Cancers of the oral cavity, oropharynx, larynx and oesophagus are frequently studied as a group of cancers of the upper aerodigestive tract (UADT)(Reference Lagiou, Georgila and Minaki1), because they are believed to share, even in terms of quantitative importance, exogenous aetiological factors, notably tobacco(2), alcohol(3, 4) and diet(4, 5), as well as genetic predisposition(Reference Canova, Hashibe and Simonato6). Worldwide, UADT cancers as a group are very common, with fatality generally exceeding 50 %(Reference Ferlay, Parkin and Bray7). In Greece, incidence of and mortality from UADT cancers are substantially lower than the corresponding indices in Western and Northern Europe and in the United States(Reference Ferlay, Parkin and Bray7). Because the prevalence of tobacco smoking is very high in Greece and consumption of alcoholic beverages is only slightly lower than that in the rest of Europe(8), we hypothesised that the traditional Greek Mediterranean diet, which is still adhered to by large segments of the adult Greek population, may be responsible by conveying protection against UADT cancers. To evaluate this hypothesis, we have used data from Greece collected in the context of the Alcohol-Related Cancers and Genetic Susceptibility in Europe (ARCAGE) study(Reference Lagiou, Georgila and Minaki1).
Materials and methods
Study subjects
ARCAGE is a multicentre case–control study. The study was approved by the ethical review board of IARC, as well as by the respective local boards in the participating centres across Europe. All subjects provided written informed consent for their participation in the study. Details on the overall study design are given elsewhere(Reference Lagiou, Georgila and Minaki1).
The Greek component of the ARCAGE study was conducted from 2002 to 2005 in four major hospitals in Athens. Incident cases were identified in the hospitals, and were pathologically confirmed. Among eligible cases, approximately 4 % were unable or not willing to participate. Eventually, a total of 239 cases were included in the study: 109 with cancer of the oral cavity or pharynx (excluding nasopharynx), 108 with cancer of the larynx and 22 with cancer of the oesophagus. In order to facilitate collection of blood samples, hospital controls were used and they were frequency matched to cases by sex and age (in 5-year groups). Only controls with a recently diagnosed non-malignant disease were accepted, and admission diagnoses related to alcohol, tobacco or dietary practices were not eligible. The proportion of controls within a specific diagnostic group could not exceed 33 % of the total(Reference Lagiou, Georgila and Minaki1). Approximately 11 % of the eligible controls were unable or not willing to participate. Eventually, a total of 194 controls were included in the study. Overall, the most common diagnoses among controls were gastrointestinal diseases (by protocol, appendicitis, anal fissure and fistula, perianal abscess, ischiorectal abscess and cholangitis) (14 %); diseases of the upper respiratory tract (13 %); skin, subcutaneous tissue and musculoskeletal conditions (12 %); trauma (12 %); circulatory system diseases (10 %); benign conditions of oral cavity, salivary gland and jaw (8 %); and genitourinary conditions (7 %).
Cases and controls underwent identical interviews in person during which a lifestyle questionnaire was completed with the help of a trained health professional. The questionnaire included information on socio-demographic variables, as well as detailed smoking and alcohol drinking histories. Height and weight at recruitment, as well as 2 years before the current hospitalisation, were also recorded, and BMI was calculated.
Dietary intake and Mediterranean diet score
In the Greek ARCAGE study, dietary habits were assessed using an extensive semi-quantitative FFQ, regularly used in case–control studies in Greece, and allowing for the calculation of total energy intake. Cases and controls were asked to indicate the average frequency of consumption per month, per week or per day of about 120 food items or beverage categories during a period of 1 year before the onset of the present disease. Using food composition tables that accommodate the particularities of the Greek diet(Reference Trichopoulou and Georga9), frequencies of consumption were translated into quantities on the basis of typical portion sizes, and quantities were converted into average daily nutrient intakes. Consumption of alcoholic beverages, on account of its known association with UADT cancer risk, was recorded in detail using the analytical ARCAGE questionnaire on drinking habits, and ethanol content per type of drink was estimated according to the overall ARCAGE protocol(Reference Lagiou, Georgila and Minaki1).
To evaluate adherence to the traditional Mediterranean diet, a score that was originally developed by Trichopoulou et al. (Reference Trichopoulou, Kouris-Blazos and Wahlqvist10) and later revised to include fish intake(Reference Trichopoulou, Costacou and Bamia11) was used. In brief, a value of 0 or 1 was assigned to each of nine components with the use of the sex-specific median as the cut-off. For beneficial components (vegetables, legumes, fruits and nuts, cereals and fish), persons whose consumption was below the median were assigned a value of 0, and persons whose consumption was at or above the median were assigned a value of 1. For components presumed not to be beneficial (meat and dairy products), persons whose consumption was below the median were assigned a value of 1, and persons whose consumption was at or above the median were assigned a value of 0. To assess lipid intake, the ratio of monounsaturated to saturated lipids was used, and the value of 1 was assigned to those with a ratio equal to or above the sex-specific median. For ethanol, we assigned a value of 1 to men who consumed between 10 and 50 g/d and to women who consumed between 5 and 25 g/d, based on average glasses of alcoholic beverages per day among current or former drinkers. The cut-off points for alcohol consumption in the context of the Mediterranean diet have been proposed in 1995(Reference Trichopoulou, Kouris-Blazos and Wahlqvist10), and have been consistently used since(Reference Trichopoulou, Costacou and Bamia11). Thus, the total Mediterranean diet score (MDS) ranged from 0 (minimal adherence to the traditional Mediterranean diet) to 9 (maximal adherence).
Statistical analysis
For the statistical analysis, all UADT cancer cases were grouped together, because they were considered to share common aetiological factors in tobacco smoking, consumption of alcoholic beverages and diet. Simple tabulations were used to examine the distribution of cases and controls by sex, years of schooling, tobacco smoking and alcohol drinking. For age, height and BMI 2 years before diagnosis, mean values with their standard errors were calculated. With respect to consumption of the components of the MDS, energy-adjusted (to 8368 kJ/d for men and to 7113 kJ/d for women) mean daily intakes by sex and case–control status were estimated.
The association of Mediterranean diet with UADT cancer risk was assessed by multiple logistic regression. We evaluated all nine components, considered as dichotomous, using the sex-specific medians as cut-offs (except for alcohol), at first alternatively and then simultaneously. Subsequently, we evaluated the association of the MDS with UADT cancer risk for two- and three-unit increments. Due to the established detrimental effect of ethanol intake in the occurrence of UADT cancers(4), we also evaluated the risk associated with MDS by excluding the ethanol component (thus reducing the ten-point score to a nine-point score), and estimated the OR associated with a two- and three-unit increment in the score minus ethanol. To preserve comparability, we multiplied the logarithm of the estimated OR by 9/10 before exponentiating.
In all models, we controlled for age (in years, continuous), sex, BMI 2 years before diagnosis (kg/m2, ordered in quintiles), height (in cm, continuous), educational level (categorical: primary as baseline, higher but not university-level education and university education) and smoking status (never smokers, former smokers and current smokers; for former and current smokers, further control was undertaken for pack-years as an ordered variable with 1 = < 20, 2 = 20–39, 3 = 40–59, 4 = 60–79 and 5 = 80+pack-years). Pack-years among ex-smokers and current smokers were assessed as ordinal variables to avoid disproportional influence of a few individuals with extreme values. We also controlled for alcohol intake (moderate v. others) in all models except in the ones that focused on it as a distinct variable and the model evaluating the association of the overall MDS (which includes a component for alcohol) with UADT. In the design of the analytical strategy, we considered known, or suspected, risk factors as the possible confounding variables for the disease outcome. Although many of the potential confounders had minimal confounding influence, we consistently adjusted for them in the models to preserve comparability of the estimates. Statistical analysis was conducted using the statistical package for social sciences 16.0 statistical software (SPSS version 16; SPSS, Inc., Chicago, IL, USA).
Results
Table 1 presents basic demographic characteristics, as well as patterns of tobacco smoking and alcohol consumption, for 239 participants with UADT cancer and 194 control subjects. These data serve only descriptive purposes because the indicated variables are not mutually adjusted for. In comparison to control subjects, cancer patients were somewhat less educated, heavier smokers and more frequently drinkers. All these differences were accounted for in the statistical modelling of the nutritional data.
Table 1 Characteristics* of incident cases of upper aerodigestive tract cancers and hospital controls in the Greek segment of the Alcohol-Related Cancers and Genetic Susceptibility in Europe study

* For quantitative variables, mean values with their standard errors are presented; for qualitative variables, number and percentage are presented.
† For quantitative variables, P values were derived from t tests; for qualitative variables, P values were derived from χ2 for (2 × 2) tables or χ2 for trend for (n × 2) tables.
Table 2 allows an inspection of differences between cases and controls in the energy-adjusted daily intakes of components of the MDS by sex. These comparisons are not adjusted for demographic or lifestyle variables. There is evidence that intake of meat is associated with increased risk of UADT cancers, whereas consumption of vegetables or fruits is inversely associated with UADT cancer risk.
Table 2 Energy-adjusted* mean daily intake of the nine components of the Mediterranean diet score by sex and upper aerodigestive tract cancer case–control status in the Greek segment of the Alcohol-Related Cancers and Genetic Susceptibility in Europe study

* To 8368 kJ/d for men and to 7113 kJ/d for women.
† For males, < 10, 10–49·99 and 50+ g/d. For females, < 5, 5–24·99 and 25+ g/d; classification was based on average glasses of alcoholic beverages/d among current or past drinkers.
Table 3 presents mutually adjusted OR and 95 % CI for UADT cancers by anthropometric as well as non-dietary lifestyle characteristics. There appears to be no important association of UADT cancer risk with educational level or somatometric characteristics. As expected, smoking is a major risk factor for developing UADT cancers, and heavy (former or current) drinkers are at increased risk than non-drinkers or light drinkers.
Table 3 Logistic regression-derived OR and 95 % CI* for upper aerodigestive tract cancers by specified variables in the Greek segment of the Alcohol-Related Cancers and Genetic Susceptibility in Europe study
(Odds ratios and 95 % confidence intervals)

* Adjusted for age and sex, as well as mutually among the indicated variables.
† For males, < 10, 10–49·99 and 50+ g/d. For females, < 5, 5–24·99 and 25+ g/d; classification was based on average glasses of alcoholic beverages/d among current or past drinkers.
Table 4 presents the OR and 95 % CI for UADT cancers by components of the MDS, controlling for the anthropometric and lifestyle characteristics listed in Table 3. Results are given with and without mutual adjustment among the indicated nutritional variables. Higher consumption of vegetables (OR = 0·62, 95 % CI 0·39, 1·00) and legumes (OR = 0·61, 95 % CI 0·39, 0·95) was significantly associated with reduced UADT cancer risk, but after mutual adjustment among the nine components of the MDS, no association remained significant at the 0·05 level, although associations were generally in the predicted directions.
Table 4 Logistic regression-derived OR and 95 % CI for upper aerodigestive tract cancers by components of the Mediterranean diet score in the Greek segment of the Alcohol-Related Cancers and Genetic Susceptibility in Europe study
(Odds ratios and 95 % confidence intervals)

* Controlling for the variables listed in Table 4 as well as for energy intake.
† As in footnote (*), but also mutually.
‡ For males, non-drinkers, < 10 g/d; moderate drinkers, 10–49·99 g/d; and heavy drinkers, 50+ g/d. For females, non-drinkers, < 5 g/d; moderate drinkers, 5–24·99 g/d; and heavy drinkers, 25+ g/d.
Table 5 presents the OR and 95 % CI for UADT cancers per two- and three-unit increase in the MDS. It also provides information on how the association of MDS with UADT cancer risk changes, when ethanol intake is not included in the score, but it is controlled for separately in the models (due to the known association of alcohol intake with UADT cancers, any benefit from adherence to the Mediterranean diet would be expected to increase after exclusion of ethanol from the score). Stricter adherence to the Mediterranean diet is associated with a substantial and significant decrease in UADT cancer risk (30 % for a two-unit increase and 41 % for a three-unit increase in MDS), which becomes more evident (31 % for a two-unit increase and 42 % for a three-unit increase in MDS without ethanol) and statistically more significant after exclusion of ethanol intake from the score.
Table 5 Logistic regression-derived OR and 95 % CI for upper aerodigestive tract cancers associated with two- or three-unit increase in the Mediterranean diet score* in the Greek segment of the Alcohol-Related Cancers and Genetic Susceptibility in Europe study
(Odds ratios and 95 % confidence intervals)
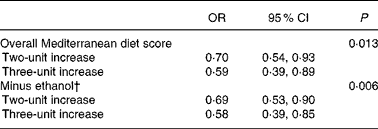
* Adjusted for age, sex, height, BMI, educational level, smoking status and pack-years, and energy intake.
† Adjusted as in footnote (*), as well as for alcohol drinking (non- or light, moderate and heavy drinkers). Originally estimated logarithm of OR was multiplied by 9/10, and then exponentiated to account for the proportionally larger increment in the minus ethanol score.
Discussion
In a moderately sized case–control study of UADT cancers in the greater Athens area, we found that closer adherence to the traditional Mediterranean diet was strongly and significantly inversely associated with UADT cancer risk. In the present study, though the associations of each of the components of this diet with UADT cancer risk were generally in the expected direction, none reached statistical significance. We interpret the present finding as indicating that traditional Mediterranean diet is more important than its individual components in conveying protection against UADT cancers, and that adherence to this diet could be largely responsible for the relatively low incidence of and mortality from these cancers in Greece.
The MDS is an a priori developed score(Reference Trichopoulos and Lagiou12) relying on the salient characteristics of the traditional Mediterranean diet and the empirically derived evidence on the association of its components with overall mortality(Reference Trichopoulou, Kouris-Blazos and Wahlqvist10, Reference Trichopoulou, Costacou and Bamia11, Reference Trichopoulou, Bamia and Trichopoulos13). In this score, moderate consumption of alcohol, mostly in the form of wine during meals, is considered as conducive to health, mainly on account of the well-established U-shaped association of alcohol intake with cardiovascular mortality(Reference Di Castelnuovo, Costanzo and Bagnardi14, Reference Mukamal and Rimm15), which is a common cause of death. For UADT cancer risk, however, the association with alcohol drinking is monotonic, which suggests that exclusion of the alcohol component from the MDS would strengthen the inverse association between the score and UADT cancer risk. This is indeed what happens (Table 5), although the change is marginal, probably because moderate – as contrasted to heavy – drinking only slightly increases UADT cancer risk in comparison to non-drinking (Table 4).
There are several reasons why the MDS is more strongly associated with UADT cancer risk than its individual components(Reference Trichopoulou, Costacou and Bamia11, Reference Trichopoulou, Bamia and Trichopoulos13, Reference Jacques and Tucker16). It may be that chance, non-differential misclassification and residual confounding have more important consequences when a single food is evaluated rather than a multicomponent, unidimensional score, as their effects are more likely to disruptively operate on a single food group rather than simultaneously on several of them. Moreover, in an analysis focusing on individual components, effects are examined against the background of average risk associated with other nutritional components, whereas a dietary score can account for extremes of cumulative exposures in the absence of other major nutritional effects(Reference Jacques and Tucker16).
There has been another and larger study examining the association of adherence to Mediterranean diet with cancers of the UADT(Reference Bosetti, Gallus and Trichopoulou17). The results were compatible with those of the present study. The Bosetti et al. study, however, relied on populations of northern Italy, where dietary practices are not typically Mediterranean. In any case, the convergence of the results of these two studies indicates that the inverse association between adherence to Mediterranean diet and UADT cancer risk is probably genuine and quite strong. Although there are several variations of the traditional diet in the olive oil-producing areas of the Mediterranean region, the MDS we have used captures the salient characteristics of this traditional diet and has been found to be robust to these variations(Reference Sofi, Cesari and Abbate18).
The strengths of our investigation are the high participation rates among both cases and controls, the reliance on a strict protocol for case ascertainment and control selection, and the use of an extensive dietary questionnaire, shorter versions of which have been frequently employed in Greece in case–control studies with results in line with those of subsequent major cohort investigations(Reference Manousos, Day and Trichopoulos19, Reference Manousos, Day and Tzonou20). Another strength of our investigation is also its undertaking in a population where large segments still adhere to the traditional Mediterranean diet. Weaknesses of our investigation include the modest study size and reliance on a hospital-based case–control design. Hospital-based case–control studies are criticised for being susceptible to selection and information bias. However, control selection relied on the very strict ARCAGE protocol, which excluded admission diagnoses related to alcohol, smoking or diet, thus, limiting the potential for diagnosis-related selection bias. Moreover, choice of hospital controls maximised response rates and, thus, minimised participation-related selection bias. Of note, the results concerning the relationship of individual components of the Mediterranean diet with UADT cancer risk were compatible with the collective evidence concerning the association of these factors with UADT cancer risk(4, Reference Lagiou, Talamini and Samoli21).
In conclusion, we have found evidence that adherence to the traditional Mediterranean diet is associated with reduced risk of cancers of the UADT. The association is statistically significant and stronger than the associations of the individual components of this diet with UADT cancer risk. Closer adherence to the traditional Mediterranean diet could explain the lower incidence of UADT cancers in Greece, in spite of the smoking and drinking habits of this population.
Acknowledgements
The financial support for the present study was provided by the European Community (Fifth Framework Programme) grant no. QLK1-CT-2001-00182 and the University of Athens Medical School. None of the authors has any conflict of interest. E. S. was responsible for the statistical analysis. A. L. was responsible for the nutritional epidemiological analysis. E. N., G. L. and D. L. contributed to the data collection, and provided significant advice regarding clinical issues and the ascertainment of case diagnosis. A. B. contributed to the data processing and analysis. D. T. is a senior consultant epidemiologist. P. B. is the principal investigator of the overall ARCAGE project. P. L. is the principal investigator of the Greek ARCAGE study, and supervised the present study. P. L., E. S., A. L. and D. T. were responsible for the drafting of the manuscript with input from all authors. All authors have approved the submitted version of the manuscript.







