Vitamin D has long been known for its importance in bone health and development, especially during growth from infancy through adolescence. It has been realized that vitamin D has diverse actions beyond its role in endocrine functions, intestinal Ca uptake and bone mineralization as a paracrine and apacrine hormone at the tissue and cellular levels( Reference Haussler, Jurutka and Mizwicki 1 , Reference Norman 2 ). Recent studies suggest that vitamin D has an important role beyond the skeleton in diverse diseases and health conditions as well. For instance, inadequate vitamin D status has been associated with CVD, metabolic syndrome, autoimmune conditions and several types of malignancy( Reference Guadarrama-Lopez, Valdes-Ramos and Martinez-Carrillo 3 – Reference Grant 7 ), but the direction of causality is always uncertain( Reference Mangin, Sinha and Fincher 8 ).
It has been established that the majority of vitamin D is not derived from the diet, but rather is produced by exposure to solar radiation( 9 ). A precursor in the skin, 7-hydroxycholesterol, is converted to cholecalciferol by the action of UVB radiation from the sun or other sources. Darker skin pigmentation acts as a natural UVB radiation block, with melanin decreasing the penetration of radiation to the dermal level of the skin and thus reducing the amount of active vitamin D formation( Reference Holick, MacLaughlin and Doppelt 10 ). For darker-skinned migrants in temperate latitudes with lower solar intensity, melanin blockage of UVB light is a recognized and well-studied problem( Reference Durazo-Arvizu, Aloia and Dugas 11 , Reference Farrar, Webb and Kift 12 ). On the other hand, in regions with higher sun exposure in the tropical latitudes, vitamin D deficiency was not considered to be a public health concern. However, a geographic review by Brito et al.( Reference Brito, Cori and Olivares 13 ) of indicators of vitamin D intake and status in Latin America and the Caribbean described widespread inadequacy and insufficiency in survey data that had been published from the region. Moreover, unlike in Western countries, milk is not an important beverage among children and adolescents in Guatemala( Reference Montenegro-Bethancourt, Vossenaar and Doak 14 ) and is not readily fortified with vitamin D( Reference Fiedler and Helleranta 15 ). Also in Guatemala, our group documented insufficient and deficient vitamin D status among elderly people of Mayan descendancy living in the country’s western highlands( Reference Sud, Montenegro-Bethancourt and Bermúdez 16 ).
As ageing of the skin is a well-recognized factor for reduced dermal vitamin D synthesis( Reference MacLaughlin and Holick 17 ), we turned to the region with presumably the most tropical setting and the population with theoretically the greatest outdoor exposure, namely adolescents residing on the Caribbean coast of Guatemala at a latitude of 15°49′N and longitude of 88°45′W. Two ethnic groups inhabit this area, from the mouth of the Rio Dulce to ~30 km upriver to its origin in Lake Izabal. The former, in the town of Livingston, are Garifunas, a mixture of indigenous Caribes and African heritage, and the latter is a community of Kekchi Mayans, internally displaced from the Sierra Madre highlands to the banks of the river during the internal conflict of the 1980s. Both groups, with very little intermixing, have pigmented skins, with the Garifunas darker in complexion than their Kekchi neighbours. To confirm or refute the hypothesis that problematic vitamin D status may be concentrated only in the older Mayan population, we conducted a field survey, with a secondary comparative context, in these two population centres of the Rio Dulce. We present the findings of this inquiry here.
Methods
Population setting
The study was conducted in the province of Izabal on the Caribbean coast of Guatemala, in two different locations. The first location was Rio Dulce, a town in the north-east of Izabal, which is inhabited by the Kekchi population. The second was Livingston, which is located at the mouth of the Rio Dulce at the Gulf of Honduras. This area is inhabited largely by the Garifuna, who are predominantly of African descent. Livingston sits at sea level at a latitude and longitude of 15°49′N and 88°45′W, respectively. Rio Dulce has a similar latitude and longitude of 15°46′N and 88°49′W, respectively.
Study population and enrolment
Investigators and field workers from the Center for Studies of Sensory Impairment, Aging, and Metabolism established collaborations in the two towns, Livingston and Rio Dulce. In each town, arrangements were made for identification and recruitment of the adolescents at public secondary schools. In Rio Dulce, the study was conducted at an orphanage, which also functions as a school that serves all of the Kekchi adolescents. In Livingston, a local physician found four different secondary schools from which we were able to recruit participants. Ethnic origin was based on the surname of indigenous parlance, language spoken, and father and mother who identified themselves of a particular group. Based on self-reporting, the specific attire worn by study adolescents was deemed to be their traditional outfit during the dry season and generally what is customarily worn on most days during the year.
The primary inclusion criteria for adolescents to participate in this survey were as follows: to be either of Kekchi or Garifuna descent and to be aged 12 to 18 years at the time of study in February 2014. Exclusion criteria included the following: a history of chronic malabsorptive disease; failure to provide assent from the adolescent and consent from one parent; and failure to sample blood from the adolescent.
The study was approved by the Human Studies Committee of the Center for Studies of Sensory Impairment, Aging, and Metabolism. All parents of participants were required to provide informed consent after a complete discussion of the purpose, benefits and risks of the study. This was conducted in Spanish or the native dialect. Each adolescent was also required to provide assent for the study. The goal of the study was to collect blood samples for analysis of 25-hydroxyvitamin D (25(OH)D) from eligible adolescents. Recruitment was conducted by voluntary, convenience sampling through a variety of methods. All data collection was conducted during a three-day period in each study site. All parties involved understood the voluntary nature of the study and no incentives were provided.
Survey questionnaire on vitamin D-conditioning factors
Adolescents arrived on their scheduled days to their respective locations. A survey questionnaire (see online supplementary material) was administered to each participant to assess various factors and behaviours that may impact vitamin D status. The survey questions were derived from a noted review on vitamin D surveillance by McCarty( Reference McCarty 18 ). The questionnaire was conducted by a Spanish-speaking individual and was translated into either Kekchi or Garifuna dialects by an interpreter if necessary. The questions assessed various aspects of vitamin D exposure including sun protection practices and level of sun exposure. To estimate skin exposure of study adolescents, photographs were taken of all participants and kept in a secured locked cabinet.
Anthropometry
Height (centimetres) was measured on a wall stadiometer and weight (kilograms) was assessed on a portable, calibrated digital scale (Tanita model BC522; Tanita, Tokyo, Japan) with shoes removed. Both measurements were obtained by the same researcher for all participants. Blood pressure and heart rate were also recorded for each participant using a standard electronic sphygmomanometer. Lastly, a trained phlebotomist extracted blood from each participant.
Photographic images and estimation of body surface exposure
The photographs were taken on the same day as the rest of data were collected with a digital camera (Sony Cybershot model DSC-S2000; Sony Corp., Tokyo, Japan). During the height assessment, a member of the team took a full-length frontal or semi-sagittal exposure photograph to document the clothing worn to the measuring. The photograph was labelled with the participant’s ID and stored in a digital file until analysis.
The coded digital image was uploaded to a computer screen and the areas of the body not covered were estimated by the ‘rule of nines’, which was adapted from surface burns and scalds management and adjusted for children older than 12 years as a percentage of ‘exposed’ area( 19 ). To estimate the absolute skin area in centimetres squared, the weight and height data were entered into a formula( Reference Halls 20 ) to provide total body skin surface in metres squared and then transformed into centimetres squared. Finally, the total area was related to an estimation of exposed surface to provide an expression of the percentage of body surface exposed to the sun.
Blood sample collection, processing and quantitative analysis
Participants were not required to be in the fasting state for the blood collection. Approximately 7 ml of whole blood was drawn from the antecubital vein using a 0·20-gauge hypodermic needle into a 10 ml syringe. The blood was transferred to a 10 ml clotting tube and allowed to coagulate at room temperature for 10 min for formation and contraction of the red cell clot. The tubes were placed in a clinical centrifuge and spun at 5000 rpm for approximately 10 min. The serum separated and was collected manually using a pipette. Following this step, serum for each participant was stored in 2 ml coded plastic vials and frozen. The vials were then transported to Guatemala City on dry ice and shipped to the Osteoporosis Center at Creighton University in Omaha, Nebraska, USA for laboratory analysis. The remainder of the blood samples was stored for additional analysis, including parathyroid hormone and Ca measurements.
Quantitative assay of circulating 25-hydroxyvitamin D
Serum 25(OH)D levels were measured by RIA using the LIAISON 25 OH Vitamin D 100 TOTAL Assay (DiaSorin, Stillwater, MN, USA). It was run in duplicate with CV of 2·6 %. The laboratory facilities at Creighton University are certified by the Vitamin D External Quality Assessment Scheme and assess vitamin D levels using standardized protocols and guidelines. The serum concentrations of 25(OH)D were reported by the Osteoporosis Center at Creighton University in nanograms per millilitre. To date, there is no consensus in the current literature regarding the classification of vitamin D definitions and their cut-off values. However, for the present study, a 25(OH)D level of greater than 30 ng/ml was defined as normal, 20–30 ng/ml was defined as insufficient, and less than 20 ng/ml was defined as deficient. These definitions have been established based on vitamin D levels that lead to biochemical changes in parathyroid hormone and Ca levels, as well as predispose an individual to physical symptoms( Reference Holick 21 ).
Statistical analysis
All statistical analyses were conducted with the statistical software package IBM SPSS Statistics Version 22. Descriptive statistics included means and standard deviations (age, BMI, sex, ethnicity, skin exposure) and demographic information (sex ratio) for both study samples. The Student’s t test for two independent samples was performed to assess the significance of any differences.
To ascertain differences in the survey responses between the two groups, the Mann–Whitney U test was used to assess the ordinal questions. The χ 2 test was used for the binary survey questions 1 and 4. Linear regression analysis was used to assess the predictive capacity for 25(OH)D level (as a continuous variable) for each relevant question. Stepwise regression was utilized to identify those variables that contributed to explaining the variability in circulating 25(OH)D levels. Also, with the variables identified as predictors from the stepwise regression and the univariate regression analysis, we tested a multivariate model. All statistical tests were two-sided and P value of 0·05 was defined as significant.
Results
Demographic information and descriptive statistics
Descriptive information regarding each ethnic group is provided in Table 1, including the sex distribution and mean age, mean BMI, mean exposed skin area, mean percentage of exposed skin area and mean 25(OH)D concentration. The sample was evenly divided by ethnicity with forty-three adolescents of Garifuna or African descent and forty-three adolescents of Kekchi or Mayan descent. The mean age of the entire group was 13·5 (sd 1·6) years. The Garifuna group was, on average, a year older than the Kekchi group (P=0·003). The sex distribution was approximately even between the two groups with a slightly higher female predominance: 54 % and 58 % for Kekchi and Garifuna, respectively.
Table 1 Descriptive information by ethnicity (Kekchi, indigenous Mayan; Garifuna, Afro-Caribe) among the sample of adolescents (n 86), mean age 14 years, residing on the Rio Dulce at the Caribbean coast in Izabal Province, Guatemala, February 2014
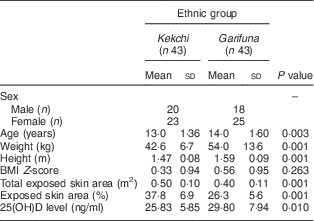
25(OH)D, 25-hydroxyvitamin D.
Mean height differed significantly (P=0·001), with the Garifuna adolescents being 12 cm taller than their Kekchi counterparts. Mean weight correspondingly differed in the same direction (P=0·001), with the difference between the ethnicities being 11·5 kg. Mean BMI for the Kekchi and Garifuna adolescents was 19·6 kg/m2 and 21·2 kg/m2, respectively. The total body surface area, derived from height and weight data, was 1·32 (sd 0·13) m2 and 1·53 (sd 0·23) m2, respectively (P=0·001), reflecting the larger body size of the latter. In the eighty-six adolescents, all with adequate photographic images for assessment, the total exposed body surface area (i.e. that not covered by clothing) differed across ethnicities (Table 1), with the larger-sized Afro-Caribe group having 11·5 % greater total surface area (P=0·001). The overall percentage of skin exposed across the whole group ranged from a minimum of 20·0 % to a maximum of 49·4 % of the body surface, with a mean of 32·0 (sd 8·5) %. When compared by ethnicity, however, the Mayan indigenous sub-sample had both a greater percentage (P=0·001) and a greater absolute surface area exposed (P=0·001) than their Afro-Caribe counterparts.
A total of eighty-six serum samples were collected and analysed. The mean 25(OH)D level for the Kekchi adolescents was 25·8 (sd 5·9) ng/ml. In contrast, the mean 25(OH)D level for the Garifuna adolescents was 29·8 (sd 7·9) ng/ml (P=0·01). Figure 1 illustrates the vitamin D status and the proportion of Kekchi and Garifuna adolescents who belonged to each classification. Approximately 21 % of Kekchi adolescents were in the deficient range as compared with 5 % of Garifuna. There were equal proportions of Kekchi and Garifuna adolescents in the insufficient category at about 51 % each. Approximately 43 % of Garifuna adolescents had normal 25(OH)D levels as compared with only 28 % in the Kekchi group.

Fig. 1 Vitamin D status by ethnicity (![]() , indigenous Mayan (Kekchi);
, indigenous Mayan (Kekchi); ![]() , Afro-Caribe (Garifuna)) among adolescents (n 86), mean age 14 years, residing on the Rio Dulce at the Caribbean coast in Izabal Province, Guatemala, February 2014. Differences between the two ethnic groups were statistically significant (P=0.01)
, Afro-Caribe (Garifuna)) among adolescents (n 86), mean age 14 years, residing on the Rio Dulce at the Caribbean coast in Izabal Province, Guatemala, February 2014. Differences between the two ethnic groups were statistically significant (P=0.01)
Survey analysis
Each numbered survey question was analysed to determine if there were significant differences between the two ethnic groups; in questions that revealed sufficient variance, the corresponding variable was subsequently tested for prediction of 25(OH)D levels. Both questions 1 and 4 had binary responses about drinking milk and use of multivitamin supplements, respectively; a χ 2 test revealed no statistically significant difference between the two groups.
Question 2 assessed time spent outdoors, whereas question 3 assessed the relative intensity of sun exposure. The latter question revealed no difference across the two groups, but the former question revealed a significant difference between the two groups according to the Mann–Whitney U test (P=0·001). The Kekchi group spent less time outdoors by a wide margin: approximately 75 % of the Kekchi adolescents spent less than 1 h outdoors in the sun daily while, in contrast, approximately 30 % of Garifuna adolescents spent 1–2 h and another 40 % spent more than 2 h outdoors in the sun daily. Figure 2 illustrates these differences. In order to run the regression analysis, the last two categories (‘>4h/d’ and ‘3–4 h/d’) were combined with the ‘2–3 h/d’ category and renamed ‘>2 h/d’. This was done because a very limited number of participants answered those particular responses. The reference category was the ‘0–1 h/d’.

Fig. 2 Daily hours spent outdoors by ethnicity (![]() , indigenous Mayan (Kekchi);
, indigenous Mayan (Kekchi); ![]() , Afro-Caribe (Garifuna)) among adolescents (n 86), mean age 14 years, residing on the Rio Dulce at the Caribbean coast in Izabal Province, Guatemala, February 2014
, Afro-Caribe (Garifuna)) among adolescents (n 86), mean age 14 years, residing on the Rio Dulce at the Caribbean coast in Izabal Province, Guatemala, February 2014
Questions 5, 6 and 7, relating to solar protection behaviours through the use of hats, long sleeves or sunscreen, respectively, were so sharply skewed to the extreme of ‘rarely’ or ‘never’ in both groups that the lack of variance precluded their useful entry into regression analyses.
Linear and multivariate regression analyses
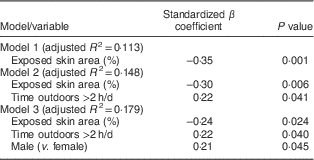
Univariate analysis was conducted to assess which of the nine variables were independently predictive of 25(OH)D levels (Table 2). Each variable was tested individually in the simple linear regression model to assess its association with 25(OH)D level. As demonstrated, age, being male, being Garifuna, spending >2 h/d outdoors, skin area exposed and percentage of skin exposed were statistically significant predictors of 25(OH)D levels; however, the association of exposed skin area and percentage with 25(OH)D concentration was inverse, which is prima facie paradoxical. The same variables were then entered into a forward, stepwise regression model as shown in Table 3. The modelling phased through three sequential models, each of which retained the exposed skin percentage as a significant factor with a negative β coefficient, but as the process was completed the significance declined (P=0·024). In the third and final model, two additional significant determinants remained: being male (P=0·045) and spending more time outdoors (P=0·040). With an additional multivariate model we found that sex (being male, P=0·034) and exposed skin percentage (P=0·044), but not age (P=0·165) nor >2 h/d outdoors (P=0·077), remained as predictors of 25(OH)D concentrations (data not shown).
Table 2 Associations of plasma 25-hydroxyvitamin D with independent factors among adolescents (n 86), mean age 14 years, residing on the Rio Dulce at the Caribbean coast in Izabal Province, Guatemala, February 2014
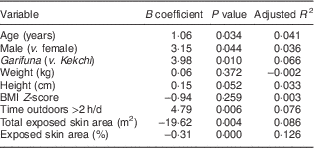
Table 3 Predictors of plasma 25-hydroxyvitamin D, obtained with stepwise regression models, among adolescents (n 86), mean age 14 years, residing on the Rio Dulce at the Caribbean coast in Izabal Province, Guatemala, February 2014
Discussion
Guatemala is a country that has concerned itself with the study of nutrient deficiencies, has found adverse issues related to vitamin A, riboflavin, vitamin B12, Fe, iodine and Zn, and has devised and implemented remedial action for a few of these deficiencies. Vitamin D has only very recently entered the list of problem nutrients for the country( Reference Sud, Montenegro-Bethancourt and Bermúdez 16 ). The main findings of the present study included a high prevalence of deficient and insufficient circulating levels of 25(OH)D in both lighter-skinned, Mayan adolescents and darker-skinned, Afro-Caribe adolescents, with the former having the lower concentrations. Also, of nine variables assessed with stepwise and multiple regression models, only four were deemed to be independent determinant factors for lower 25(OH)D status.
Between the extrinsic vitamin D in foods and beverages in the diet and the intrinsic vitamin D produced by UVB radiation on the skin, it is almost universally the case that the solar-derived variety is dominant. However, and as indicated before, dietary vitamin D was not formally assessed in the current study, which limited our ability to explore these issues further among our study group of Guatemalan adolescents.
Seasonality (summer months) and proximity to the equator are both conditioning factors for higher intensity of solar exposure. The present study was conducted during the northern hemisphere winter (February 2014). At 15°N, Guatemala’s Caribbean coast is relatively close in a tropical sense to the latitudes of the Seychelles (4°S) and Ghana (6°N) in a recent comparative study of vitamin D status by latitude in African-descendant populations( Reference Durazo-Arvizu, Aloia and Dugas 11 ). Our coastal site is much more tropical than the 27°S in south-east Queensland, Australia described as ‘a sunny climate’( Reference Kimlin, Sun and Sinclair 22 ). Clothing, protecting the skin from the sun, can be another potent determinant of vitamin D status as demonstrated in Turkey, where women wearing an Islamic veil had three times the rate of deficient 25(OH)D concentrations compared with women in secular dress( Reference Buyukuslu, Esin and Hizli 23 ). On the other hand, consumption of vitamin D from either foods or supplement contributes to the nutrition status of this vitamin. However, as mentioned before, vitamin D supplementation is not mandatory in Guatemala and the population does not consume important sources of vitamin D. Obesity, as reflected by an elevated BMI, has also been inversely related to vitamin D status, including in adolescent populations as demonstrated in Pennsylvania, USA( Reference Rajakumar, Holick and Moore 24 ).
Melanin pigmentation can reduce the efficiency of UVB production of vitamin D in the skin as shown in controlled tanning experiments with different intensities of UVB exposure through a range of lighter- to darker-skinned volunteers( Reference Armas, Dowell and Akhter 25 ); radiation intensities and skin tone explained 80 % of the variance in changes in circulating 25(OH)D. The effect of skin pigmentation on vitamin D status can be seen in a study of 526 prisoners of different ethnic backgrounds scattered across facilities of the Massachusetts Department of Correction in the north-east USA, latitude 42°N( Reference Nwosu, Maranda and Berry 26 ). Whereas approximately 50 % of black prisoners had a 25(OH)D of <20 ng/ml, only 29 % of white prisoners did. There was no difference in circulating vitamin D levels for blacks and whites in minimal-security settings, but blacks in moderate- or maximum-security custody had significantly lower 25(OH)D levels, such that the risk of deficiency was four times higher for a black prisoner in maximum-security facilities compared with a white peer. This conclusion is matched by cross-sectional studies in Queenslanders that found: ‘25(OH)D levels were also lower in participants who had black hair, dark/olive skin, or brown eyes, when compared with participants who had brown or fair hair, fair skin, or blue/green eyes’( Reference Kimlin, Sun and Sinclair 22 ). A host of additional studies from different locations have confirmed a significant skin-colour differential in circulating 25(OH)D( Reference Hirani, Mosdol and Mishra 27 – Reference Ohlund, Silfverdal and Hernell 32 ), whereas a substantial number have failed to confirm this finding( Reference Malvy, Guinot and Preziosi 33 – Reference Martini, Verly and Marchioni 39 ).
Univariate and multiple regression models are often generated with the variables to determine what are often considered mutually independent predictors or determinants of 25(OH)D. This analysis, of course, depends on what variables the investigators thought to – or could afford to – include in the study design or retrieve in secondary survey analysis. Needless to say, accurate and reliable measurements are essential to reduce misclassification errors and attenuation of effects( Reference Willett 40 ). Of the nine variables entered into the independent univariate analysis here, we found significant predictions from six of them; and from the stepwise regression, found three as predictive of 25(OH)D levels. Although we acknowledge the limitations of these exploratory analyses, including the small sample size, we have selected seven recent publications presenting regression analyses among the variables measured( Reference Hirani, Mosdol and Mishra 27 , Reference Unger, Cuppari and Titan 28 , Reference Pazaitou-Panayiotou, Papapetrou and Chrisoulidou 34 – Reference Alyahya, Lee and Al-Mazidi 36 , Reference Guo, Gies and King 38 , Reference Martini, Verly and Marchioni 39 ) (Table 4). Making certain rough equivalencies such as veiling and body surfaces exposed, ethnicity and skin colour, and age and life stage, we identified thirty-one different variables among the seven citations. Except for the percentage of exposed skin, the other eight variables tested in our models had also been measured and modelled in the comparative literature.
Table 4 Comparison of risk factors for low 25-hydroxyvitamin D status among seven selected international publications

PTH, parathyroid hormone; PA, physical activity; WHR, waist-to-hip ratio; WC, waist circumference.
The variables in italic font were measured but found not to be determinant in the final model, whereas those in bold font within parentheses were published as significant predictors by the authors.
Male sex was prominent in our models, and was a predictive variable in all three papers that reported entering it. Men also had significantly higher 25(OH)D among elderly Mayans( Reference Sud, Montenegro-Bethancourt and Bermúdez 16 ). Age was an individual predictor in Guatemalan adolescents, and it appeared significant in two of the three reports. More time outdoors entered as a predictive factor as it had in Greece( Reference Pazaitou-Panayiotou, Papapetrou and Chrisoulidou 34 ), the other study with this variable. Height and weight were not predictive of 25(OH)D levels in Guatemala; weight never rose to prediction status in the two studies reporting it as a variable, whereas height was significant in one of the same two studies. BMI was not predictive in our study as it was in only one of the four comparative studies in Table 4 reporting this variable.
Our study offered two unexpected and paradoxical findings. The group that was more scantily dressed in the photo image variable had a greater percentage of their skin exposed and had the lower vitamin D status, in regression analysis in a scaled manner. This is counterintuitive and contrary to the study among adolescent girls in Kuwait for whom wearing a headscarf and veil interfered with vitamin D status( Reference Alyahya, Lee and Al-Mazidi 36 ). The strength of the association declined through stepwise regression (Table 3). Total skin area exposed, arguably a better indicator of net solar exposure, was less contrasted between ethnicities, as the Garifunas had a larger total skin surface (Table 1). However, it matters little how much skin is exposed if the time spent outdoors is minimal: the differential outdoor time shown in Fig. 1 may mitigate skin exposure, however it is assessed.
What remains unexplained is the apparently paradoxical skin-colour effect, in which the darker-skinned sample had higher circulating 25(OH)D than those of lighter skin tone. Ethnicity or skin colour was a candidate variable in all seven citations in Table 4. It failed to predict 25(OH)D in three publications but was significant in the other four. Being Kekchi or Garifuna was a predictor in the independent linear series, but in an unexpected manner. Everywhere yet reported where ethnicity or skin colour was a factor, the darker-skinned group had a disadvantage in vitamin D status; here, by contrast, our lighter-hued ethnicity (Mayan) had lower circulating 25(OH)D. As reviewed, the data are mixed between an insignificant( Reference Malvy, Guinot and Preziosi 33 – Reference Martini, Verly and Marchioni 39 ) or a significant effect of skin colour( Reference Kimlin, Sun and Sinclair 22 , Reference Armas, Dowell and Akhter 25 – Reference Ohlund, Silfverdal and Hernell 32 ), but to our knowledge, the present study is the first one in which the darker-hued group was apparently relatively more vitamin D replete. Tallness was a differential and favourable attributable determinant of vitamin D status in the Greek population( Reference Pazaitou-Panayiotou, Papapetrou and Chrisoulidou 34 ); height was a marginal predictor (P=0·052) in our univariate model and could be preserving vitamin D status in the taller – but darker-skinned – coastal sub-sample. Moreover, an effect of inflammation on apparent vitamin D status may have been overlooked as no inflammatory biomarkers have been measured; Mangin et al.( Reference Mangin, Sinha and Fincher 8 ) have suggested that background inflammation may depress circulating 25(OH)D and the more jungle environment of the Mayans’ residence may produce more immuno-stimulation.
Strengths and limitations of the study
A strength of the present study is that it sought out arguably the most outdoor-active segment of children in the intensely sunny region of the nation, half-way through the non-rainy season in the month of February. Although this month is technically in the northern hemisphere’s winter months, the coastal summer corresponds to the rainy season with daily cloud cover and precipitation. Since blood samples were collected within 14 d of one another, any seasonality factor is removed, and the nutrient assays were conducted in a reliable and certified laboratory.
The representativeness of clothing used to estimate sun-exposed skin may be called into question as the estimation was made with school attire and this may not be what is worn during after-school outdoor activities. The outdoor exposure-scale variable, although adopted from a generic questionnaire scheme( Reference McCarty 18 ), is somewhat crude and inexact. The fallacy of BMI as a sensitive measure of body composition is well exemplified in the current study in which huge differences in absolute body size (data not shown) were seen in the context of comparable average BMI. The failure to measure inflammatory biomarkers precludes investigation of differential inflammatory stress as a mediator of 25(OH)D levels. Finally, without any estimation of differential vitamin D intake from the diet, we cannot exclude this factor in the contrasting status even if diet seems unlikely to be an important determinant, particularly in Guatemala, where food sources of vitamin D, naturally occurring in foods or from supplementation, are scarce.
Conclusions
The previous finding of vitamin D insufficiency and deficiency in elderly Mayans in the western highlands of Guatemala( Reference Sud, Montenegro-Bethancourt and Bermúdez 16 ) seems not to be an anomaly of the adverse effect of ageing on vitamin D status. Inadequate vitamin D status occurs widely among adolescents of two ethnicities inhabiting the tropical Caribbean coast of Guatemala. The apparent paradox of higher 25(OH)D levels in the darker-pigmented ethnic group is intriguing, and further elucidation is warranted. Meanwhile, Guatemala should take steps to survey vitamin D status in a nationally representative sample to assess whether public health action may be warranted in order to address yet another micronutrient deficiency in this nation.
Acknowledgements
Acknowledgements: The authors thank Ms Sheny Romero and Mr Carlos Tánchez who assisted with sample collection and processing. They acknowledge the Creighton Osteoporosis Research Center Laboratory for conducting measurements of 25(OH)D. Ms June Bierman is acknowledged for her assay work with 25(OH)D in Nebraska, USA. Also acknowledged are Angelina de Galdámez, Heather Graham, Michelle Adyniec and the personnel of Casa Guatemala, Río Dulce, Izabal for their kind assistance. The authors also acknowledge the important efforts of Dr Elizabeth Stokes, María Elena Ferguson, Dr Gladys Contreras, Raquel Leiva and Ingrid Ciego from Livingston, Izabal. Special thanks go to the adolescents and their parents who kindly participated in the study. Financial support: This research received no specific grant from any funding agency in the public, commercial of not-for-profit sectors. Conflict of interest: None. Authorship: A.N. developed the study protocols, participated in data collection and analysis, and drafted the manuscript. N.W.S. participated in the conceptualization of the study, did the overall coordination of the field work, and participated in data analysis and interpretation and in writing the final version of the manuscript. R.C. coordinated with local organizations and schools for participant recruitment, assisted in the development of study protocols, and in IRB documentation. M.J.S.-M. participated in the study design and data collection, and assisted with statistical analysis. E.C. developed and implemented equations for sun exposure and participated in statistical analysis. L.A. directed the biochemical analysis, interpreted them and participated in statistical analysis. O.I.B. participated in the conceptualization of the study, data analysis and interpretation, and in writing the final version of the manuscript. All co-authors have read and approved the final version. Ethics of human subject participation: The study was approved by the Human Studies Committee of the Center for Studies of Sensory Impairment, Aging, and Metabolism. All parents of participants were required to provide informed consent and each adolescent participant was also required to provide assent for the study.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1368980016000598









