding the multiple causes of childhood obesity is essential to develop targeted and effective treatment and prevention. The influence on the whole population of environmental factors such as sedentary lifestyles and constant availability of cheap energy-dense food is undoubtedly powerful(Reference Hill, Wyatt, Reed and Peters 3 , Reference Jeffery and Utter 4 ), but one remarkable feature of the epidemic is the persistence of enormous individual variation in body weight throughout the population. Individual differences may even be increasing in both children and adults, with the lean staying lean while the fat get fatter(Reference Romon, Duhamel, Collinet and Weill 5 –Reference Wardle and Boniface 7 ), which indicates that individuals interact differently with the pervasively ‘obesogenic’ environment. Given that common obesity results from an imbalance between energy intake and expenditure, it is highly likely that this interaction occurs partly through individual variation in appetite and eating behaviours, and given that obesity is occurring in younger and younger children these individual differences might be expected to emerge early in life.
Appetitive traits and obesity in children
The present article will review evidence for associations between individual differences in appetite and adiposity, focusing specifically on the paediatric literature. Evidence relating to a small number of appetitive traits with potential associations with obesity will be addressed, i.e. low responsiveness to internal satiety signals such as gastric distension and changes in gut hormones (satiety responsiveness), high responsiveness to external food cues such as the smell, taste or sight of palatable food (food-cue responsiveness), the subjective reward experienced when eating liked foods (reinforcing value of food) and preferences for energy-dense foods (food preferences).
Satiety responsiveness and child obesity
Energy compensation
Case–control studies comparing obese and normal-weight adults have found support for impaired satiety responsiveness among obese individuals using a variety of behavioural methods(Reference Stunkard and Fox 8 –Reference Stunkard and Kaplan 11 ), and many of these methods have now been employed in children. One test of satiety responsiveness uses a preloading paradigm and is based on the assumption that an individual who is responsive to internal satiety cues will adjust their intake at a subsequent meal according to the energy content of the preload. When presented with a test meal immediately after a preload obese 8–12 year olds show no down-regulation of intake compared with a no preload condition, while normal-weight children show ‘energy compensation’, indicative of greater satiety responsiveness(Reference Jansen, Theunissen, Slechten, Nederkoorn, Boon, Mulkens and Roefs 12 ).
A number of other studies have used community samples, enabling the examination of distribution-wide relationships between compensation ability and weight. This method may be more relevant for 21st century obesity, which is no longer confined to an ‘abnormal’ few, but instead represents a large proportion of the upper end of a weight continuum(Reference Haworth, Plomin, Carnell and Wardle 13 ). Heavier individuals are at higher risk of excess weight and future health problems than leaner individuals, and a good theory should therefore be able to explain all weight variation, not just obesity. Taking a correlational approach one study has tested adjustment of lunch meal intake following consumption of high-energy drinks compared with low-energy drinks equated for taste and has found that poorer compensation is associated with greater adiposity in 3–5-year-old girls(Reference Johnson and Birch 14 ). However, despite using very similar methods another study has failed to find a relationship with weight in a sample of 3–7 year olds (n 64)(Reference Faith, Keller, Johnson, Pietrobelli, Matz, Must, Jorge, Cooperberg, Heymsfield and Allison 15 ), and in a study using solid food preloads followed by self-selected lunches 90 min later no association between compensation and weight was found in 6–9 year olds (n 74)(Reference Cecil, Palmer, Wrieden, Murrie, Bolton-Smith, Watt, Wallis and Hetherington 16 ).
In a study by the authors the aim was to match the child age-group (3–5 years) used in the previously mentioned study that found a significant relationship(Reference Johnson and Birch 14 ) and increase the reliability of the compensation index by conducting two different preloading tests. In one test the preloads consisted of a low-energy drink compared with a high-energy version created by adding polycose, a form of carbohydrate that increases energy without affecting taste (n 95). In the other test preloads were a familiar low-energy drink (water) compared with a familiar high-energy drink (strawberry-flavoured milkshake; n 77). To maximise ecological validity, testing was conducted at the children's schools and test meals were standardised lunches eaten with peers 30 min later. BMI z scores adjusted for child age and gender were calculated using measured height and weight data, and results revealed an association between higher adiposity and poorer average compensation (S Carnell, EL Gibson and J Wardle, unpublished results).
Eating rate
Another presumed indicator of satiety responsiveness is eating rate. Slower speed of eating and deceleration of intake throughout the meal have been considered a response to the progressive triggering of internal satiety cues with consumption. Several studies comparing obese and normal-weight adults have demonstrated faster eating(Reference Meyer and Pudel 10 , Reference Stunkard and Kaplan 11 ) and in some cases a relative lack of deceleration through the meal(Reference Meyer and Pudel 10 ), with a small number of studies documenting positive associations between BMI and self-reported eating rate(Reference Otsuka, Tamakoshi and Yatsuya 17 , Reference Sasaki, Katagiri, Tsuji, Shimoda and Amano 18 ).
Results in children are mixed. In one study (n 60) school cafeteria meals were observed and obese 6 year olds were found to eat faster, take more bites and chew each bite fewer times(Reference Drabman, Cordua, Hammer, Jarvie and Horton 19 ). In a laboratory study (n 43) using a computerised eating monitor obese 11 year olds were found to eat faster over two lunchtime meals and show no deceleration towards the end of the meal(Reference Barkeling, Ekman and Rossner 20 ). This lack of deceleration has since been replicated in a wider age-range of obese children using the same methodology. Obese–normal differences in average eating rate were not found to be significant in this study (n 40), but evidence for a trend towards faster eating emerged in the obese group(Reference Lindgren, Barkeling, Hagg, Ritzen, Marcus and Rossner 21 ). Eating rate may also be influenced by the eating situation and choice of meal; in a repeated-measures study (n 80) using computerised monitoring of a standard yoghurt meal overweight children were reported to display a faster eating rate only when their mothers were present in the laboratory(Reference Laessle, Uhl and Lindel 22 ).
In a large sample (n 252) of 9–12-year-old twins participating in a cohort study of eating behaviour, physical activity and adiposity, eating rate has been examined across the weight distribution. Children were drawn from the Twins Early Development Study, a large (approximately 10 000) population-representative cohort of families with twin children. Each child was given a standard lunch meal of six sandwiches made with their preferred spread and filling and twelve slices of bread, accompanied by a mixed portion of fruit. Testing sessions were conducted within the family home and children were seated next to their twin, allowing relatively natural eating behaviour to be assessed. Lunches took place in a separate room from the researchers and were videotaped for later coding. Analysis of the number of bites taken for each minute of the meal has shown that eating rate increases systematically with BMI z score. However, obese, overweight and normal-weight children over a range of body weights display a similar pattern of deceleration (CH Llewellyn, CHM Van Jaarsveld, D Boniface, S Carnell and J Wardle, unpublished results). The findings support a pattern of lower satiety responsiveness in heavier children, although faster eating and a lack of deceleration throughout a meal could also reflect other aspects of appetite (e.g. heightened food-cue responsiveness).
Food-cue responsiveness and child obesity
Evidence for greater food-cue responsiveness in obese adults has come from a variety of sources, including behavioural studies demonstrating that obese individuals have a higher intake of palatable foods than less-palatable foods and eat in response to the time of day rather than internal hunger sensations(Reference Schachter 23 ). Studies of biomarkers have also implicated differential responsiveness in terms of greater salivary responses to tastes of palatable food(Reference Epstein, Paluch and Coleman 24 ), differential basal levels and eating-related changes in gut hormones such as leptin, ghrelin, peptide YY, cholecystokinin and glucagon-like peptide-1(Reference Huda, Wilding and Pinkney 25 ) and different patterns of brain activation in response to food stimuli(Reference Tataranni and Delparigi 26 –Reference Delparigi, Chen, Salbe, Reiman and Tataranni 28 ).
To test the hypothesis in children, the ‘eating in the absence of hunger’ paradigm has been used to assess the extent to which the presence of palatable food overrides participants' internal satiety sensations(Reference Birch, Fisher and Davison 29 ). After eating to satiety at a provided meal, children are left alone for 10 min with free access to a variety of palatable snack foods (e.g. potato chips, cookies, ice cream) and a selection of toys and games, and intake is recorded. Compared with normal-weight children, intake during this paradigm is higher among children who are overweight at 5 and 7 years(Reference Birch and Fisher 30 ), among overweight children and adolescents ranging from 4 years to 19 years(Reference Fisher, Cai, Jaramillo, Cole, Comuzzie and Butte 31 ) and among children who are at high risk of obesity based on parental weight(Reference Faith, Berkowitz, Stallings, Kerns, Storey and Stunkard 32 ).
A simplified version of this paradigm is being developed for use in a non-laboratory environment. In a school-based sample of 7–9 year olds participating in the Physical Exercise and Appetite in Children Study, a large (n 348) study of eating and physical activity phenotypes relating to child weight, each participating child was presented with a bag of packaged sweet snack foods and a puzzle book for 10 min in their classrooms, directly after their normal school lunch. A similar procedure was used within home-based testing sessions in a subsample (n 316) of 9–12-year-old twins from the Twin Early Development Study, who were each provided with a snack pack and puzzle book for 10 min, after eating a standardised meal to satiety. In both studies intake was found to be positively correlated with BMI sd scores. However, weaker associations were found among females than males, possibly as a result of social desirability perceptions among the overweight girls(Reference Hill, Saxton, Webber, Purslow and Wardle 33 ).
Measurement of biological indicators of food-cue responsiveness is promising in adults and even more so in children, for whom relevant biomarkers may predict later obesity risk. Ethical approval for invasive protocols such as taking blood samples is not easily granted, however, and fewer studies exist. Measuring salivary flow via oral swabs is relatively acceptable to both children and parents, and may provide an index of food-cue responsivity. However, one study has revealed no increases in flow following exposure to the sight and smell of large dishes of palatable snack foods in either obese or normal-weight children(Reference Jansen, Theunissen, Slechten, Nederkoorn, Boon, Mulkens and Roefs 12 ), although obese children subsequently show greater intake. Neuroimaging studies in children present the methodological problems of anatomical differences and restricting motion in the scanner(Reference Davidson, Thomas and Casey 34 ), but children with Prader-Willi syndrome have shown post-meal increases in activity on functional MRI scans in the orbitofrontal cortex, medial prefrontal cortex, insula, hippocampus and parahippocampal gyrus compared with decreases in normal-weight children(Reference Holsen, Zarcone, Thompson, Brooks, Anderson, Ahluwalia, Nollen and Savage 35 ), and the same research group has recently found similar hyperactivation of motivation and reward areas in children with common obesity (AS Bruce, LM Holsen, R Chambers, L Martin, WM Brooks and CR Savage, unpublished results).
Food reward, food choices and child obesity
Food reward
The subjective reward experienced when consuming palatable foods is likely to be a potent motivator for intake. One method for measuring subjective reward is based on behaviour economic principles and tests how much work (e.g. key presses) the participant will do to win a specified ‘reward’ (e.g. palatable food) compared with an alternative reward (e.g. less-palatable food, physical activity). A concurrent schedule of reinforcement is applied, in which winning the item of interest becomes progressively harder while the work required to win the alternative item remains constant. The point at which the participant begins to work for the alternative reward provides an index of the reinforcing value of the item of interest(Reference Epstein, Leddy, Temple and Faith 36 ). Few studies have used this paradigm to compare obese and normal-weight individuals, but one study has found that obese adults work longer for palatable snack foods before switching to liked sedentary activities(Reference Saelens and Epstein 37 ), and another study has found that overweight children show a slower decline in responding for food over a 20 min period than do normal-weight children(Reference Temple, Giacomelli, Roemmich and Epstein 38 ).
In terms of biological indicators it is interesting that the heightened brain activation in obese compared with normal-weight adults(Reference Tataranni and Delparigi 26 –Reference Delparigi, Chen, Salbe, Reiman and Tataranni 28 ) and children (AS Bruce, LM Holsen, R Chambers, L Martin, WM Brooks and CR Savage, unpublished results) is centred on reward-related areas including the orbitofrontal cortex and insular cortex. There is also evidence for higher general reward sensitivity in obese individuals(Reference Davis, Strachan and Berkson 39 ), possibly reflected in levels of dopamine receptors and activity(Reference Epstein and Leddy 40 , Reference Wang, Volkow, Thanos and Fowler 41 ), and this association may extend to adolescents as well as adults(Reference Nederkoorn, Braet, Van Eijs, Tanghe and Jansen 42 ).
Food preferences
Another way in which obese individuals may differ from normal-weight individuals is in patterns of food preference. It is easier to overconsume energy-dense foods than foods of low energy density, e.g. fruits and vegetables(Reference Rolls, Drewnowski and Ledikwe 43 ), and numerous studies document higher weight and risk of overweight among individuals with a high-energy-density diet(Reference Ledikwe, Blanck, Kettel, Serdula, Seymour, Tohill and Rolls 44 –Reference Quatromoni, Copenhafer, D'Agostino and Millen 46 ). Since liking is an important predictor of intake, obese individuals might therefore be expected to like energy-dense foods more and choose them over less-energy-dense alternatives.
Surprisingly little evidence is available to support higher liking of energy-dense foods in obese adults. This position could be because evolutionary forces have led to an innate or easily learned preference, for tastes associated with high energy (e.g. sweet, fatty)(Reference Levine, Kotz and Gosnell 47 ), and thus traits determining the amount eaten rather than the type of food chosen are more discriminatory. Concern for social desirability might also lead obese individuals to dampen their liking for ‘unhealthy’ foods while inflating their appraisals of ‘healthy’ alternatives. Finally, the lack of evidence could also reflect the important distinction between ‘liking’ and ‘wanting’ a food(Reference Berridge and Robinson 48 ), the latter being the more salient determinant of eating. ‘Wanting’ a food may not be captured by palatability ratings, and different paradigms could well be needed to tap into a food's relative reward value.
Relative liking of various foods could be more important in young children, who have more dislikes than adults and avoid eating those foods. In a study of families with 4–5-year-old twins selected for high obesity risk (both parents obese) or low risk (both parents lean), lower child liking for various vegetables was reported by mothers of high-risk children compared with mothers of low-risk children. Mothers' reports were indirect (presumably based on observations of their child's food choices), which makes them a questionable measure of actual child liking but potentially a better reflection of behavioural preferences jointly motivated by liking and wanting. In order to obtain direct measures of preference children were also given tastes of high-fat (cheese, chocolate, pastry) and low-fat (rye cracker, candy, carrot) foods from a range of food groups. The higher ranking of the high-fat foods by the high-risk children than by the low-risk group suggests that preferences for energy-dense foods may be a behavioural marker for future obesity(Reference Wardle, Guthrie, Sanderson, Birch and Plomin 49 ).
Psychometric measures of appetite and child obesity
Most of the research that has been described has used behavioural tests to assess appetitive traits. These tests provide objective measures of eating behaviour but cannot claim to be true trait measures as they capture behaviour on only one occasion, and often in an artificial laboratory-based context, which could elicit atypical or socially-desirable behaviour. Sample sizes are often small and the power to detect relationships with weight is consequently diminished. One alternative is to use psychometric instruments. This method could be more vulnerable to social desirability and presents the new challenges of insight and accurate recall, but may be better at tapping consistent behavioural styles. Psychometric instruments can be administered to large samples, maximising statistical power, and can be completed by parents of children who are too young to report on themselves. Parental evaluations may also reflect some social desirability bias, especially when reports concern overweight children or those with unusual eating behaviour, but have the unrivalled asset of being founded on repeated observations of their child eating.
Most studies using psychometric measures of eating behaviour are designed to detect eating disorder symptomatology, and have therefore used self-report questionnaires (Dutch eating-behaviour questionnaire(Reference Van Strien, Frijters, Bergers and Defares 50 ) and three-factor eating questionnaire(Reference Stunkard and Messick 51 )) to find that eating styles such as restraint, disinhibited eating, emotional eating and external eating differ in obese adolescents and children compared with normal-weight counterparts in middle childhood(Reference Shunk and Birch 52 –Reference Lluch, Herbeth, Mejean and Siest 54 ). However, the direction of the associations is mixed, with some studies reporting lower scores on emotional and external eating scales in overweight individuals(Reference Snoek, Van Strien, Janssens and Engels 53 , Reference Lluch, Herbeth, Mejean and Siest 54 ). This disparity is likely to reflect dieting behaviour or aspirational responding, since those individuals who are concerned about their weight are known to modify their eating behaviour and reports. Examining appetite and weight in young children, using parent-report scales, may give a better reflection of the relationship between appetitive traits and weight, because children are less likely to have internalised social norms and prejudices, and therefore less likely to be dieting as a result of weight-related distress. However, only a small number of studies have followed this approach. One study has found higher external cue responsiveness in a clinical sample of obese children using a parent-report version of the Dutch eating-behaviour questionnaire(Reference Braet and Van Strien 55 ), but this result has not been replicated in a more recent community study(Reference Wardle, Guthrie, Sanderson and Rapoport 57 ).
The present authors have been using the child eating-behaviour questionnaire (CEBQ)(Reference Wardle, Guthrie, Sanderson, Birch and Plomin 49 ), a parent-report instrument designed to assess eating-behaviour styles in the normal and clinical range. Along with scales assessing other aspects of eating style (e.g. fussiness about food, emotional undereating), the CEBQ incorporates scales tapping into a number of appetitive traits with potential links to obesity. Between them the satiety responsiveness (e.g. ‘my child gets full up easily’), slowness in eating (e.g. ‘my child takes a long time to eat a meal’), food responsiveness (e.g. ‘given the choice, my child would eat most of the time’) and enjoyment of food (e.g. ‘my child enjoys eating’) items are close to the concepts of satiety responsiveness and food-cue responsiveness, and may also touch on the rewarding value of food. Each scale correlates well with behavioural tests designed to tap similar constructs (r 2 approximately 0·2 for satiety responsiveness(Reference Carnell and Wardle 58 )), and shows continuity in children from 4 years to 11 years (r approximately 0·5)(Reference Ashcroft, Semmler, Carnell, Van Jaarsveld and Wardle 59 ).
This questionnaire has been administered in two samples large enough to allow detailed exploration of relationships (3–5 year olds, n 549; 8–11 year olds, n 10 359). Preschool children and their parents were recruited from a diverse selection of preschools in London, England, and children's heights and weights were measured by trained researchers at school visits. The older children were drawn from the Twins Early Development Study and children's height, weight and waist measurements were reported by parents in a postal questionnaire. All parents completed selected scales from the CEBQ. For both groups higher adiposity was found to be associated with lower satiety responsiveness and slowness in eating scores and higher enjoyment of food, with the linearity of the association clearest in the waist circumference data for the older children(Reference Carnell and Wardle 60 ) (Fig. 1 (a and b)). Similar associations have also been observed in other cultures and child age-groups(Reference Viana, Sinde and Saxton 61 ). These results support the idea that appetitive traits are not merely abnormal in obese individuals but are also associated with weight variation throughout the population.
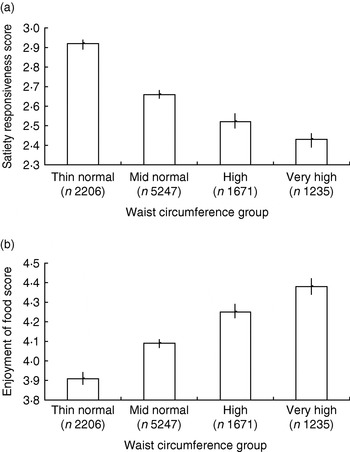
Fig. 1. Child eating-behaviour questionnaire satiety responsiveness scores (a) and enjoyment of food scores (b) by waist circumference group for an 8–11-year-old twin sample drawn from the Twins Early Development Study. Waist sd scores were based on UK 1990 reference data(Reference McCarthy, Jarrett and Crawley 127 ) and used to derive centile scores: thin normal group, 0th–50th centile; mid normal group, 51st–90th; high group, 91st–97th; very-high group, 98th–100th. Values are means and 95% CI represented by vertical bars. (From Carnell & Wardle(Reference Carnell and Wardle 60 ).)
Future research on child appetite and adiposity
The research that has been described is predominantly cross-sectional, and it is not possible to establish whether differences in appetitive traits cause variation in body weight. Prospective studies are needed, and should begin early, since there is evidence that infant indicators of satiety responsiveness such as milk sucking rate in the first month of life may go on to predict adiposity at 1–2 years(Reference Stunkard, Berkowitz, Stallings and Schoeller 62 ), and establishing risk status early could facilitate early intervention. Long-term prospective studies may benefit from measuring promising biomarkers. For example, one study has demonstrated lower levels of the satiety hormone peptide YY in obese children, which increase after successful weight loss(Reference Roth, Enriori, Harz, Woelfle, Cowley and Reinehr 63 ). If individual differences in fasting or pre- or postprandial levels of gut hormones linked to hunger and satiety (e.g. peptide YY, polypeptide Y, cholecystokinin, ghrelin) are found to predict subsequent weight gain, this factor would be consistent with a causal role for appetitive traits. Pre-obese differences in brain activation responses to food stimuli would provide further support.
To explore some of these possibilities, families are currently being recruited to participate in a large (approximately 2000) cohort study examining genetic and environmental influences on children's appetite and weight from birth (Genes, Environment and Maturation IN Infancy Study). Parents will complete questionnaires on anthropometric measures and children's eating style throughout development, while more detailed biological and behavioural data will be obtained in an intensively-studied subsample. Future support for appetitive traits as determinants of adiposity may also emerge from studies demonstrating weight change following appetite-targeted interventions.
Determinants of appetitive traits
If appetitive traits are determinants (rather than mere correlates or byproducts) of body weight, the crucial questions are how do they develop and can this process be influenced? Parental feeding has attracted by far the most attention as a potential determinant of children's eating behaviour. Literature in this area will be summarised and new evidence that genetic influences play an influential role will be reviewed.
Parental influences on appetitive traits
Satiety responsiveness
A central idea within the parental-feeding literature is that parents who exert high levels of control over their child's eating may unintentionally disregulate their appetite by encouraging them to eat according to external rather than internal satiety cues(Reference Savage, Fisher and Birch 64 , Reference Faith, Scanlon, Birch, Francis and Sherry 65 ). One study has found that 3–5 year olds whose parents have higher scores on a control scale (measuring pressure to eat and rigidity around mealtimes and feeding) show poorer compensation for the preload energy content(Reference Johnson and Birch 14 ). This negative association between parental control and compensation has been replicated in other samples using explicit measures of restrictive feeding(Reference Birch and Fisher 30 ).
Other studies have examined relationships between parental feeding and eating rate, hypothesising that parental pressure to eat promotes faster eating and overweight in children. In support, one observational study has found that higher eating rate in 3–5 year olds is associated with more maternal prompts to eat(Reference Drucker, Hammer, Agras and Bryson 66 ). The theory is also consistent with the finding that overweight children eat faster only when their mothers are present in the laboratory(Reference Laessle, Uhl and Lindel 22 ).
Food-cue responsiveness
Restricting children's access to fatty sugary snack foods is a common parental behaviour, but it has been hypothesised that over-restricting could increase preferences for and responses to those foods. Experimental studies emulating parental restriction by limiting children's access to a snack food have demonstrated greater intake and selection of the limited food immediately after it becomes available(Reference Fisher and Birch 67 , Reference Jansen, Mulkens and Jansen 68 ), and this response occurs both when the restricted food is initially preferred(Reference Fisher and Birch 67 ) and when it is liked no more than the non-restricted food(Reference Jansen, Mulkens and Jansen 68 ). This form of restriction may be an imperfect analogue for the ongoing limitation of energy-dense snacks practised by parents. However, child and maternal reports of restriction predict higher intake in the eating in the absence of hunger paradigm both cross-sectionally(Reference Fisher and Birch 69 ) and longitudinally(Reference Fisher and Birch 70 ) supporting some generisability of the laboratory findings. Restriction may also interact with overweight status such that girls who are overweight and whose parents restrict them are most vulnerable to the paradoxical effect(Reference Birch, Fisher and Davison 29 ).
Food preferences
Perhaps the clearest experimental support for an influence of parental feeding on children's eating comes from research on instrumental feeding. Parents commonly employ healthy foods as ‘means’ to obtain less-healthy foods or ‘ends’, sometimes simultaneously (e.g. ‘If you eat your peas, you can have ice-cream’). This approach may increase preference for the ‘end’, and have the opposite effect on the ‘means'. For example, when a teacher presents a snack food to a sample of preschool children to reward certain behaviours, preferences for that food increase(Reference Birch, Zimmerman and Hind 71 ). However, when they are repeatedly offered non-food rewards for consuming a new milk beverage, preferences decrease(Reference Birch, Marlin and Rotter 72 ). Another study in which consumption of a target snack (snack A) was rewarded with consumption of another snack (snack B) has found that children show relatively decreased preferences for snack A compared with participants given the snacks in a matching temporal order or an order of their choice(Reference Newman and Taylor 73 ).
Psychometric measures of disordered eating style
Obesity among children is a relatively new concern. Until recently, eating disorders were considered the primary scourge to be guarded against, especially among girls. Studies testing whether parental feeding could promote disordered eating have therefore assessed eating behaviour using scales from the Dutch eating-behaviour questionnaire and three-factor eating questionnaire, with mixed results. One study in a sizeable cohort of 4–6-year-old girls (n 197) has revealed positive associations between pressure to eat and restriction on one hand, and dietary restraint, emotional eating and external eating on the other(Reference Carper, Orlet and Birch 74 , Reference Van Strien and Bazelier 75 ). However, one large (n 596) survey of 7–12 year olds has shown that higher restriction is associated with lower external and emotional eating among girls(Reference Van Strien and Bazelier 75 ), suggesting that associations may differ for older children. Evidence has also come from retrospective designs. For example, one study has found that adults who recall their parents using food to reward behaviour and enforcing strict restriction are more likely to report disordered eating such as bingeing and unsuccessful dietary restraint(Reference Puhl and Schwartz 76 ). However, another study of university students has found no associations between recalled parental feeding and current eating behaviour(Reference Brunstrom, Mitchell and Baguley 77 ).
Psychometric measures of appetitive traits
The CEBQ and a combination of parental feeding questionnaires have been used to test associations between parental feeding and appetitive traits in a series of studies, including a preschool-based sample of 3–5-year-old children (n 541), a school-based sample of 9–11 year olds (n 348) and a large cohort of twins (Twins Early Development Study) surveyed at 9–11 years (n 10 359) with more detailed measures for a subsample of these families taken when twins were 4–5 years (n 400) and again when they were 9–11 years (n 340; S Carnell and J Wardle, unpublished results). Robust associations (r 0·2–0·4) have emerged across all samples, with positive relationships apparent between CEBQ food responsiveness scores and restriction, emotional feeding and instrumental feeding, and between CEBQ satiety responsiveness and parental pressure to eat (see Fig. 2 (a and b) for twin subsample data).
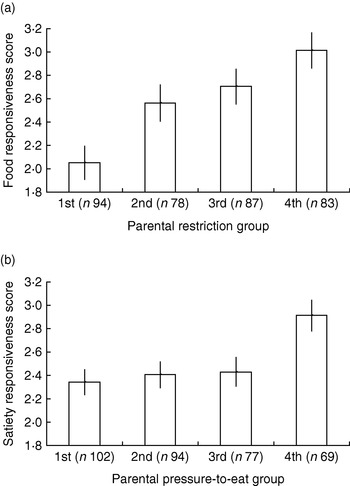
Fig. 2. Child eating-behaviour questionnaire food responsiveness scores by parental restriction group (a) and satiety responsiveness scores by parental pressure-to-eat group (b) for a 9–11-year-old twin sample drawn from the Twins Early Development Study. Parental feeding groups were created by dividing scores on the restriction and pressure-to-eat scales from the child feeding questionnaire(Reference Birch, Fisher, Grimm-Thomas, Markey, Sawyer and Johnson 128 ) into quartiles, with 1st group representing the lowest quartile and 4th group the highest quartile. Values are means and 95% CI represented by vertical bars.
These findings demonstrate some support for a model in which some parental feeding practices influence children's appetitive traits, but the exact nature of the relevant practices and the causal relationships between variables remain uncertain. Experimental studies use short-term substitutes for parental control, and observational and behavioural studies capture only snapshots of children's eating behaviour. The questionnaire studies have used diverse parental feeding measures preventing generalisation across studies, and have primarily sought to detect disordered eating styles, which simultaneously assess appetitive traits and individuals' attempts to control them. The majority of the studies are cross-sectional, precluding causal inference, while the retrospective evidence is vulnerable to bias and inaccuracy. The small number of existing prospective studies in the field have yet to span time periods sufficient for causes and effects to play out, and risk having ‘missed the boat’ by starting at an age at which parental influence could already have had its strongest impact.
Future research on parental feeding and child appetite
Improved parental feeding measures, many of which assess child-responsive authoritative feeding strategies, in addition to overt control of feeding, are likely to illuminate the role of parental feeding in the development of child appetite(Reference Hughes, Power, Orlet, Mueller and Nicklas 78 –Reference Musher-Eizenman and Holub 81 ). Long-term prospective studies using genetically-sensitive designs may help to reveal whether feeding strategies actually influence children's appetite or simply reflect parents' responses to their child's developing eating behaviour and weight(Reference Plomin, DeFries, McClearn and McGuffin 82 ).
Consistent with growing consensus within the research community that obesity risk processes may begin to unfold at birth, or even perinatally(Reference Vickers, Krechowec and Breier 83 , Reference Wells, Chomtho and Fewtrell 84 ), studies should start as early in development as possible and incorporate detailed measures of early appetite and feeding behaviours. Breast-feeding may be associated with lower infant weight(Reference Owen, Martin, Whincup, Davey-Smith, Gillman and Cook 85 ), but the mechanisms are unknown. Some researchers have suggested that the different macronutrient composition of formula compared with breast milk could explain disregulated intake and weight gain(Reference Vickers, Breier, Cutfield, Hofman and Gluckman 86 ), but behavioural explanations should also be considered; breast-feeding may promote maternal responsiveness in feeding, allowing infants to learn how to attend to internal satiety cues(Reference Gillman, Rifas-Shiman, Camargo, Berkey, Frazier, Rockett, Field and Colditz 87 ). Other established findings also merit further investigation of mechanisms. For example, an influential hypothesis holds that foetal and early-postnatal malnutrition could lead to adaptations in glucose metabolism that increase risk of metabolic syndrome and obesity(Reference Hales and Barker 88 ). This response could occur partly through epigenetic processes whereby nutritional influences affect foetal DNA(Reference Waterland and Jirtle 89 ), but low birth weight could also elicit overfeeding in mothers responding to concern about weight.
The new cohort study of families with twins followed from birth (Genes, Environment and Maturation IN Infancy Study) will provide more information about the role of parental feeding in the development of appetite. However, this and similar studies must account for the fact that even prospective associations between parental feeding and child appetite may be the result of other variables. For example, as the child grows older and energy-dense snack foods become more accessible the parent may adjust their feeding style and the child's appetitive characteristics may also change, without being directly related to parental behaviour. It may also prove difficult to assess parents and children frequently enough to capture the bidirectional causal interplay between them although careful planning of assessments to coincide with critical periods may help.
Genetic influences on appetitive traits
Feeding is a dynamic bidirectional process. Children are not merely passive responders to parental influences, but contribute substantially to the feeding interaction(Reference Satter 90 ). Consistent with this premise, parents notice differences in eating styles between siblings and report adjusting their feeding to children's appetites and preferred ways of eating(Reference Moore, Tapper and Murphy 91 ). This dynamic adjustment begins at the milk-feeding stage and is typified by the ‘feeding on demand’ pattern adopted by many mothers(Reference Renfrew, Lang, Martin and Woolridge 92 ). It seems sensible, therefore, to consider the possibility that eating styles may show genetic influence, which could be expressed at an early stage and underlie some of the observed associations between parent feeding and child eating behaviour.
Genetic influence on adult eating style
The genetic contribution to a phenotype can be established using family or twin designs in which correlations between relatives are compared with those that would be expected from their genetic similarity. Twin designs are built on the premise that monozygotic (identical) twins share all their genes, whereas dizygotic (non-identical or fraternal) twins on average share half. If a trait is entirely genetic it would therefore be expected that the correlation would be 1 for monozygotic twins and 0·5 for dizygotic twins, and if it is entirely environmental similar correlations would be expected in dizygotic twins and monozygotic twins. Using this assumption, together with a number of others (including equally similar environments for monozygotic and dizygotic twins), twin correlations can be used to estimate the proportion of the variance in a trait that is attributable to genetic v. environmental influences, and to further separate shared environmental influences (common to each twin and making children growing up in the same family more similar) and non-shared environmental influences (unique to each twin and making children growing up in the same family different)(Reference Plomin, Asbury and Dunn 93 ).
Genetic influence on child appetite
Existing research in this area is largely confined to adults. Behavioural twin studies have indicated heritability of between 14% and 69% for indices of eating style such as daily energy intake, meal intake, energy density of the diet, pre- and post-meal hunger and pre-meal stomach contents(Reference de Castro 94 –Reference de Castro 96 ), while heritability for three-factor eating questionnaire and Dutch eating-behaviour questionnaire scales range from 0% to 59% depending on study population and the measure used(Reference Neale, Mazzeo and Bulik 97 –Reference Tholin, Rasmussen, Tynelius and Karlsson 99 ). However, since there is evidence for heritability of obesity in children(Reference Wardle, Carnell, Haworth and Plomin 100 ) as well as adults(Reference Maes, Neale and Eaves 101 ), and some suggestion that shared environmental influence may be stronger in young children, who still share the family home(Reference Koeppen-Schomerus, Wardle and Plomin 102 ), independent investigation of heritability of appetite in children is warranted.
One recent family study containing 801 children and adolescents from 300 Hispanic families has assessed the heritability of intake in the ‘eating in the absence of hunger’ paradigm, and reports 51% genetic influence compared with 49% environmental influence, suggesting that genes influence appetitive behaviour as early as 5 years(Reference Fisher, Cai, Jaramillo, Cole, Comuzzie and Butte 31 ). Twin studies are additionally able to distinguish between shared and non-shared environmental influences, but very few focus on behavioural measures of eating in childhood. However, the previously described study of 252 9–12-year-old twins in which standardised lunch meals administered in the family home were observed, shows high heritability of eating rate, with a modest non-shared environment effect and no evidence for a shared environment effect (CH Llewellyn, CHM Van Jaarsveld, D Boniface, S Carnell and J Wardle, unpublished results).
Psychometric questionnaires allow large amounts of data to be gathered, allowing more-accurate estimates of genetic and environmental influences. However, the scales used in the adult studies were designed to assess eating disordered behaviour and hence reflect attitudinal as well as behavioural components, making them rather blunt measures of basic appetitive dispositions, confounded by dieting behaviour and social desirability bias. The CEBQ was designed to assess fundamental appetitive traits in children via parental reports. Two CEBQ subscales (satiety responsiveness and enjoyment of food) have been administered to mothers with 8–11-year-old twins in 5435 families from Twins Early Development Study. Standard genetic model fitting has produced estimates for genetic influence, shared environment influence and non-shared environmental influence of 63%, 21% and 16% respectively for satiety responsiveness and 75%, 10% and 15% respectively for enjoyment of food(Reference Carnell, Haworth, Van Jaarsveld, Plomin and Wardle 103 ).
In another study using a subsample of 4–5-year-old twins from the same cohort (n 214) parents' reports of children's preferences for seventy-seven different foods were obtained(Reference Breen, Plomin and Wardle 104 ). Analyses by food group reveal a pattern of differing heritability estimates of 0·20 for dessert foods rising to 0·37 for vegetables, 0·51 for fruits and 0·78 for protein foods. Shared environmental effects are much higher than those for the other appetitive traits (0·64 for desserts, 0·34 for fruits, 0·51 for vegetables, 0·12 for protein foods) suggesting that exposure to certain foods and feeding styles may markedly influence food preferences at preschool age. Future research will show whether the shared childhood environments continue to influence food choices into adulthood.
Future research on genetics of child appetite
Research into the molecular genetic basis of appetite has highlighted gene loci and candidate genes meriting further investigation(Reference Steinle, Hsueh, Snitker, Pollin, Sakul, St Jean, Bell, Mitchell and Shuldiner 105 –Reference Epstein, Wright, Paluch, Leddy, Hawk, Jaroni, Saad, Crystal-Mansour, Shields and Lerman 110 ), but remains in its infancy. A number of common genetic variants with links to obesity have been identified(Reference Saunders, Chiodini, Sham, Lewis, Abkevich, Adeyemo and de Andrade 111 –Reference Herbert 115 ), and some of their mechanisms of action may be appetitive. Robust associations with weight have been demonstrated for the FTO gene (a gene on human chromosome 16) in children and adults(Reference Frayling, Timpson and Weedon 114 ), Children and adults who are carry two copies of the high risk allele are 3 kg heavier than non-carriers, and represent about 16 % of the population, making FTO the first common obesity gene. Mounting evidence suggests that FTO exerts its effects in part through appetite control(Reference Gerken, Girard and Tung 116 –Reference Speakman, Rance and Johnstone 118 ), and a recent study by the authors provides further support, finding that FTO is highly associated with satiety responsiveness and food-cue responsiveness in children(Reference Wardle, Carnell, Haworth, Farooqi, O'Rahilly and Plomin 119 ). Further gene association studies using well-defined phenotypic measures may help to reveal the role of other obesity-associated genes in appetite.
A behavioural susceptibility theory of obesity
The evidence that has been outlined is consistent with a behavioural susceptibility model of obesity in which individuals' appetitive traits influence risk of weight gain in the obesogenic environment. This model may be thought of as a modernised expanded version of Schachter's externality theory(Reference Schachter 23 ), which was the first to suggest that obese individuals differ on appetitive traits. The difference is that behavioural susceptibility is conceived as a continuum explaining variation across the continuum of weight.
Fig. 3 illustrates the proposed model. All things being equal, individual factors such as appetitive traits and traits affecting physical activity (e.g. activity preferences) will influence eating behaviour and therefore energy intake, energy balance and body weight. The development of these traits will depend on both nature (genetics) and nurture (parent feeding and other early influences). Broad environmental factors are also assumed to exert a direct effect. In addition, individual and environmental factors will interact to influence weight. For example, heightened food-cue responsiveness will be expressed to a greater extent with more cues present in the environment, while large portion sizes may inspire greater intake in individuals with low satiety responsiveness. Individual and environmental factors could also directly influence each other. For example, an individual who finds food rewarding and prefers energy-dense foods may seek out environments with high availability and affordability of palatable energy-dense foods, whereas long-term exposure to large portions could decrease satiety responsiveness by expanding gastric capacity and changing consumption norms.
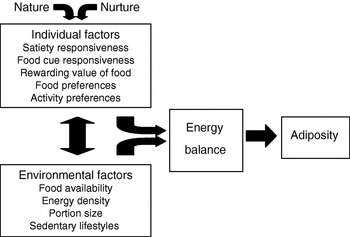
Fig. 3. Behavioural susceptibility theory of obesity.
Conclusions and implications
Evidence has been presented that suggests that satiety responsiveness, food-cue responsiveness, the reinforcing value of food and preferences for energy-dense foods are associated with adiposity even at an early age, and that both genetic and environmental influences, including parental feeding style, may contribute to the development of these traits. Prospective studies combining anthropometric, behavioural, biological and genetic measures are needed to establish whether appetitive traits influence food intake and body weight, reveal biological mechanisms and understand the inter-relationships between constructs.
If weight-related appetitive traits can be identified early in life, targeted behavioural or biological interventions that can either alter traits or ameliorate their impact on weight become plausible. For example, if research into the biological roots of satiety responsiveness reveals hormonal differences between individuals, it may become possible to regulate appetite through pharmaceutical means, although the multi-faceted nature of appetite may oppose a ‘quick fix’. Interventions at the behavioural level may be more immediately useful, and one study using a doll model to increase attention to internal satiety cues has successfully improved intake regulation in preschool children(Reference Johnson 120 ), while teaching regulation of eating rate using computerised feedback to allow activation of satiety signals also holds some promise(Reference Bergh, Sabin, Shield, Hellers, Zandian, Palmberg, Olofsson, Lindberg, Bjornstrom, Sodersten and Blass 121 ). Even if appetitive traits turn out to be immutable, environmental changes could enable ‘damage limitation’. For example, managed portion sizes, an emphasis on low-energy-density foods and conscious control of eating rate may be of help.
Heightened food-cue responsiveness may require different approaches. The principles of behaviour therapy suggest that limiting exposure to palatable food cues should be useful, so highly-responsive adults may benefit from keeping tempting foods out of sight, out of reach or out of the home, and parents could also help cue-responsive children in this way. Adults and children who prefer energy-dense foods could try substituting a number of their favourites with palatable low-energy versions. Parents, siblings and peers may also be able to influence children's liking of fruits and vegetables via exposure and modelling(Reference Cooke, Wardle and Gibson 122 , Reference Cooke, Wardle, Gibson, Sapochnik, Sheiham and Lawson 123 ), and early preferences may persist, making healthy choices easier in adulthood.
If the relative reward value of food is high, it may help to consciously seek other activities providing similar reinforcement. Parents could pay greater attention to the alternatives they offer their child. For example, an obese child may eat their favourite snack in preference to most forms of physical activity, but offering the choice of a less-favoured snack and a well-liked activity may yield a more-desirable outcome. Research into the hormonal and neurological correlates of food reward may also suggest new pharmaceutical targets, although the intimate connection between food reward and other kinds of reward may make the search for drugs with specific actions challenging.
Aside from the possibility of targeted intervention, the mere knowledge that eating patterns are partly driven by genetically-influenced traits may be of clinical value. Internalisation of anti-fat attitudes causes distress in many overweight individuals(Reference Puhl and Brownell 124 ), and although it has been suggested that this response is vital for initiating weight loss, negative emotions often make dieting more difficult by promoting unrealistic aspirations and diet–binge cycling(Reference Puhl, Moss-Racusin and Schwartz 125 , Reference Teixeira, Palmeira, Branco, Martins, Minderico, Barata, Silva and Sardinha 126 ). Parents of overweight children may also feel victimised by a mainstream media keen to apportion blame, and the knowledge that it is harder for some children to maintain a healthy weight could provide relief from unhelpful anxiety. Providing information on genetic behavioural risk could encourage individuals to believe that weight is predetermined and immutable, but complementing feedback with education on how to work with and around their or their child's genetic endowment may guard against this possibility.
Finally, a common misconception about genetic explanations is that they argue against the importance of environmental influences. In fact, without certain permissive environmental conditions many genes could not be expressed. Irrespective of individual dispositions, changing the wider environment to make it easier to limit energy intake and expend energy through physical activity is likely to benefit everyone. Environmental changes have the potential to reduce the obesity epidemic not only by protecting individuals burdened with the strongest trait obesity risk, but also by lowering overall population weight and preventing many normal-weight individuals from crossing the threshold for weight-related health complications. A fuller understanding of the interaction between genetic and environmental influences on appetite and body weight could therefore be useful not only for children, parents, adults and clinicians, but also at the level of community and government.
Acknowledgements
This paper is based on the symposium presentation by S.C. but has been subsequently updated to reflect relevant recent publications. S. C. was supported by an Economic and Social Research Council/Medical Research Council inter-disciplinary postdoctoral fellowship and J. W. is funded by Cancer Research UK. The work described was funded by grants from the Biotechnology and Biological Sciences Research Council, Cancer Research UK, the Economic and Social Research Council and the Medical Research Council. S. C. wrote the first draft of the manuscript and both authors contributed to subsequent drafts.
Neither author has any conflicts of interest to declare.
Many thanks are due to colleagues at the Health Behaviour Research Centre and collaborators at the Social, Developmental and Genetic Psychiatry Centre, particularly Claire Haworth and Robert Plomin for their expertise in quantitative genetics. We are also very grateful to the Nutrition Society for the opportunity to present our thoughts on this topic.





