Anaemia is one of the most common nutritional disorders, affecting an estimated 1·62 billion people around the globe( Reference McLean, Cogswell and Egli 1 ). Fe deficiency is an important cause of anaemia, but can also be present without anaemia. Fe deficiency is also highly prevalent globally and non-pregnant women of reproductive age (15–49 years) are greatly affected, with over 496 million( Reference Stevens, Finucane and De-Regil 2 ) identified as Fe deficient worldwide. Anaemia and Fe deficiency are associated with impaired cognitive function and decreased work capacity( 3 ). For women of reproductive age who may become pregnant, additional adverse effects include increased risk for preterm delivery, low-birth-weight infants and decreased Fe stores for the baby, which may lead to impaired development( Reference Allen 4 ). Populations in developing countries are particularly susceptible to anaemia and Fe deficiency due to increased risk of infections and poor diet quality( 3 ).
There is uncertainty regarding the ideal biomarkers to assess Fe status during states of infection and/or inflammation( Reference Thurnham, McCabe and Haldar 5 ). Serum ferritin is greatly affected by increased inflammation, while other Fe status markers such as soluble transferrin receptor, Zn protoporphyrin and erythropoietin may be less affected, although these indicators have other drawbacks( Reference Kung’u, Wright and Haji 6 ). Serum ferritin is the WHO-recommended indicator to assess Fe deficiency in populations in the absence of inflammation. However, there is no clear guidance on how to account for inflammation in assessing Fe status in populations with a high prevalence of inflammation( 7 ).
Concurrently, overweight and obesity have become a global epidemic with over a third of adult women considered overweight or obese and almost two-thirds in central Latin America( Reference Caballero 8 , Reference Stevens, Singh and Lu 9 ). A growing number of developing countries are experiencing a dual burden of malnutrition with undernutrition and overnutrition present at the same time( Reference Satia 10 – Reference Fernald and Neufeld 13 ). Obesity is associated with CVD, diabetes and some cancers( 14 ). In addition, obesity, anaemia and Fe deficiency can result in overlapping consequences, including adverse pregnancy outcomes( Reference Allen 4 , Reference Farr, Yamada and Butterfield 15 ). Using various indicators of Fe status to define Fe deficiency, several reports have found an association between obesity and Fe deficiency, with the direction of the relationship varying by the Fe indicator used( Reference Aeberli, Hurrell and Zimmermann 16 – Reference Lecube, Carrera and Losada 22 ). Serum ferritin is often used as a biomarker for detecting Fe deficiency in epidemiological studies since low serum ferritin levels are a reflection of depleted Fe stores( 3 ). Multiple studies, most carried out in high-income countries, have reported positive( Reference Ausk and Ioannou 18 , Reference Ahmed, Coyne and Dobson 23 , Reference Moschonis, Chrousos and Lionis 24 ) or non-significant associations( Reference Lecube, Carrera and Losada 22 , Reference Karl, Lieberman and Cable 25 – Reference Kordas, Centeno and Pachon 27 ) between serum ferritin and obesity or overweight status. This may be due to increased inflammation associated with obesity( Reference Greenberg and Obin 28 ) and ferritin’s role as an acute-phase protein( Reference Ausk and Ioannou 18 , Reference Means 29 ). This is in contrast to other indicators that detect later stages of Fe deficiency (e.g. soluble transferrin receptor, serum Fe, transferrin saturation), which tend to show an inverse relationship between Fe status and weight status( Reference Aeberli, Hurrell and Zimmermann 16 – Reference Ausk and Ioannou 18 , Reference Pinhas-Hamiel, Newfield and Koren 20 , Reference Lecube, Carrera and Losada 22 , Reference Menzie, Yanoff and Denkinger 30 , Reference Yanoff, Menzie and Denkinger 31 ).
During an acute-phase response, the concentration of positive acute-phase proteins increases and may lead to a decrease in Hb production and an increase in serum ferritin( Reference Finch, Bellotti and Stray 32 , Reference Baynes, Bezwoda and Bothwell 33 ), although the exact mechanism of this increase remains unclear( Reference Feelders, Vreugdenhil and Eggermont 34 ). C-reactive protein (CRP) and α1-acid glycoprotein (AGP) are commonly used to measure the acute-phase response caused by inflammation( Reference Northrop-Clewes 35 ). Both of these indicators have been positively associated with obesity( Reference Yanoff, Menzie and Denkinger 31 ) and elevated serum ferritin( 7 ). CRP has been frequently used to measure inflammation in overweight and obese populations( Reference Aeberli, Hurrell and Zimmermann 16 – Reference Ausk and Ioannou 18 , Reference Tussing-Humphreys, Liang and Nemeth 21 , Reference Yanoff, Menzie and Denkinger 31 , Reference Timpson, Nordestgaard and Harbord 36 ), although AGP, a marker of chronic low-grade inflammation, may be a more appropriate indicator for assessing the chronic inflammation associated with obesity( Reference Kung’u, Wright and Haji 6 ). The use of CRP as a marker of acute inflammation and AGP as a marker of chronic inflammation is based on the behaviours of these proteins in response to an acute or chronic infection( Reference Thurnham, Mburu and Mwaniki 37 ). The behaviours of these proteins in response to chronic excess of adipose tissue and elevation of adipokines have been less studied, although it appears they are associated( Reference Maachi, Pieroni and Bruckert 38 ).
In developing countries where the double burden of undernutrition and overnutrition is increasing, understanding the potential influence of overweight and obesity on serum ferritin, a key indicator of Fe deficiency, may be important to understand the prevalence of and changes in Fe deficiency in these countries over time. The objective of the current analysis was to examine the relationship between overweight and obesity and serum ferritin among a population of women of reproductive age from Nicaragua. We also consider the impact of AGP on these relationships.
Experimental methods
Participants
In Nicaragua, the Sistema Integrado de Vigilancia de Intervenciones Nutricionales (SIVIN; Integrated Surveillance System for Nutrition Interventions) surveys a nationally representative sample of households with children 6–59 months of age. SIVIN employs a complex, multistage, probability cluster design to continuously sample selected households throughout the year, resulting in a national sample after 12 months of data collection; multiple years can be combined for increased sample size. In each household, a single child (6–59 months) as well as his/her mother or caregiver are invited to participate. The participants included in the current analysis were the non-pregnant mother/caregivers (aged 15–49 years) surveyed in 2004–2005. These women reported information about the household, family, herself and her child; anthropometric and biological measures were also collected.
Ethical review
Participation in the interview and biological sample collection was voluntary and informed consent by signature was obtained from each woman. The Nicaraguan Ministry of Health approved the data collection. A de-identified data set was used for the current analysis. Emory University Institutional Review Board (IRB) determined no IRB review was required for this secondary analysis.
Anthropometric measures
Height and weight measurements were taken according to recommended standards( 39 ). Shorr measuring boards were used to measure each woman’s height in centimetres and millimetres. Weight was measured by SECA 881 scales in kilograms. BMI (kg/m2) values were categorized as: underweight (<18·5), normal weight (18·5–24·9), overweight (25·0–29·9) and obese (≥30·0)( 40 ). Weight values of <12·9 kg or >140·0 kg, height values of <110·0 cm or >200·0 cm and BMI values of <3·2 kg/m2 or >55·0 kg/m2 were excluded as implausible( 41 ).
Biochemical measures
Venous sampling was used to collect blood samples. Vacutainers were placed in a cold box during the day and processed each night. After processing, samples were frozen and stored in freezers in the health clinic until being transported to the central laboratory within a few days. Samples were then shipped to the Instituto de Nutricion de Centro America y Panama (INCAP) in Guatemala for analysis of AGP and serum ferritin. Serum AGP was measured by the Dade-Behring turbidimetric method( 41 ) and serum ferritin by the Ramco enzymatic immunoassay. Elevated AGP was defined as >1·0 g/l( Reference Thurnham, McCabe and Northrop-Clewes 42 ) and low serum ferritin as <15 μg/l( Reference Hallberg, Bengtsson and Lapidus 43 ).
Covariates
Covariates included age (15·0–19·9 years, 20·0–29·9 years, 30·0–39·9 years and 40·0–49·9 years), urban/rural residence, and education attained (none, primary school, secondary school, university).
Statistical analyses
From 2004–2005, 1000 women were surveyed. Analysis was limited to participants with complete data for serum ferritin, AGP, anthropometry and covariates (n 861). Those excluded because of insufficient data were more likely to be older (40·0–49·9 years) and with no formal education. Underweight women were excluded from analysis due to small sample size (n 29). This brought the final sample size to 832. Descriptive statistics and the prevalence of low serum ferritin in each BMI and AGP group were calculated. Normality assessment showed AGP and serum ferritin to have non-normal distributions; therefore geometric means were presented and Pearson correlations used log-transformed variables.
Logistic regression modelling was implemented accounting for weighting and complex sample design using the procedure SURVEYLOGISTIC in the statistical software package SAS version 9·2. Collinearity was assessed by a macro accounting for sample design using PROC SURVEYLOGISTIC (M Zack, J Singleton, C Satterwhite et al., unpublished results). Three models with low serum ferritin as the dependent variable and categorized BMI as the primary independent variable were analysed. The first model included only the weight status variables and covariates, the second model additionally adjusted for AGP, and the third model was the same as the first model except that the sample was limited to only those participants without elevated AGP (n 663). All models were adjusted for age, urban/rural residence and education. Interactions were assessed for weight status with each covariate and considered significant at P<0·05. In the primary model the interaction between age and weight status was statistically significant but was dropped because of the low sample size in one subset (n 4). No other two-way interactions were significant.
Results
As shown in Table 1, 49·0 % of the women in the final sample were categorized as normal weight, 31·8 % as overweight and 19·2 % as obese, with mean BMI of 26·1 (95 % CI 25·7, 26·4) kg/m2. Obese and overweight women were more likely to have elevated AGP levels. Only 15·4 % of normal-weight women had elevated AGP compared with 22·7 % of overweight and 29·6 % of obese women (P<0·0001). The geometric mean of AGP among the women was 0·82 (95 % CI 0·80, 0·84) g/l (data not shown).
Table 1 Descriptive characteristics and crude odds ratios of low serum ferritin by covariates among non-pregnant women aged 15–49 years with a child 6–59 months of age (n 832), Nicaragua SIVIN, 2004–2005
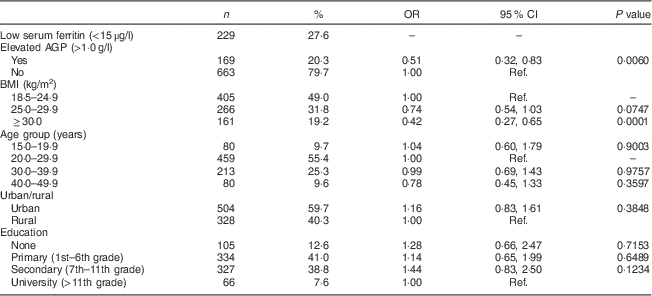
SIVIN, Sistema Integrado de Vigilancia de Intervenciones Nutricionales (Integrated Surveillance System for Nutrition Interventions); AGP, α1-acid glycoprotein; Ref., reference category.
Women with BMI<18·5 kg/m2 were excluded (n 29).
Just over a quarter of women had low serum ferritin (Table 1) with a geometric mean of 26·1 (95 % CI 24·3, 28·1) μg/l. Overweight (26·4 %) and obese (17·0 %) women were less likely to have low serum ferritin compared with normal-weight women (32·6 %; Table 2), with crude prevalence odds ratio for overweight v. normal weight of 0·74 (95 % CI 0·54, 1·03) and obese v. overweight of 0·42 (95 % CI 0·27, 0·65; Table 1). Serum ferritin and AGP were both significantly correlated with BMI and with each other, with Pearson’s r of 0·20–0·22 (means and correlation data not shown).
Table 2 Prevalence of low serum ferritin by weight status and AGP level among non-pregnant women aged 15–49 years with a child 6–59 months of age, Nicaragua SIVIN, 2004–2005
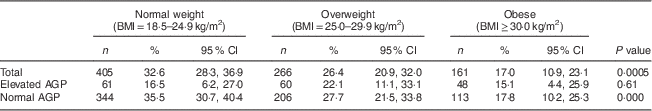
AGP, α1-acid glycoprotein; SIVIN, Sistema Integrado de Vigilancia de Intervenciones Nutricionales (Integrated Surveillance System for Nutrition Interventions).
Elevated AGP is AGP>1·0 g/l; normal AGP is AGP≤1·0 g/l.
P values indicate a difference in prevalence of serum ferritin by weight status and are based on Rao–Scott χ 2 test.
In order to explore the effect of inflammation (AGP) and weight status on low serum ferritin, three models were constructed. In the first model, all women were included (elevated and normal AGP). Only obese status was a significant predictor of low serum ferritin with an adjusted odds ratio (AOR) of 0·42 (95 % CI 0·26, 0·65). In the second model, AGP was included and was significantly associated with low serum ferritin with AOR of 0·56 (95 % CI 0·34, 0·92). However, the relationship between weight status and serum ferritin remained largely unchanged with an overweight AOR of 0·77 (95 % CI 0·54, 1·10) and an obese AOR of 0·45 (95 % CI 0·28, 0·71). In the third model, all women with elevated AGP (n 169) were excluded from the analysis. This also had little effect on the relationship between weight status and serum ferritin. Overweight women had an OR of 0·69 (95 % CI 0·47, 1·03) and obese women had an OR of 0·39 (95 % CI 0·22, 0·67; Table 3). Overall, women with a BMI≥30·0 kg/m2 were significantly less likely to have low serum ferritin. This relationship was not appreciably affected when accounting for inflammation as indicated by AGP.
Table 3 Association between low serum ferritin and weight status among women aged 15–49 years with a child 6–59 months of age, Nicaragua SIVIN, 2004–2005
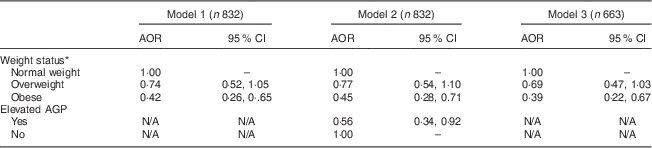
SIVIN, Sistema Integrado de Vigilancia de Intervenciones Nutricionales (Integrated Surveillance System for Nutrition Interventions); AOR, adjusted odds ratio; AGP, α1-acid glycoprotein; N/A, not applicable.
* Weight status is based on BMI: normal weight is BMI=18·5–24·9 kg/m2; overweight is BMI=25·0–29·9 kg/m2; and obese is BMI≥30·0 kg/m2. Estimates are odds ratios from logistic regression models. Low serum ferritin <15 μg/l is the outcome in all models.
Model 1 shows the association between BMI and low serum ferritin, adjusting for age, education and region.
Model 2 is the same as model 1, additionally adjusting for AGP.
Model 3 is the same as model 1, except the sample is restricted to only those participants with normal AGP levels.
Discussion
Low serum ferritin and obesity were significantly inversely associated in this nationally representative sample of non-pregnant, reproductive-age women with children under 5 years in Nicaragua, indicating that obese women were less likely to have low serum ferritin levels (an indicator of Fe deficiency) when compared with normal-weight women. Age, region and education did not modify or confound this relationship nor independently predict Fe status. In addition, when the population was restricted to women with normal AGP values or when AGP was added to the model, the relationship between BMI and low serum ferritin remained largely unchanged. After adjusting for AGP in the model, the AOR for overweight and obese women did not meaningfully change. This is in contrast to Ausk and Ioannou in which including CRP in the model with serum Fe status markers decreased the association between BMI and Fe status by 30–50 %( Reference Ausk and Ioannou 18 ); however, this may be related to the use of a different inflammation indicator. In Cepeda-Lopez et al., obesity no longer independently predicted Fe deficiency when CRP was added to the model( Reference Cepeda-Lopez, Osendarp and Melse-Boonstra 17 ). Although AGP has been shown to be useful in adjusting for the acute-phase response in chronic infection( Reference Kung’u, Wright and Haji 6 ), we show a lack of impact on the relationship between obesity and Fe deficiency in our population. The continued influence of BMI on serum ferritin, independent of AGP, may be due to other inflammatory events that are not signalled by AGP, or it may be through a currently unknown mechanism.
The current study had several strengths. The data set was a nationally representative sample of women with children under 5 years of age in a developing country setting. Low serum ferritin prevalence was approximately twice that of a similar US population (26·1 % v. 13·6 %)( 44 ). Inflammation was measured through AGP, a marker of low-grade, chronic inflammation( Reference Kung’u, Wright and Haji 6 ), which may be an appropriate choice for assessing inflammation associated with overweight and obesity. The analysis took into account the complex sample design of the survey.
There were also several limitations in the study. Only one marker of Fe status was measured in this population. While serum ferritin is the recommended indicator for assessing Fe deficiency by WHO( 7 ), its role as an acute-phase protein makes assessing Fe deficiency in a population with inflammation or infection challenging. Measuring additional indicators that are less affected by inflammation may be useful. Underweight women were excluded due to small sample size and women interviewed were from households with pre-school children, which may not be representative of all women of childbearing age in Nicaragua. There was no information on dietary intake or parasitic infections available, which may also influence Fe status. Dietary Fe is a primary determining factor of body Fe stores( 7 ) and in this case it was not possible to compare dietary Fe intakes across BMI groups or in relation to low serum ferritin. The study was cross-sectional and therefore temporal associations could not be examined.
Overall, in this population of reproductive-age women, obese women had a lower prevalence of low serum ferritin and this lower prevalence was independent of inflammation as measured by AGP. Obesity is an independent predictor of serum ferritin even when controlling for the inflammatory marker AGP. Measuring height and weight is common in nutrition surveys in both developing and developed country settings, although Fe deficiency prevalence is not always reported across BMI categories. Regularly reporting this information would be useful to allow further examination of the impact of weight status on Fe status. Further studies in different populations should continue to explore the possible additional impact of inflammation on this relationship. It may also be important to collect data on anthropometry in Fe supplementation trials, as BMI has been shown to affect Fe absorption( Reference Zimmermann, Zeder and Muthayya 45 – Reference Baumgartner, Smuts and Aeberli 47 ). Documenting the prevalence of Fe deficiency across BMI categories should be considered when describing the prevalence of Fe deficiency in a given population.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The primary author was supported by a T-32 Reproductive, Perinatal, Pediatric Predoctoral Fellowship through the National Institutes of Health (Grant # 2T32HD052460). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention. Conflict of interest: None. Authorship: A.S.W. is primary author and primary data analyst. M.E.J., C.G.P. and K.M.S. are advisors to analyses and contributed to editing and revision of the manuscript. P.H. contributed to revision of the manuscript. Ethics of human subject participation: Participation in the interview and biological sample collection was voluntary and informed consent by signature was obtained from each woman. The Nicaraguan Ministry of Health approved the data collection. A de-identified data set was used for this analysis. Emory University Institutional Review Board (IRB) determined no IRB review was required for this secondary analysis.





