Age-related cataract (ARC) is a common eye disease in the middle-aged and elderly that is characterized by lens opacities and visual impairment due to the oxidation of lens proteins and degenerative changes to the lens caused by ageing( Reference Congdon, Friedman and Lietman 1 , Reference Klein and Klein 2 ). According to the WHO’s latest assessment, ARC is responsible for 51 % of world blindness, which represents about 20 million people( Reference Pascolini and Mariotti 3 ). Despite its high prevalence and high cost of treatment, the aetiology of ARC is still unclear. Laboratory and animal data point to a causal role for oxidative mechanisms and suggest a possible beneficial role for antioxidant nutrients, especially vitamin E, in delaying ARC onset and progression( Reference Varma, Devamanoharan and Ali 4 – Reference Taylor and Hobbs 6 ).
Vitamin E, a lipid-soluble antioxidant concentrated in lens fibres and membranes, is postulated to inhibit ARC formation by reducing photoperoxidation of lens lipids and stabilizing lens cell membranes( Reference Bunce, Kinoshita and Horwitz 7 , Reference Sies, Stahl and Sundquist 8 ). A number of epidemiological studies( Reference Jacques and Taylor 9 – Reference Theodoropoulou, Samoli and Theodossiadis 15 ) generally support an inverse association between vitamin E and the risk of ARC. As yet, however, the protective effect of vitamin E is still controversial because other studies( Reference Hankinson, Stampfer and Seddon 16 – Reference Zheng Selin, Rautiainen and Lindblad 21 ) show that there is no relationship between vitamin E and the risk of ARC. Therefore, we conducted a meta-analysis to quantitatively assess the associations between dietary vitamin E intake, supplemental vitamin E intake, dietary and supplemental vitamin E intake, and serum tocopherol levels and the risk of ARC.
Materials and methods
We referred to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting of meta-analyses in the present analysis.
Search strategy
There were two investigators who independently performed a literature search to May 2014 using both PubMed and the Cochrane Library without restrictions using the following search terms: (vitamin E or tocopherol) and (cataract or lens opacities). Moreover, we reviewed the reference lists from retrieved articles to search for further relevant studies.
Inclusion criteria
Studies were included in the meta-analysis if they met the following criteria: (i) cohort, case–control, cross-sectional studies or randomized controlled trials published as an original study to evaluate the association between vitamin E and ARC; (ii) the exposure of interest was dietary vitamin E intake (i.e. vitamin E from foods), supplemental vitamin E intake (i.e. vitamin E from supplements), dietary and supplemental vitamin E intake (i.e. vitamin E from foods and supplements) or serum tocopherol levels; (iii) the outcome of interest was clearly diagnosed as ARC; and (iv) the relative risk (RR) with 95 % confidence interval was provided. If studies had overlapping patients or controls, only the latest or the most complete one was included.
Data extraction
Data were independently extracted by two investigators who reached a consensus on all of the items. Information extracted from each study was as follows: first author’s name, year of publication, area in which the study was conducted, study design, age, gender and number of cases and controls (participants for cohort studies), the highest and the lowest levels of vitamin E intake or serum tocopherol, and multivariate-adjusted RR (we present all results with RR for simplicity) with corresponding 95 % CI for the highest v. the lowest category of dietary vitamin E intake, supplemental vitamin E intake, dietary and supplemental vitamin E intake, or serum tocopherol level, respectively. For dose–response analysis, the number of cases and participants (person-years) and the RR (95 % CI) for each category of dietary vitamin E intake were also extracted. The median or mean level of dietary vitamin E intake for each category was assigned to the corresponding RR for every study. If the upper or lower boundary of the exposure category was open-ended, we assumed that the boundary had the same amplitude as the adjacent category.
Statistical analysis
Two (highest v. lowest, dose–response) types of meta-analysis were performed. For highest v. lowest analyses, the pooled measure was calculated as the inverse variance-weighted mean of the logarithm of the multivariate-adjusted RR with 95 % CI to evaluate the relationship between ARC risk and vitamin E status. Statistical heterogeneity among studies was assessed by using the Q and I 2 statistics( Reference Higgins and Thompson 22 ). If substantial heterogeneity was present (I 2>50 %)( Reference Higgins, Thompson and Deeks 23 ), the DerSimonian and Laird( Reference DerSimonian and Laird 24 ) random-effect model was adopted as the pooling method; otherwise, the fixed-effect model was used as the pooling method. A sensitivity analysis was performed with one study removed at a time to assess whether the results could have been affected markedly by a single study. Meta-regression with restricted maximum likelihood estimation was performed to explore the potentially important covariates that might have substantial impacts on between-study heterogeneity. Publication bias was estimated using Begg’s test( Reference Begg and Mazumdar 25 ) and the funnel plot( Reference Egger, Davey Smith and Schneider 26 ).
For dose–response analysis, a two-stage, random-effects, dose–response meta-analysis( Reference Orsini, Li and Wolk 27 ) was performed. In the first stage, a restricted cubic spline model with three knots at the 10th, 50th and 90th centiles( Reference Harrell, Lee and Pollock 28 ) of the dietary vitamin E intake was estimated using generalised least square regression, taking into account the correlation within each set of published RR( Reference Orsini, Li and Wolk 27 ). Then the study-specific estimates were combined using the restricted maximum likelihood method in a multivariate random-effects meta-analysis( Reference Jackson, White and Thompson 29 ). A P value for non-linearity was calculated by testing the null hypothesis that the coefficient of the second spline is equal to 0.
All statistical analyses were conducted with the statistical software package Stata 12·0. A two-tailed P<0·05 was considered statistically significant.
Results
Literature search and study characteristics
The search strategy identified 256 articles from PubMed and forty-two articles from the Cochrane Library. Seventy-four articles were reviewed in full text after screening by reviewing titles and abstracts. Upon closer examination, forty-seven articles were excluded for the following reasons: thirty-six articles were irrelevant to the interest of the exposure or the outcome, seven articles( 30 – Reference Chylack, Brown and Bron 36 ) did not provide OR/RR and its 95 % CI, three articles( Reference Mohan, Sperduto and Angra 37 – Reference Mares-Perlman, Brady and Klein 39 ) were duplicated studies and one article( Reference Christen, Gaziano and Hennekens 40 ) was a review. Finally, twenty-seven articles( Reference Taylor and Hobbs 6 , Reference Robertson, Donner and Trevithick 10 – Reference Leske, Chylack and He 13 , Reference Theodoropoulou, Samoli and Theodossiadis 15 – Reference McCarty, Mukesh and Fu 17 , Reference McNeil, Robman and Tikellis 19 – Reference Zheng Selin, Rautiainen and Lindblad 21 , Reference Knekt, Heliovaara and Rissanen 41 – Reference Pastor-Valero 56 ) were included in the present meta-analysis. The detailed literature search for article inclusion is shown in Fig. 1; the baseline characteristics of the study participants and the design characteristics in the published articles are shown in Tables 1 and 2.
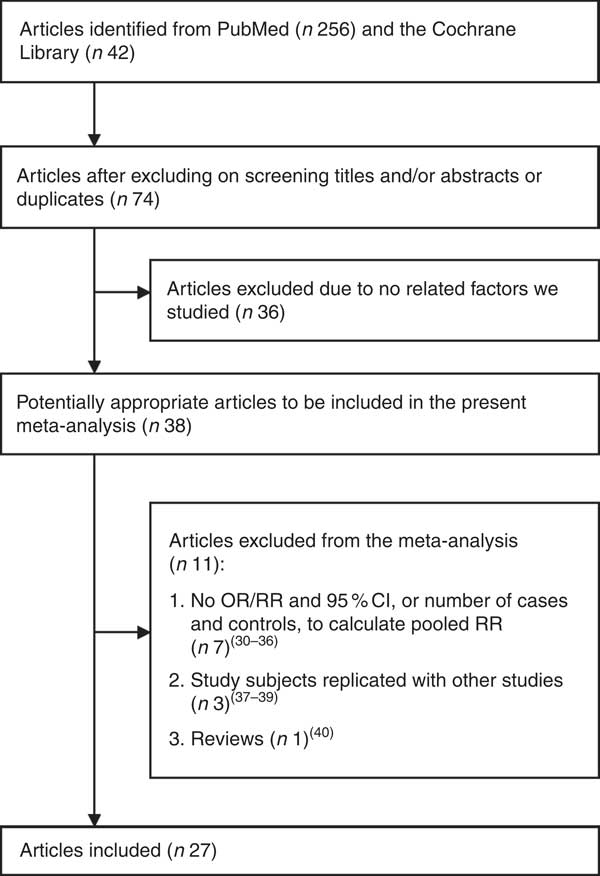
Fig. 1 Flow diagram of the literature search (RR, relative risk)
Table 1 Characteristics of the studies on vitamin E intake and age-related cataract included in the present meta-analysis

ARC, age-related cataract; RR, relative risk; RCT, randomized controlled trial; PSC, posterior subcapsular cataract; PHS, Physicians’ Health Study.
* Study ID is used in Fig. 2.
Table 2 Characteristics of the studies on serum tocopherol levels and age-related cataract included in the present meta-analysis
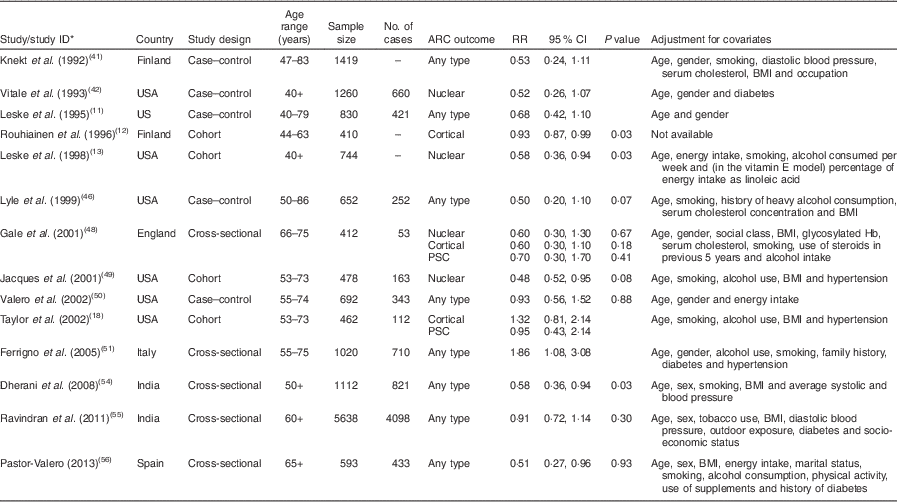
ARC, age-related cataract; RR, relative risk; PSC, posterior subcapsular cataract.
* Study ID is used in Fig. 4.
Quantitative synthesis
The main results are summarized in Table 3.
Table 3 Pooled relative risks of the relationship between vitamin E and age-related cataract, and corresponding 95 % confidence intervals
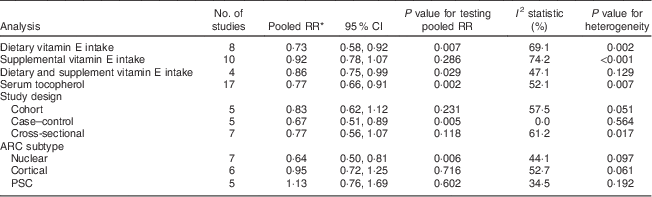
RR, relative risk; ARC, age-related cataract; PSC, posterior subcapsular cataract.
* When I 2≦50 %, pooled RR (95 % CI) was for fixed-effects model; otherwise, it was for random-effects model.
Dietary vitamin E intake and risk of age-related cataract
The association between dietary vitamin E intake and ARC risk was examined in eight articles( Reference Theodoropoulou, Samoli and Theodossiadis 15 – Reference McCarty, Mukesh and Fu 17 , Reference Tavani, Negri and La Vecchia 43 , Reference Lyle, Mares-Perlman and Klein 45 , Reference Valero, Fletcher and De Stavola 50 , Reference Christen, Liu and Glynn 53 , Reference Pastor-Valero 56 ) with eight studies including 15 021 participants and 2258 cases. The highest v. the lowest dietary vitamin E intake was statistically significantly associated with the risk of ARC (RR=0·73; 95 % CI 0·58, 0·92; I 2=69·1 %; P heterogeneity=0·002; Fig. 2).
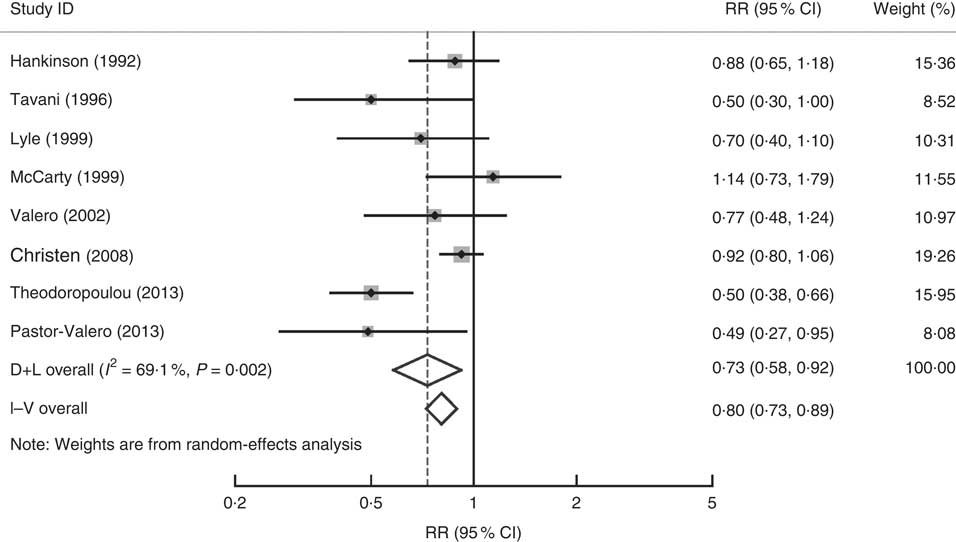
Fig. 2 Forest plot for the pooled relative risk (RR) of dietary vitamin E intake and age-related cataract. The study-specific RR and 95 % CI are represented by the grey square and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the diamond presents the pooled RR risk and its width represents the pooled 95 % CI. D+L denotes the random-effect model; I–V denotes the fixed-effect model
Dose–response analysis
Data from three studies( Reference Theodoropoulou, Samoli and Theodossiadis 15 , Reference Christen, Liu and Glynn 53 , Reference Pastor-Valero 56 ) were included in dose–response analysis. Evidence of a non-linear relationship was found (P for non-linearity=0·0009) between dietary vitamin E intake and ARC risk. The RR of ARC was 0·99 (95 % CI 0·98, 1·01), 0·97 (95 % CI 0·94, 1·00), 0·94 (95 % CI 0·90, 0·97), 0·89 (95 % CI 0·85, 0·94), 0·80 (95 % CI 0·74, 0·88) and 0·69 (95 % CI 0·59, 0·80) for dietary vitamin E intake of 5, 6, 7, 8, 9 and 10 mg/d, respectively. Figure 3 shows a statistically significant decreased risk of developing ARC with increasing dietary vitamin E intake from 7 mg/d.
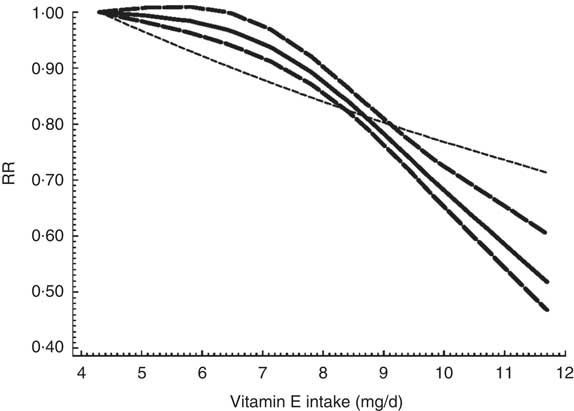
Fig. 3 The dose–response analysis between dietary vitamin E intake and risk of age-related cataract. ![]() and
and ![]() represent the estimated relative risk (RR) and its 95 % CI, respectively, from the spline model;
represent the estimated relative risk (RR) and its 95 % CI, respectively, from the spline model; ![]() represents the linear model
represents the linear model
Supplemental vitamin E intake and risk of age-related cataract
The association between supplemental vitamin E intake and ARC risk was examined in ten articles( Reference Robertson, Donner and Trevithick 10 , Reference Leske, Chylack and He 13 , Reference Hankinson, Stampfer and Seddon 16 , Reference McNeil, Robman and Tikellis 19 – Reference Zheng Selin, Rautiainen and Lindblad 21 , Reference Teikari, Rautalahti and Haukka 44 , Reference Nadalin, Robman and McCarty 47 , Reference Jacques, Chylack and Hankinson 49 , Reference Christen, Glynn and Chew 52 ) with ten studies including 358 007 participants and 5147 cases. Compared with the lowest category, the pooled RR of ARC for the highest category was 0·92 (95 % CI 0·78, 1·07; I 2=74·2 %; P heterogeneity<0·001); no statistically significant association was observed between supplemental vitamin E intake and risk of ARC.
Dietary and supplemental vitamin E intake and risk of age-related cataract
Three articles( Reference Taylor, Jacques and Chylack 18 , Reference Jacques, Chylack and Hankinson 49 , Reference Christen, Liu and Glynn 53 ) with four studies including 8512 participants and 874 cases provided the result for dietary and supplemental vitamin E intake and ARC risk. The highest v. the lowest dietary and supplemental vitamin E intake was statistically significantly associated with the risk of ARC (RR=0·86; 95 % CI 0·75, 0·99; I 2=47·1 %; P heterogeneity=0·129).
Serum tocopherol levels and the risk of age-related cataract
A forest plot of the seventeen included studies from fourteen articles( Reference Leske, Wu and Hyman 11 – Reference Leske, Chylack and He 13 , Reference Taylor, Jacques and Chylack 18 , Reference Knekt, Heliovaara and Rissanen 41 , Reference Vitale, West and Hallfrisch 42 , Reference Lyle, Mares-Perlman and Klein 46 , Reference Gale, Hall and Phillips 48 – Reference Ferrigno, Aldigeri and Rosmini 51 , Reference Dherani, Murthy and Gupta 54 – Reference Pastor-Valero 56 ) with 17 194 participants and 4179 cases is shown in Fig. 4. The highest v. the lowest level of serum tocopherol was statistically significantly associated with the risk of ARC (RR=0·77; 95 % CI 0·66, 0·91; I 2=52·1 %; P heterogeneity=0·007). A significant association was found in case–control studies (RR=0·67; 95 % CI 0·51, 0·89; I 2=0·0 %; P heterogeneity=0·564), while no significant association was found in cohort studies (RR=0·83; 95 % CI 0·62, 1·12; I 2=57·5 %; P heterogeneity =0·051) and cross-sectional studies (RR=0·77; 95 % CI 0·56, 1·07; I 2=61·2 %; P heterogeneity=0·017). For ARC subtypes, a significant association was found in nuclear (RR=0·64; 95 % CI 0·50, 0·81; I 2=44·1 %; P heterogeneity=0·097) but not in cortical (RR=0·95; 95 % CI 0·72, 1·25; I 2=52·7 %; P heterogeneity=0·061) and posterior subcapsular cataract (RR=1·13; 95 % CI 0·76, 1·69; I 2=34·5 %; P heterogeneity=0·192).
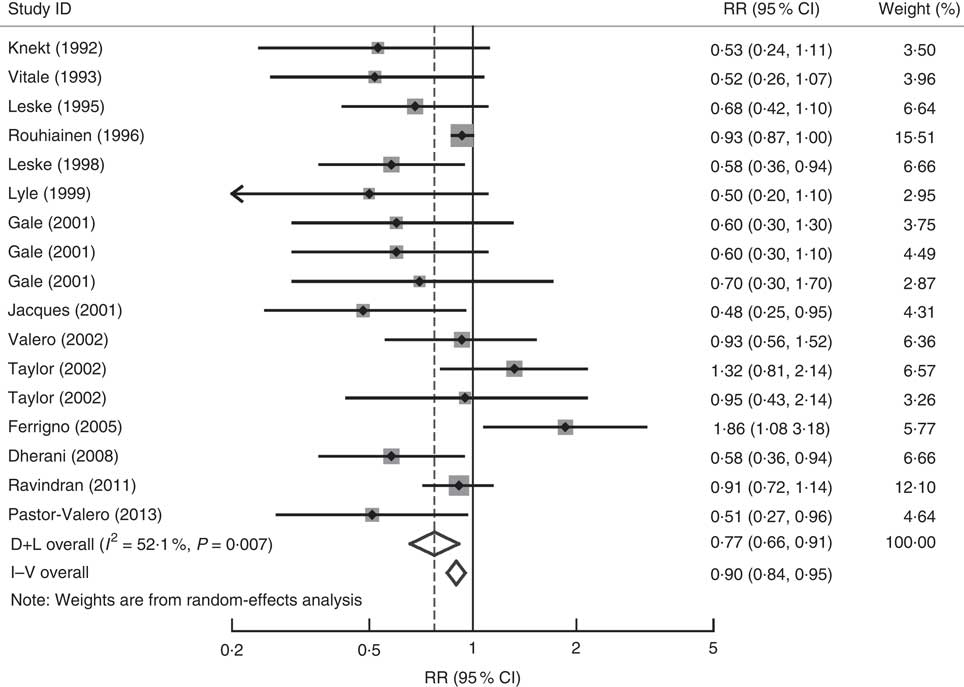
Fig. 4 Forest plot for the pooled relative risk (RR) of serum tocopherol levels and age-related cataract. The study-specific RR and 95 % CI are represented by the grey square and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the diamond presents the pooled RR risk and its width represents the pooled 95 % CI. D+L denotes the random-effect model; I–V denotes the fixed-effect model
Sources of heterogeneity and sensitivity analysis
To explore the heterogeneity, meta-regression was performed for covariate analysis for individual results. However, for the covariates publication year, study design, study conducted area and gender, the univariate meta-regression analysis showed that no covariate was significantly associated with between-study heterogeneity. Sensitivity analysis showed that no individual study had excessive influence on the above-mentioned pooled effect.
Publication bias
Visual inspection of the funnel plot and Begg’s test showed no evidence of significant publication bias for the studies of dietary vitamin E intake (P=0·174), supplemental vitamin E intake (P=0·283), dietary and supplemental vitamin E intake (P=1·000), or serum tocopherol levels (P=0·807; Fig. 5) on ARC.
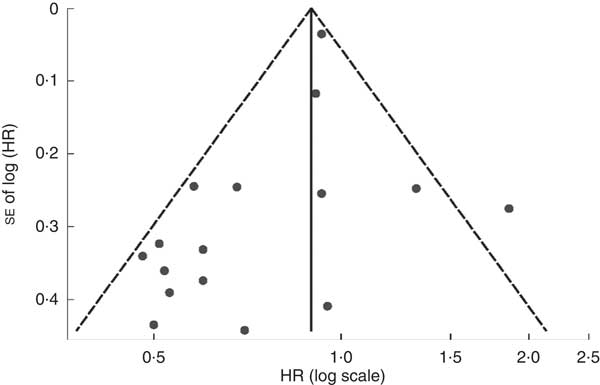
Fig. 5 Funnel plot with pseudo 95 % confidence limits (![]() ) of the associations between serum tocopherol levels and age-related cataract (HR, hazard ratio)
) of the associations between serum tocopherol levels and age-related cataract (HR, hazard ratio)
Discussion
Mechanisms of ARC are still disputed, but oxidative damage of lens proteins is believed to play an important part in the process( Reference Jacques and Taylor 9 ). Antioxidants such as vitamin E may modify antioxidant defence and the development of ARC. Vitamin E can inhibit lipid peroxidation( Reference Machlin and Bendich 57 ) and stabilize lens cell membranes( Reference Libondi, Menzione and Auricchio 58 ). Vitamin E may also affect ascorbate regeneration and enhance glutathione recycling, perhaps helping to maintain concentrations of reduced glutathione in the lens and aqueous humor( Reference Costagliola, Iuliano and Menzione 59 ).
In recent years, a large body of literature has been performed to evaluate the relationship between vitamin E and risk of ARC based on populations. The results of those studies were conflicting. Generally each individual study had a relatively small number of participants and was underpowered for detecting the effect, thus a meta-analysis should be the appropriate approach to obtain a more definitive conclusion. Our meta-analysis, of twenty-seven articles including 245 531 individuals from different countries, afforded us a much higher possibility to reach reasonable conclusions regarding the association of dietary vitamin E intake, supplemental vitamin E intake, dietary and supplemental vitamin E intake, or serum tocopherol levels and ARC risk.
Overall, we found that dietary vitamin E intake, dietary and supplemental vitamin E intake, and high serum tocopherol levels were significantly associated with decreased risk of ARC. The findings from the dose–response analysis showed evidence of a non-linear association between dietary vitamin E intake and ARC. The risk of ARC decreased with dietary vitamin E intake from 7 mg/d. In addition, a protective role was found in the stratified analyses for serum tocopherol on nuclear cataract; and high serum tocopherol was found to have a positive effect on ARC in case–control studies. However, the association of supplemental vitamin E intake with ARC risk reduction was not statistically significant. Several reasons might be taken into consideration. First, the dose of supplementation used differed in the different original studies, and the use of high-dose vitamin E supplements may be associated with increased risk of ARC. Second, in the total of ten included studies on supplemental vitamin E intake and ARC risk, five were randomized controlled trials( Reference McNeil, Robman and Tikellis 19 , Reference Christen, Glynn and Sesso 20 , Reference Teikari, Rautalahti and Haukka 44 , Reference Nadalin, Robman and McCarty 47 , Reference Christen, Glynn and Chew 52 ) that were based on populations with different nutritional status. In addition, in two randomized controlled trials( Reference McNeil, Robman and Tikellis 19 , Reference Nadalin, Robman and McCarty 47 ) the subjects were volunteers, thus volunteer bias might be introduced. Third, in three of five randomized controlled trials( Reference McNeil, Robman and Tikellis 19 , Reference Teikari, Rautalahti and Haukka 44 , Reference Nadalin, Robman and McCarty 47 ) and one cohort study( Reference Leske, Chylack and He 13 ), the intervention periods of less than 5 years are too short to influence the natural history of cataract development. Furthermore, ARC develops slowly over many years and might even require a long-term prevention rather than treatment; thus, perhaps it is better to start the preventive interventions at an earlier age within one’s lifetime.
The findings of our study have important clinical and public health implications with respect to ARC prevention. According to the dose–response analysis, a statistically significant decreased risk of developing ARC was shown with increasing dietary vitamin E intake from 7 mg/d.
Our meta-analysis suggested that vitamin E might have a significant beneficial effect on the prevention of ARC, especially nuclear cataract, in the analyses of serum tocopherol on ARC subtypes. This might be due to the fact that different cataract types are related to different risk factors and different pathophysiological processes( Reference Chang, Koo and Agron 60 , Reference Michael and Bron 61 ). Cumulative oxidative stress might be more likely to result in depletion of the endogenous antioxidant defence system in the nucleus of the lens, which would reduce antioxidants uptake in this region and lead to inability to repair the damage( Reference Fan, Zhang and Theves 62 – Reference Beebe, Holekamp and Siegfried 64 ).
Between-study heterogeneity is common in meta-analysis and it is essential to explore the potential sources of between-study heterogeneity. Hence, we conducted a meta-regression analysis on variables including publication year, study design, study conducted area and gender to explore the potential sources of between-study heterogeneity. However, these factors were not found to be sources of heterogeneity in our meta-analysis, but other possibilities related to ARC, such as variations in lifestyle and dietary practices, cannot be ruled out. The presence of heterogeneity indicates the need for consensus definitions for ARC and its subtypes in future studies.
To interpret our study results properly, it is necessary to understand several limitations. First, the potential contributions to the epidemiological criteria for causality are different in the various observational studies included in the present meta-analysis. With the exception of consistency, to which all designs contribute, and biological plausibility, to which no designs contribute directly, all three types of observational studies in our meta-analysis contribute to some but not all criteria including temporality, strength or dose–response, experimental confirmation and specificity. A prospective design meets the criteria of temporality and is less affected by biases than case–control and cross-sectional designs, so it is in the highest order of the strength of evidence in observational studies; while the other two designs usually have no temporality and are susceptible to biases, and the strength of evidence from these studies is weaker as a result. On the other hand, observational studies are closer to the real-life environment, which is more credible when making its corollary to reality, but the results are more susceptible to interference. In our meta-analysis, combined results from the three types of study design in serum tocopherol were inconsistent; stronger association was found in the combined results from case–control studies. However, an overstated association could be expected from the case–control studies because of recall or selection bias, and early symptoms in patients may have resulted in a change in dietary habits. Second, a meta-analysis is not able to solve problems with confounding factors that could be inherent in the included studies. Although most studies adjusted for other known risk factors for ARC, residual or unknown confounding cannot be excluded as a potential explanation for the observed findings. Third, the number of studies involved in the meta-analysis was insufficient and we could only perform dose–response analysis on dietary vitamin E intake. Finally, in a meta-analysis of published studies, it is possible that an observed association might suffer from publication bias because studies with null results tend not to be published. However, no significant publication bias was detected in our meta-analysis.
Conclusion
In summary, results from the present meta-analysis suggest that increasing dietary vitamin E intake, dietary and supplemental vitamin E intake, and high level of serum tocopherol might be significantly associated with reduced ARC risk.
Acknowledgements
Financial support: This research project was supported by The Natural Science Foundation of Shandong Province (grant number ZR2009CM112; Principal Investigator: W.J.). The funder provided aid to the authors to complete the literature search, but had no role in the design, data collection, analysis or writing of this article, and the reporting of this meta-analysis. Conflict of interest: None. Authorship: Y.Z. and W.J. conceived of the study, participated in its design and coordination, carried out the literature search and data extraction, and were involved in drafting the manuscript or revising it critically for important intellectual content. Z.X. and W.W. carried out the literature search, data extraction and interpretation of the data. D.Z. was involved in interpretation of the data and revising the manuscript critically for important intellectual content. Ethics of human subject participation: Ethical approval was not required.











