In the original publication of Filippone et al.,[Reference Filipone, Sun and Jaramillo1] a number of errors were introduced after a numerical error in the calculated formation energies of the Zr- and Hf-containing materials that were studied. All corrections are listed below.
Caption of Table 1, “eV/formula unit” should be “eV/unit cell.”
Page 3, right column, paragraph 2, line 8: Temperature range given as “1300–1500 °C” should be “1300–1350 °C.”
Figures 1 and 2 have been corrected and are reproduced here. The caption to Figure 2 should additionally include the sentence, “The effect of such errors is very small, and the red lines overlap the underlying black line in the plot.”
Page 5, left column, paragraph 1, line 7: “ZrSrS3” should be deleted so that the sentence reads, “Using for example 1000 °C, BaZrS3, BaHfS3, and SrHfH3 require a maximum pressure of about 10−9 torr for adsorption-limited growth.”
Page 5, left column, paragraph 1, lines 10–13: The two sentences beginning with, “BaTiS3 does not form…” and ending with “…stable solid phase TiS,” should say, “BaTiS3 and SrZrS3 do not have growth windows.”
Page 5, left column, paragraph 2, lines 3–9: The last three sentences of the paragraph should be replaced with, “The growth windows for BaZrS3, BaHfS3, BaZrSe3, and SrHfS3 are all very narrow. The widest is that for BaHfS3, extending for one order of magnitude in pressure.”
Page 5, right column, paragraph 1, line 11: “−0.447 eV/f.u.” should be “–0.112 eV/formula unit.”
Page 6, left column, paragraph 3, lines 17–18: The last sentence should end after “negligible” so that the sentence reads, “The effect on the predicted growth window is negligible.”
Page 6, right column, paragraph 1, lines 6–9: The sentence beginning, “The growth window for…” should be replaced with “As a rule, the growth windows for selenides may be more accessible than those for sulfides.”
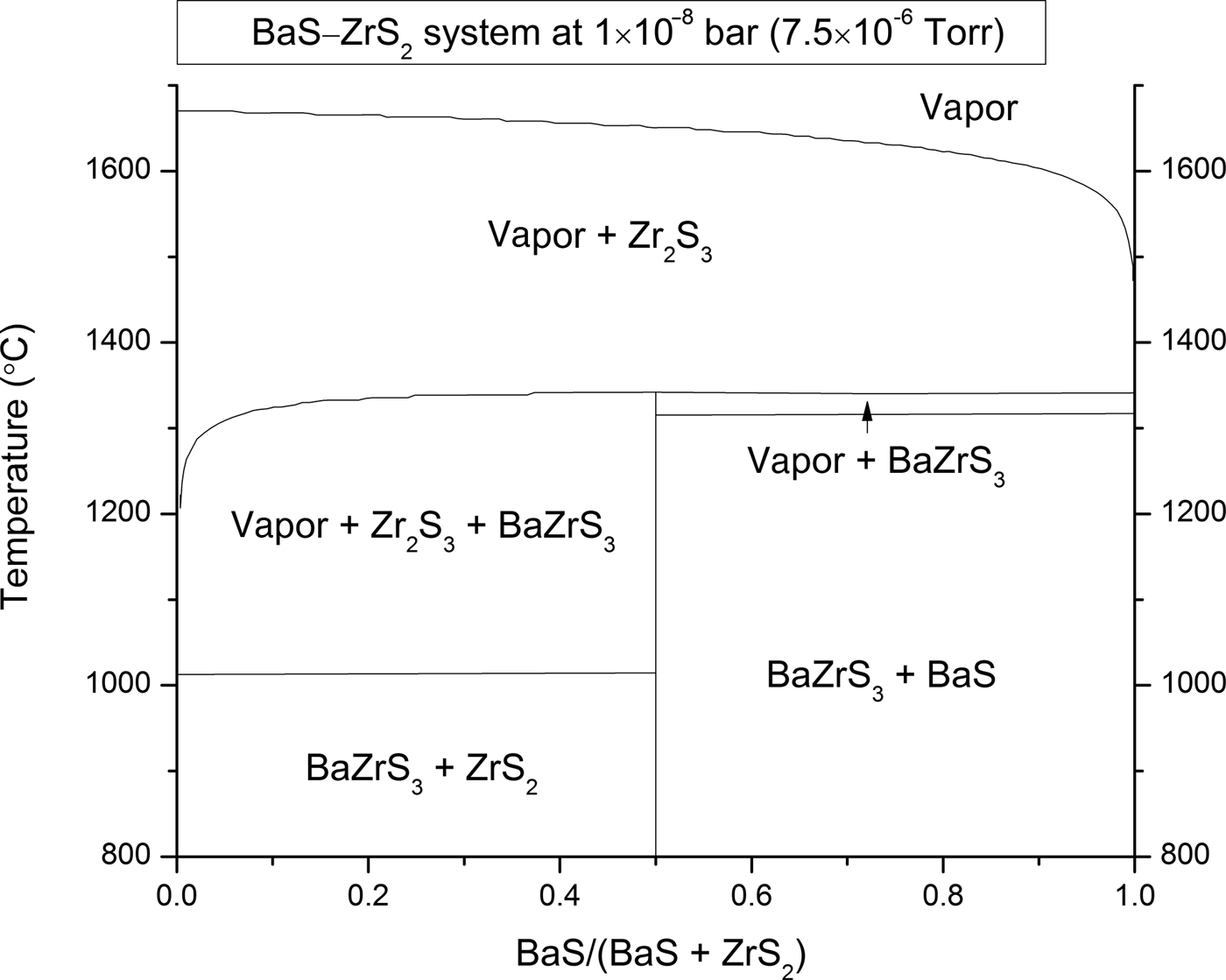
Figure 1 Composition–temperature equilibrium phase diagram for the pseudo-binary BaS–ZrS2 system at a pressure of 7.5 × 10−6 torr. The thermodynamic growth window for BaZrS3 is on the Ba-rich side of the phase diagram.

Figure 2 Temperature–pressure phase diagrams for six of chalcogenide perovskites that are calculated to be thermodynamically stable relative to decomposition into binary chalcogenides. The fixed composition in each case is a mole fraction of 0.6 of the ACh component. In each case, the thermodynamic growth window is labeled “ABCh3 + vapor”, for variable elements A, B, and Ch. Plots are ordered by the formation energy of the ternary phase from the binaries, from most to least stable. (a) BaS–ZrS2, (b) BaS–HfS2, (c) BaS–TiS2, (d) BaSe–ZrSe2, (e) SrS–HfS2, (f) SrS–ZrS2. The additional red lines in (a) show how the lower limit of the growth window would be changed by a ±5% error in the formation energy of BaZrS3. The effect of such errors is very small, and the red lines overlap the underlying black line in the plot.
The authors apologize for these errors. The message and conclusions of the paper remain unchanged. The authors predict that chalcogenide perovskites containing transition metals may be very difficult to grow by the adsorption-controlled technique, but those containing main group metals may be quite accessible.





