Aboriginal populations worldwide are disproportionally affected by type 2 diabetes mellitus( Reference Naqshbandi, Harris and Esler 1 ). Recent dramatic increases in the prevalence of type 2 diabetes in aboriginal populations have been attributed to rapid ‘westernisation’( Reference O'Dea 2 ). In Canada, epidemic rates of diabetes exist among the aboriginal population, with crude prevalence rates being 3·6 to 5·3 times higher than corresponding rates for non-aboriginal Canadians( Reference Young, Reading and Elias 3 ).
Population-based primary prevention of diabetes through efforts to change the overall nature of dietary behaviour has had limited successes thus far. However the potential remains for prevention through more targeted changes in the nature of dietary fat consumed. n-3 Fatty acids have been proposed to have a protective role against insulin resistance and consequently diabetes in aboriginal and non-aboriginal populations( Reference Ebbesson, Kennish and Ebbesson 4 , Reference Adler, Boyko and Schraer 5 ). Epidemiological studies have reported a lower prevalence of insulin resistance and type 2 diabetes in Greenland Inuit and sub-Arctic native populations whose diets vary from those typical of mainstream Western European, Australian or North American populations( Reference Adler, Boyko and Schraer 5 , Reference Bang, Dyerberg and Sinclair 6 ). These diets vary not so much in the total quantity of fat, but in its quality, being higher in n-3 fatty acids and lower in SFA( Reference Adler, Boyko and Schraer 5 , Reference Schraer, Risica and Ebbesson 7 ). SFA, on the other hand, have been implicated to have detrimental effects on insulin resistance( Reference Parker, Weiss and Troisi 8 ).
Little research has investigated the relationship between dietary fats and glycaemic disease for aboriginal populations residing outside the sub-Arctic zone. It is important to investigate such associations in other aboriginal populations similarly at risk for the development of type 2 diabetes, but for which the food supply is not dominated to such a degree by local seafood high in n-3 fatty acids. Therefore the present study sought to assess the relationship between total n-3 fatty acid and SFA intakes and insulin resistance in a Canadian First Nation sample at high risk for the development of diabetes. Such research has important public health implications for influencing dietary recommendations in First Nations populations suffering an excess burden of insulin resistance and glycaemic disease.
Methods
Data collection
Data analysed in the present observational study were drawn from a diabetes screening initiative conducted among on-reserve First Nation people (Interior Salishan, Plateau Area) in the rural Okanagan region of British Columbia, Canada. The sampling and methodology of the study have been reported previously( Reference Daniel, Cargo and Lifshay 9 ). The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the University of British Columbia. Written informed consent was obtained from all participants.
Participants were recruited through community meetings. Individual volunteers for screening were required to be aged 18 years or older and at high risk for diabetes (overweight or obese, and having a family member with type 2 diabetes). Pregnant women and persons with known diabetes were excluded from screening. Fifty-seven per cent of eligible persons (n 198) identified from community health records as being overweight (BMI ≥25 kg/m2) participated in the survey. The main reason for non-participation was lack of interest. Male sex and age less than 30 years were the main correlates of non-participation. All testing was done in meeting halls between 07.30 and 12.00 hours by laboratory technicians and registered nurses from a local hospital. Glycaemic status was determined using a 2-h oral glucose tolerance test with a 75-g carbohydrate load. Participants were classified using WHO criteria as normoglycaemic or having glycaemic disease (2-h glucose ≥ 11·1 mmol/l)( 10 ). Only normoglycaemic participants were considered in the present study.
Measures
Insulin resistance was calculated using the homeostasis model assessment (HOMA-IR) algorithm( Reference Matthews, Hosker and Rudenski 11 ) from blood glucose and insulin concentrations determined from 12 h fasting venous blood samples. HOMA-IR is used widely in epidemiological studies and has been validated against ‘gold standard’ hyper-euglycaemic and hyper-glycaemic clamps respectively( Reference Matthews, Hosker and Rudenski 11 , Reference Haffner, Miettinen and Stern 12 ). Assays were performed on serum specimens obtained on-site and stored at 4°C until analysis on the day of collection. Glucose was assessed via enzymatic assay kits (Kodak Ektachem®; Eastman Kodak Co., Rochester, NY, USA); insulin was measured via microparticle immunoassay kits (IMx®; Abbott Laboratories, Abbott Park, IL, USA). Intra- and inter-assay coefficients of variation were respectively 1·2 % and 1·8 % for glucose and 3·8 % and 4·2 % for insulin.
Dietary food and drink intake was assessed for the week following screening using self-completed records maintained over three consecutive days, including one weekend day. Participants received training by registered nurses and dietitians on how to record their food consumption. Three-dimensional models and illustrations were used to portray serving sizes. All records were rigorously reviewed for completeness and then coded following clarification, verification and/or revision of unusual or likely incorrect responses. Nutrient intakes were computed by a registered dietitian-nutritionist using the Canadian Dietary Information System (CANDI) version 4·0 for food intake analysis, which includes traditional foods consumed by First Nations in Canada( Reference Thompson and Brulé 13 ). Total grams of n-3 fatty acids and SFA consumed over the 3 d period were determined. The reliability and validity of self-report 3 d estimate food records are established( Reference Crawford, Obarzanek and Morrison 14 ).
Anthropometric, lipid, sociodemographic and behavioural measures were also obtained. All anthropometric measures were taken three times by the same two registered nurses. Only the median of the three values was retained for analyses. Body mass and height were measured using a beam balance scale and stadiometer. BMI was calculated as body mass (in kilograms) divided by the square of height (in metres). Waist circumference measures were taken where the waist was best defined, halfway between the costal border and the iliac crest. High waist circumference was defined as ≥88 cm for women and ≥102 cm for men. Concentrations of TAG and HDL-cholesterol (HDL-C) were determined from 12 h fasting venous blood samples and assessed via enzymatic assay kits (Kodak Ektachem®; Eastman Kodak Co.). Sociodemographic measures including age, gender and high school graduation were all obtained in a self-reported demographic questionnaire. Behavioural measures included physical activity, smoking status and alcohol consumption. These were surveyed in a lifestyle questionnaire based on the Canadian National Population Health Survey. Each behavioural measure was self-reported and required a ‘yes’ or ‘no’ answer. The following questions were used: ‘At least once a week, did you engage in any regular activity similar to brisk walking, jogging, bicycling, etc., long enough to work up a sweat?’, ‘Do you currently smoke cigarettes daily?’ and ‘Do you drink alcohol?’.
Statistical analyses
Of 198 persons screened with an 2-h oral glucose tolerance test, complete data were available for 142 of whom ten had impaired glucose tolerance (2-h glucose ≥ 7·0 and <11·1 mmol/l) and six had previously unknown diabetes (2-h glucose ≥11·1 mmol/l)( 10 ). These sixteen individuals were excluded from statistical analyses. HOMA-IR values were log transformed and modelled against total n-3 fatty acid and SFA intakes. The linear regression model accounted for age, gender, community, education, physical activity, waist circumference, HDL-C and TAG concentrations. Analyses also accounted for additional measures of diet including fibre, protein and carbohydrate intakes expressed as a proportion of total energy intake. Total fat intake, however, was not included in models given its high correlation with total SFA intake already included in models (r = 0·95). Accounting for the above covariates, especially additional measures of dietary intake, is important to illustrate that total n-3 fatty acid intake is not merely a marker for low SFA intake, high fibre intake and/or more physical activity( Reference Cundiff, Lanou and Nigg 15 ). Smoking was considered as a covariate but was not found to be statistically significantly related to HOMA-IR and was thus removed to conserve statistical power. Continuous independent variables were standardised prior to analyses. Statistical significance was set at α = 0·05. Analyses were conducted using the statistical software package SPSS® version 17·0.
Results and discussion
Characteristics of the participants and results from the regression analysis are reported in Tables 1 and 2, respectively. In this sample of First Nation individuals in rural British Columbia, insulin resistance was inversely associated with n-3 fatty acid intake and positively associated with SFA intake (Table 2).
Table 1 Characteristics of participants: on-reserve First Nation individuals (Interior Salishan) aged 18 years and over (n 126), rural Okanagan region of British Columbia, Canada
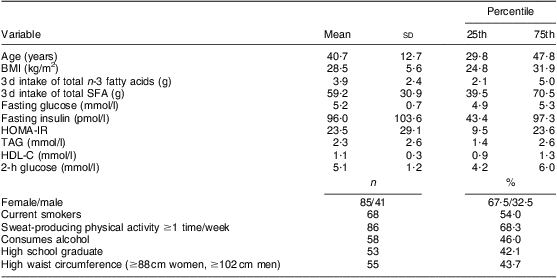
HOMA-IR, homeostasis model assessment of insulin resistance; HDL-C, HDL-cholesterol.
Table 2 Parameter estimates for multivariable analyses of relationships between HOMA-IR, dietary n-3 fatty acid and SFA intakes* among on-reserve First Nation individuals (Interior Salishan) aged 18 years and over (n 126), rural Okanagan region of British Columbia, Canada
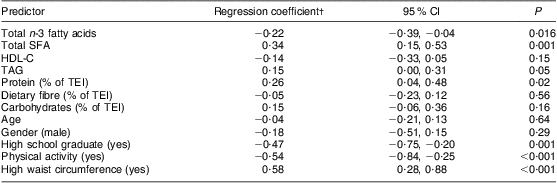
HOMA-IR, homeostasis model assessment of insulin resistance; HDL-C, HDL-cholesterol; TEI, total energy intake.
*The model also adjusts for community.
†Regression coefficients for continuous measures are for 1 sd increments.
A comprehensive literature review suggests that the current study is the first one published reporting such associations for an aboriginal sample at risk for the development of diabetes, outside the Arctic zone. The results are consistent with observational studies( Reference Ebbesson, Risica and Ebbesson 16 , Reference Thorseng, Witte and Vistisen 17 ) of Greenland Inuit and Alaska Natives that reported an inverse association between dietary n-3 fat intake and insulin resistance. However, the results of these studies may to some degree be confounded by overall macronutrient profile, particularly other dietary components that correlate with both n-3 fatty acid intake and insulin resistance. The current study does not suffer the same limitation.
Our results are consistent with findings from studies conducted in non-aboriginal populations, which generally support an association between n-3 fatty acids consumption and insulin resistance and sensitivity. For instance, Tsitouras and colleagues( Reference Tsitouras, Gucciardo and Salbe 18 ) have reported improvement in insulin sensitivity following an 8-week diet rich in n-3 fatty acids. Three randomised controlled trials found that n-3 fatty acids had a lowering effect on insulin resistance( Reference Ramel, Martinez and Kiely 19 ) and improved insulin sensitivity( Reference Browning, Krebs and Moore 20 , Reference Navas-Carretero, Pérez-Granados and Schoppen 21 ). Other randomised controlled trials( Reference Griffin, Sanders and Davies 22 , Reference Gustafsson, Öhrvall and Ekstrand 23 ) yielded changes in insulin resistance in the expected direction, although these results were not statistically significant.
Furthermore, it appears that previous dietary studies of aboriginal populations have not yet assessed the relationship between insulin resistance and SFA. The results of the present study are consistent with speculations previously made for aboriginal populations, that a lower dietary intake of SFA might correspond to a lower level of glycaemic disease( Reference Bang, Dyerberg and Sinclair 6 ). Some work has attributed increasing rates of diabetes in Arctic and other aboriginal populations to a change from traditional to westernised diets( Reference O'Dea 2 , Reference Bang, Dyerberg and Sinclair 6 ), due in part to the increase in dietary SFA, but any such changes have yet to be investigated longitudinally. The results of randomised controlled trials in non-aboriginal populations both support( Reference Vessby, Uusitupa and Hermansen 24 , Reference Xiao, Giacca and Carpentier 25 ) and contradict( Reference Fasching, Ratheiser and Schneeweiss 26 , Reference Lovejoy, Smith and Champagne 27 ) a link between insulin resistance and SFA.
The limited detailed dietary research conducted thus far on diet and insulin resistance in aboriginal populations in field settings supports the value of the present study. Research in First Nations settings is challenging, and designs such as randomised controlled trials are widely resisted by aboriginal people( Reference McDonald, Priest and Doyle 28 ). The associations observed serve to provide a stronger basis for the hypothesised protective role of n-3 fatty acids that might subsequently be pursued with stronger, more internally valid, albeit less generalisable, research designs removed from field settings in future research with aboriginal people.
The main limitation of the present study is its cross-sectional design, which precludes knowing the directionality of the observed relationships and whether the associations are causal. It is, however, an initial step to revealing these relationships. Future research is needed to determine the temporal relationship of change in n-3 fatty acids and SFA in relation to change in insulin resistance and β-cell function. A further limitation was the limited sample size of 126 participants. Although small for an epidemiological study, this sample size still provided adequate power to detect statistically significant associations. The focus of the study on normoglycaemic individuals at high risk of diabetes (relatives of persons with diabetes and/or overweight or obese) precludes inference that the results are representative of the overall base population. Finally, gold-standard methods for the assessment of insulin resistance, the hyper-euglycaemic and the hyperglycaemic clamp techniques, could not be used in this research. Both such methods are expensive, labour intensive, invasive and time consuming, and thus impracticable for field-based epidemiological studies. The measurement method used in the present study, HOMA-IR, is appropriate for such studies and has been validated against gold standards.
Conclusions
Our results, which account for a spectrum of confounding influences including macronutrient profile, suggest that total dietary n-3 fatty acids may be protective against insulin resistance, and that SFA may promote insulin resistance which may precede the development of diabetes in First Nations individuals. These results, specific to a non-Arctic high-risk aboriginal group, hold a key position for furthering dietary research relevant to the prevention of diabetes in such populations. Our data suggest that the nature or quality of fat consumed is of key importance in relation to insulin resistance and glycaemic risk for aboriginal people.
Acknowledgements
Sources of funding: This research was funded by the National Health Research and Development Program, Health Canada (NHRDP #6610-2022-ND). C.P. was funded by a National Health and Medical Research Council (NHMRC) Post-doctoral Training Research Fellowship (#570139). Conflicts of interest: The authors declare that they have no competing interests. Authors’ contributions: All authors contributed to the conception and design of the study and to the analysis and interpretation of the data. M.D. collected the data. C.P. and S.L.P. drafted the manuscript. M.D. provided critical review. All authors approved the final version of the manuscript. Acknowledgements: The authors appreciate the support and participation of the Interior Salishan peoples of the Okanagan Valley, British Columbia, and all Aboriginal Advisory Committee members. Appreciation is also extended to the Nursing Faculty of Okanagan University College for its assistance in the conduct of the study.




