Fe deficiency is reported to be the world’s most common single nutrient deficiency. One-quarter of the world’s population is affected by anaemia and pre-school children are among the risk groups. The WHO estimates that 47 % of children under 5 years of age are anaemic and that half of the anaemia prevalence can be attributed to Fe deficiency( 1 ). The deficiency of Fe in children is associated with adverse mental, motor and socio-emotional development( Reference Konofal, Lecendreux and Arnulf 2 , Reference Black, Quigg and Hurley 3 ). In addition, Fe deficiency during early life is a risk factor for poorer cognitive performance in adolescence even after children are Fe replete for several years( Reference Lozoff, Jimenez and Smith 4 ).
Diets of rural populations in low-income countries are dominated by non-refined cereals and legumes which are rich in phytates that form insoluble complexes with Fe, diminishing its bioavailability. Consumption of animal-source foods which contain readily bioavailable Fe and by themselves are Fe-absorption enhancers is low in these populations( Reference Neumann, Harris and Rogers 5 ). Thus, in addition to inadequate dietary intake, poor Fe bioavailability could be a major factor contributing to Fe-deficiency anaemia (IDA)( Reference Adish, Esrey and Gyorkos 6 ). Diets of rural Ethiopian populations are predominantly plant based with low intakes of animal-source foods( Reference Umeta, West and Fufa 7 ). Researchers have recognized the presence of mild to moderate IDA in lactating( Reference Haidar, Muroki and Omwega 8 ) and pregnant( Reference Gibson, Abebe and Stabler 9 ) women, as well as other women of reproductive age( Reference Umeta, Haidar and Demissie 10 – Reference Haidar 12 ), from Ethiopia. Recent studies have shown a high dietary intake of Fe by pregnant women( Reference Abebe, Bogale and Hambidge 13 ) and young children( Reference Baye, Guyot and Icard-Verniére 14 ).
Haider et al. ( Reference Haidar, Nekatibeb and Urga 15 ) reported that in Ethiopia the distribution of IDA varied by region and was dictated by the type of staple foods. Thus, as the country is agro-ecologically diverse and staple foods differ by area, the available data are not representative of the situation of the country. At present, except for small activities, Fe intervention programmes are not in place at the national scale. The national Fe advisory group suggested the need for more data for decision making. There is only one biochemical study available on Fe status of Ethiopian children( Reference Adish, Esrey and Gyorkos 6 ). However, ferritin was not adjusted for inflammation or infection, which may underestimate the prevalence. That study reported presence of 18·6 % of IDA but the estimation for IDA was based on a sub-sample of anaemic individuals.
It is generally assumed that anaemia and IDA are very common in populations from low-income countries with primarily plant-based diets but little animal-source foods. However, given the available reports showing only mild to moderate Fe deficiency in the country, this general assumption may not be appropriate for the Ethiopian context.
The present study investigated the Fe status of pre-school children from resource-poor settings of the Amhara region who consumed a predominantly unrefined plant-based diet with little animal-source foods.
Methods
Description of the research location
The study was conducted in rural areas of the Amhara region, Ethiopia. The Amhara region is located in the north-western and north-central part of Ethiopia situated within 9° and 13′45N and 36° and 40′30E. It covers a total land area of 170 000 km2. The region has a mean annual temperature in the range of 10 to 27°C and elevation between 500 and 4550 m. According to the 2013 population projection for Ethiopia, the region has a total population of 20 399 004 and more than 90 % live in rural areas. Agriculture is the main economic activity for the majority (85 %) of the population( 16 , Reference Motbainor, Worku and Kumie 17 ).
Study design
The present study was part of a large randomized controlled trial investigating the effect of iodized salt on cognitive development of iodine-deficient children. First, sixty districts from six zones (West Gojjam, East Gojjam, North Gondar, North Wollo, South Wollo and Wagehmera) of the Amhara region of Ethiopia were selected randomly. Subsequently, one rural kebele (the smallest administrative unit) from each of the selected sixty districts was randomly selected. In the selected kebeles, a house-to-house census was conducted to register all children aged 6–60 months. Then they were classified into three age groups (6–10 months, 18–22 months and 54–60 months) to control for age difference as a source of variation in cognitive performance, which was the primary objective of the randomized controlled trial. Child age was obtained from the immunization card or from the parents or guardian. An event calendar was prepared to help respondents remember birth dates. All children from the selected kebeles in the specified age range were included in the study. The single population proportion formula was used to determine sample size with 95 % confidence level, 5 % margin of error, design effect of 1·5 and 5 % non-response rate. To maximize the sample size, 50 % prevalence of Fe deficiency was considered. A sub-sample of all children aged 54–60 months (n 628) from twenty-six kebeles randomly selected from the original sixty kebeles were included. Data were collected between October 2011 and May 2012. Baseline data from the 54- to 60-month-old children are presented in the current paper.
Data collection
Research assistants were recruited and trained to standardized methods using structured protocols for data collection. A structured questionnaire that included questions about dietary intake, child morbidity, deworming medication, water and sanitation facilities available to households, and educational status of the mother and father or guardians of the selected children was used. The questionnaire was pre-tested in non-study households outside the study area before application.
Dietary assessment
A 24 h recall was used to assess dietary intake of the study children. Portion sizes were not collected. A list of thirty-one food items that could potentially be consumed by the study children was developed based on informal interviews and market and household observations. In addition, space was provided to record other food items consumed by the children but not listed. A food log book containing coloured pictures of different food items was prepared to be used as a reference to confirm responses of the mother or caregiver during a child feeding interview. Respondents were asked if the child consumed foods or foods prepared from any of the listed food items during the 24 h preceding the interview.
Child morbidity
Mothers or caregivers were asked whether their children experienced an episode of diarrhoea (passing watery stools three or more times per day), fever (fever with no other symptoms) or acute respiratory infection (episode of cough with short and rapid breathing) in the last 7 d and 14 d preceding the data collection. Malaria morbidity of children was also estimated by asking the mothers if they experienced common malaria symptoms (chills, fever, sweats and vomiting) and if the malaria infection was confirmed in the health post.
Water and sanitation facilities
Respondents were asked about the source of drinking-water and the type of toilet used by their household. Source of drinking-water and toilet facilities were categorized as improved or unimproved following the recommendation of the WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation( 18 ).
Educational status of guardians
Respondents were asked if the mother, father or guardian of the selected children had formal education. In addition, for those with formal education, the respondents were asked the highest grade they attended.
Anthropometric measurement
Height was measured in erect position using a calibrated wooden height board (Stadiometer; Shorr Productions, Olney, MD, USA) with a sliding head bar while children were barefoot. Weight was measured using a battery-powered digital scale with a precision of 0·1 kg (TANITA WB-100; Tanita Corporation, Arlington Heights, IL, USA) while children wore light clothes, emptied pockets and no shoes. The weight scale was calibrated at least twice daily. The measurement was done twice and recorded to the nearest 0·1 kg. Height-for-age, weight-for-age and weight-for-height were converted into standardized Z-scores using WHO Anthro software and the data were used to classify the study children into categories of nutritional status, using the WHO reference curves( 19 ). Children with height-for-age Z-score (HAZ) <−2, weight-for-age Z-score (WAZ) <−2 and weight-for-height Z-score (WHZ) <−2 were classified as stunted, underweight and wasted, respectively. Mid upper-arm circumference (MUAC) was also measured using a non-stretchable tape (MUAC Body Circumference Measuring Tape QM200; Quick Medical, Issaquah, WA, USA).
Blood collection, handling and analyses
Blood was collected by experienced nurses from the regional referral hospital (Felege Hiwote Hospital at Bahir Dar). Blood was drawn from the antecubital vein with disposable butterfly Vacutainer needles (BD Vacutainer butterfly needles, 23G,
![]() ${\raise0.5ex\hbox{$\scriptstyle 3$}\kern-0.1em/\kern-0.15em\lower0.25ex\hbox{$\scriptstyle 4$}}$
in; Franklin Lakes, NJ, USA) using standard safety measures. The Hb level was measured immediately onsite using a digital photometer (HemoCue® Hb 201 DM; Ängelholm, Sweden). Altitude of the study kebeles was measured using an altimeter (Sunartis BKT 381, Germany). The blood samples were allowed to clot at ambient temperature and centrifuged in the field. The duplicate serum samples were transported to Bahir Dar in an ice box and kept at −20°C until they could be transferred to Addis Ababa’s Ethiopian Public Health Institute (EPHI) on dry ice, for storage at −80°C until analysis. One vial of each frozen sample was shipped on dry ice to the Department of Nutritional Sciences at Oklahoma State University, USA, for Fe analysis.
${\raise0.5ex\hbox{$\scriptstyle 3$}\kern-0.1em/\kern-0.15em\lower0.25ex\hbox{$\scriptstyle 4$}}$
in; Franklin Lakes, NJ, USA) using standard safety measures. The Hb level was measured immediately onsite using a digital photometer (HemoCue® Hb 201 DM; Ängelholm, Sweden). Altitude of the study kebeles was measured using an altimeter (Sunartis BKT 381, Germany). The blood samples were allowed to clot at ambient temperature and centrifuged in the field. The duplicate serum samples were transported to Bahir Dar in an ice box and kept at −20°C until they could be transferred to Addis Ababa’s Ethiopian Public Health Institute (EPHI) on dry ice, for storage at −80°C until analysis. One vial of each frozen sample was shipped on dry ice to the Department of Nutritional Sciences at Oklahoma State University, USA, for Fe analysis.
Serum Fe was analysed by inductively coupled plasma–mass spectrometry (ELAN9000; PerkinElmer, Norwalk, CT, USA). Serum samples were diluted in 0·1 % (v/v) trace metal grade HNO3 (Fisher Scientific, Fair Lawn, NJ, USA). Working standards were freshly prepared by diluting 100 ppm multi-element stock solution (Atomic Spectroscopy Standard PerkinElmer Pure Plus) in 0·1 % (v/v) HNO3 and 0·5 % (v/v) Triton-X-100 (SIGMA Chemical Company, St. Louis, MO, USA) solution. Gallium (PerkinElmer) was used as an internal standard. A reference standard of freeze-dried human serum (Utak Laboratories, Inc., Valencia, CA, USA) was used to verify the method performance.
Serum ferritin was analysed using a fully automated clinical analyser (Electrochemilumenescense Immuno Assay (ECLIA) Elecsys® 2010 analyser, Cobas e 411; Roche Diagnostics GmbH, Mannheim, Germany) at EPHI. Soluble transferrin receptor (sTFR) and α1-acid glycoprotein (AGP) were determined by immunoturbidimetric methods with a clinical chemistry analyser (Cobas Integra 400 system; Roche Diagnostics GmbH) at Saint Paulos Hospital, Addis Ababa. Fe deficiency was defined as serum ferritin <12 µg/l, and low Hb (<110 g/l) with Fe deficiency was used to define IDA in children. Serum ferritin was adjusted for the presence of infection or inflammation using AGP. In the presence of infection or inflammation (AGP >1·2 g/l), a serum ferritin value <30 µg/l was used as a cut-off for Fe deficiency. An elevated serum sTFR indicates the presence of Fe-deficiency erythropoiesis (IDE)( 20 ). The normal reference range for sTFR for latex-enhanced immunoturbidimetric assay on the Cobas Integra 400 system is 2·2 to 5 mg/l as recommended by the manufacturer; >5 mg/l was used as an indicator of IDE in the present study.
Statistical analyses
The statistical analysis of data was performed using the statistical software package PASW Statistics for Windows Version 18. Descriptive statistics were used to present results. Normal distribution of data was checked with the Kolmogorov–Smirnov test. Non-normally distributed data were analysed by non-parametric tests. The FAO guideline for measuring individual dietary diversity was used to assess diversity of children’s food intake( Reference Kennedy, Ballard and Dop 21 ). The dietary diversity score (DDS) was created by summing the different food groups (grain, roots or tubers; legumes, nuts or seeds; dark green leafy vegetables; other vitamin A-rich fruits and vegetables; other fruits and vegetables; organ meats; meat and fish; milk and milk products; eggs) consumed over the 24 h recall period. The DDS assigned 1 point for consumption of one or more food items in a group or 0 if no foods were consumed from the group. The children’s DDS was further classified into three intake levels as low (1–2 food groups), medium (3–4 food groups) and high (5–8 food groups). Prevalence of stunting, underweight and wasting was compared between boys and girls using Student’s t test. Hb values were adjusted for altitude according to the formula by Sullivan et al. ( Reference Sullivan, Mei and Grummer‐Strawn 22 ). Body Fe store was calculated according to the formula by Cook et al.( Reference Cook, Flowers and Skikne 23 ). The level of sTFR in anaemic and non-anaemic children was compared using the Mann–Whitney test. Pearson correlation was used to identify bivariate correlations. A probability level of P <0·05 was considered statistically significant.
Results
Characteristics of study participants are indicated in Table 1. The study children (n 628) had a male-to-female ratio of 1·01:1. The mean age of the children was 56·9 (sd 1·8) months with a range of 54–60 months. Of the total, 81·7 % (n 513) of fathers and 92·4 % (n 580) of mothers had no formal education. A majority (54·2 %) of households had unimproved sources of drinking-water such that 16·6 % of households used drinking-water from a lake, pond or river; and 37·6 % from unprotected springs. In addition, 28·3 % (n 178) of the participants reported that they defecated outdoors in nature and 71·7 % (n 450) had pit latrines. About 57·6 % (n 362) of the children had deworming medication six months preceding the interview.
Table 1 Characteristics of households and child caregivers (n 628) from resource-poor rural households of the Amhara region, Ethiopia, October 2011–May 2012

* Spring water was not of protected type.
† Pit latrines were not identified as whether covered or not.
Dietary intake of the children is indicated in Table 2. The dietary diversity score of the study children was in the range of 1–5, with a mean value of 2·1 (sd 0·8). Most children, 74·8 % (n 470), were in the lowest dietary diversity group consuming 1–2 food groups. Medium dietary diversity (3–4 food groups) and high dietary diversity (5–8 food groups) scores were attained by 24·0 % (n 151) and 1·1 % (n 7) of the children, respectively. Grain, roots or tubers were consumed by 100 % of the study children, followed by the food group comprising pulses, legumes or nuts as consumed by 66·6 % of the participants. The dairy group was consumed by 21·7 % (n 136), dark green leafy vegetables by 6·8 % (n 43), other vitamin A-rich fruit and vegetables by 6·5 % (n 41), other fruits and vegetables by 14·3 % (n 90), meat, poultry and fish by 2·2 % (n 14) and eggs by 14·3 % (n 93) of the participants. None of the participants consumed organ meats in the 24 h preceding the interview.
Table 2 Percentage distribution of intake of different food items in the 24 h preceding the survey by children (n 628) from resource-poor rural households of the Amhara region, Ethiopia, October 2011–May 2012
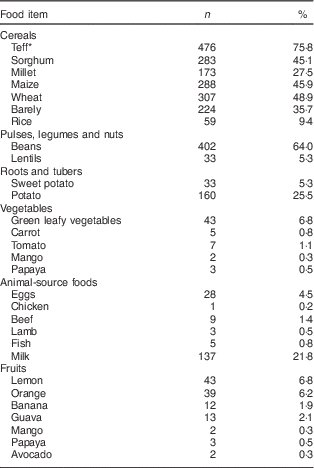
* Teff is a gluten-free tiny cereal grain about the size of a poppy seed with variety of colours from white and red to dark brown. It is a staple crop to the majority of Ethiopians.
For the two weeks preceding the survey, the overall rate of morbidity was 22·9 % (n 144), with major contributors being fever and cough (Table 3). A summary of the anthropometric characteristics of the study children is presented in Table 4. In the present study, 43·2 % (n 271) of the children were stunted, 29·6 % (n 186) were underweight and 6·8 % (n 43) were wasted. Prevalence of undernutrition was not statistically different (P>0·05) between boys and girls (Table 5). Only one child had lower MUAC value.
Table 3 Report of illness in the 7 d and 14 d preceding the interview among children (n 628) from resource-poor rural households of the Amhara region, Ethiopia, October 2011–May 2012

Table 4 Summary of anthropometric characteristics of children (n 628) from resource-poor rural households of the Amhara region, Ethiopia, October 2011–May 2012
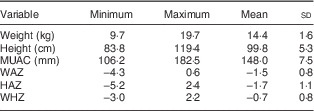
MUAC, mid upper-arm circumference; WAZ, weight-for-age Z-score; HAZ, height-for-age Z-score; WHZ, weight-for-height Z-score.
Table 5 Nutritional status of children (n 628) from resource-poor rural households of the Amhara region, Ethiopia, October 2011–May 2012

* Overall=Z-score <−2.
† Moderate=Z-score between −2 and −3.
‡ Severe=Z-score <−3.
The study children had altitude-corrected Hb levels in the range of 51·5–162·5 g/l with a mean value of 120·4 (sd 10·7) g/l. Of the total, 13·6 % (n 82) children were anaemic (Hb<110 g/l) and only two children were severely anaemic (Hb<70 g/l). Severely anaemic children were referred to the health post. A summary of Fe status parameters is indicated in Table 6. Inflammation or infection (AGP>1·2 g/l) was present in 30·2 % (n 184) of the children. Fe deficiency was found in 9·1 % (n 57) of the children. Serum ferritin concentration was positively correlated with AGP (r=0·16, P<0·01). Only 5·3 % (n 32) of the children had IDA and 2·9 % (n 18) of the children were calculated to have a negative body Fe store. Serum sTFR was positively correlated with AGP (r=0·42, P<0·01) but negatively correlated with serum Fe (r=−0·14, P<0·01). Considering only those with AGP ≤1·2 g/l, 14·2 % (n 60) of the children had elevated sTFR, suggesting the presence of IDE. The concentration of sTFR was higher in anaemic children than in those with normal Hb level (P=0·02). Using serum Fe <600 µg/l as the cut-off for low Fe transport( Reference Cook, Baynes and Skikne 24 ), 3·2 % (n 19) of the study children had low Fe transport.
Table 6 Summary of biochemical parameters for iron status of children from resource-poor rural households of the Amhara region, Ethiopia, October 2011–May 2012
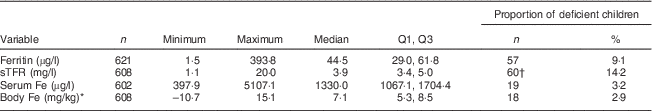
Q1, first quartile; Q3, third quartile; sTFR, soluble transferrin receptor.
* Body Fe was calculated based on the formula by Cook et al.( Reference Cook, Flowers and Skikne 23 ).
† Proportion was calculated for children (n 420) with α1-glycoprotein ≤1·2 g/l.
Discussion
The present study investigated the Fe status of children from resource-limited rural households of the Amhara region, Ethiopia. Plant-based diets are expected to contain poorly bioavailable Fe. In addition, children from low-income countries have low consumption of animal-source foods, making them at risk of anaemia and IDA. In view of the food patterns including low dietary diversity, high consumption of cereals and legumes, but very low consumption of fruit and vegetables, animal-source foods and vitamin A-rich foods, Hb concentration and Fe status of the present study children would have been predicted to be poor. However, despite the poor-quality diet, there was a very low prevalence of IDA. In addition, anaemia would be classified as only a mild public health problem.
The number of food groups consumed over a period of time is a proxy indicator for micronutrient intake. Low dietary diversity has been associated with dietary micronutrient deficiency in children( Reference Steyn, Nel and Nantel 25 , Reference Kennedy, Pedro and Seghieri 26 ). Children from low-income countries typically experience monotonous or less diversified types of diets dominated by cereals, whole grains, roots and tubers( Reference Thompson and Amoroso 27 ). Such unrefined plant-based staple foods often are rich in phytate that can bind and significantly reduce absorption of non-haem Fe due to the formation of insoluble Fe complexes( Reference Gibson, Perlas and Hotz 28 , Reference Hurrell and Egli 29 ). Meat, fish and poultry are good sources of haem Fe of high bioavailability. In addition, the protein in animal-source foods can enhance absorption of Fe from non-haem sources attributed to the formation of soluble Fe complexes that prevent the precipitation of Fe in the lumen( Reference Bæch, Hansen and Bukhave 30 ). Vitamin C from fruits and vegetables is a powerful promoter of non-haem Fe absorption by reducing ferric (Fe3+) into the more bioavailable ferrous form (Fe2+). Ascorbic acid has also the ability to chelate Fe and consequently enhance absorption( Reference Hurrell and Egli 29 ). Although the mechanism is not clear, carotenoids are also important in increasing the absorption of non-haem Fe from plant-based diets( Reference García-Casal 31 ). However, in the present study children, low consumption of animal-source foods and fruits and vegetables was observed. Milk and milk products contain low amounts of Fe. In addition, Ca and casein in cow’s milk interfere with Fe absorption( Reference Ziegler 32 ). However, only about one-fifth of the study children consumed milk. A single 24 h dietary assessment is, however, limited for capturing day-to-day variability in food consumption. In addition, it suffers from recall bias( Reference Gibson 33 ).
Because other food components and antinutitional factors interfere with Fe bioavailability, assessing only nutrient intake may not be a good indicator of Fe status. Studies in Ethiopia that assessed dietary intake of pregnant women( Reference Abebe, Bogale and Hambidge 13 ) and young children( Reference Baye, Guyot and Icard-Verniére 14 ) consuming plant-based diets have shown that Fe was not a limiting nutrient per se. The present biochemical study found a low prevalence of Fe deficiency and IDA in children from the Amhara region. Cereals grown in Ethiopia have been reported to have high Fe content( Reference Umeta, West and Fufa 7 ). Part of this Fe, particularly for teff, was attributed to an extrinsic source from soil contamination during traditional threshing( Reference Abebe, Bogale and Hambidge 34 ). Indeed, some portion of this extrinsic Fe may be bioavailable( Reference Gibson, Wawer and Fairweather-Tait 35 ) and bioaccessible( Reference Greffeuille, Kayodé and Icard-Vernière 36 ). In addition, foods are frequently fermented and it has been shown that fermentation is effective to significantly reduce the phytate content of teff and other cereals to a level that can favour bioavailability( Reference Umeta, West and Fufa 7 , Reference Abebe, Bogale and Hambidge 34 ). Further studies on identifying the factors contributing to the low prevalence of IDA in children from the Amhara region are important.
Anaemia is a major public health concern affecting one-quarter of the world’s population. Pre-school children, particularly those from low-income countries, are at risk. The WHO World Anaemia Report estimated the prevalence of severe anaemia (75·2 %) in Ethiopian children( 1 ). However, the estimation was based on a predictive regression model using the 2002 UN Human Development Index and other social and economic variables such as life expectancy, education and wealth index, which are less appropriate than Hb measurement and may not be an accurate estimation of actual Hb level on the ground.
The present study children had also a lower anaemia prevalence compared with the 2011 Ethiopian Demographic and Health Survey( 37 ) for the Amhara region (35 %). Several reasons may explain this finding, including the difference in the data collection period of the two studies. Several nutrition and health interventions have been actively promoted including delivery of insecticide-treated mosquito nets, deworming medication and vitamin A supplementation. Additionally, anaemia prevalence normally shows a marked decline as children grow but the 35 % prevalence in the Ethiopian Demographic and Health Survey report was for children under 5 years old in general, not specifically for those aged 54–60 months. In addition, even though the study districts and kebeles were selected randomly and all 54- to 60-month-old children from the selected kebeles were included, there may be variations within the Amhara region that were not captured in our data.
Dietary Fe is absorbed predominantly in the duodenum and temporarily held in the storage form of ferritin. Whenever the diet is unable to supply an adequate amount of Fe, the body’s demand for Fe is maintained by the gradual release of the mineral from storage. Low ferritin concentration stimulates duodenal absorption of Fe. However, when absorption is not sufficient, body Fe storage depletes accompanied by a shortage in supply of Fe for Hb synthesis( Reference Geissler and Singh 38 ). Serum ferritin is proportional to body Fe storage and is a reliable marker for the presence of Fe deficiency (serum ferritin <12 µg/l in children). However, independent of Fe status, ferritin concentration can increase in the presence of inflammation or infection which affects its diagnostic efficiency( Reference Cook 39 ). In the present study children, serum ferritin concentration was positively correlated with AGP (r=0·16, P<0·01), suggesting the influence of inflammation or infection on increasing serum ferritin level.
The concentration of sTFR is not as strongly affected as ferritin by the rise in acute-phase proteins due to infection or inflammation. This characteristic helps to discriminate IDA and anaemia of chronic disease. However, independent of Fe status, sTFR concentrations can increase due to clinical conditions such as megaloblastic anaemia and malaria infection that are common in children from poor-resource settings( Reference Zimmermann 40 , Reference Menendez, Quinto and Kahigwa 41 ). The associated inflammation can increase hepcidin concentration which in turn prevents the release of Fe from duodenal absorptive cells or from Fe storage in the body( Reference Zimmermann 40 ). For example, sTFR in the present study children was positively correlated with AGP, suggesting that inflammation or infection may partially explain the elevation of sTFR. An elevation of sTFR demonstrates the presence of IDE, which is characterized by a compromised plasma Fe supply to the bone marrow for erythrocyte synthesis. This condition can occur despite possessing normal or even excess storage of Fe and the term ‘functional Fe deficiency’ is commonly applied to describe the situation( Reference Cook 39 ). In the present study, anaemic children had higher levels of sTFR than normal children, which suggests that IDE could be one potential cause for low Hb concentration and a risk factor for anaemia. The influence of IDE on anaemia in the present study children warrants further study.
High incidence of infection or inflammation (AGP>1·2 g/l) was evident in the present study children. As a reaction to infection or inflammation, the concentration of acute-phase proteins is elevated( Reference Wieringa, Dijkhuizen and West 42 ). Similarly, serum ferritin concentration increases in response to inflammation and can cause an underestimate of the overall Fe deficiency prevalence by up to 14 %. C-reactive protein (CRP) elevates and reaches its peak within 24 to 48 h of infection while AGP requires 4–5 d to become elevated. On the other hand, CRP declines rapidly a short time after the infection clears whereas AGP remains elevated. The combined use of CRP and AGP to catch such gaps and narrow underestimation of Fe deficiency has been recommended. It was estimated that the use of CRP or AGP alone can leave 9 % and 5 %, respectively, of Fe deficiency undetected. However, the combined use of both CRP and AGP can reduce underestimation of Fe deficiency prevalence essentially to zero( Reference Thurnham, McCabe and Haldar 43 ). Soil helminthes, gastrointestinal parasitic infestation, malaria( Reference Alemu, Atnafu and Addis 44 , Reference Alemu, Shiferaw and Ambachew 45 ) or indoor air pollution( Reference Bruce, Perez-Padilla and Albalak 46 , Reference Fullerton, Bruce and Gordon 47 ) could be some of the factors contributing to infection or inflammation in children from rural Ethiopia. In addition, 22·9 % of children in the present study reported illness during the two weeks preceding the survey. However, the assessment of morbidity was not confirmed by medical tests and thus should be interpreted with caution. Only limited numbers of households in the present study had improved sources of drinking-water. In addition, even though the study was not able to identify covered v. open latrines, a vast majority of the households had pit latrines and at least 28·3 % of the households defecated outdoors in nature; thus, limited access to quality water and sanitary facilities could be additional plausible explanations for the high incidence of infection or inflammation.
In general, the children in the present study had plant-based dietary patterns with very low intakes of animal-source foods; yet they had low prevalence of IDA. Thus, the assumption that monotonous plant-based diets with low intakes of animal-source foods will invariably lead to a high prevalence of IDA in populations from low-income countries may not be always correct. Furthermore, the high prevalence of infection and inflammation among children living in settings similar to our research site may be further exacerbated by Fe provision. Our findings have important programmatic implications in that programmes intended to correct Fe status must go beyond assumptions and predictive models. Screening for Fe status biomarkers needs to be considered, especially given the potential adverse consequences of Fe provision to Fe-replete children( Reference Lind, Seswandhana and Persson 48 – Reference Jaeggi, Kortman and Moretti 50 ).
Acknowledgements
Acknowledgements: The authors would like to thank the data collectors, health extension workers and study participants; Sandra Peterson for assistance with the serum Fe analyses; and EPHI and Saint Paulos Hospital for the analysis of Fe biomarkers. Financial support: This work was supported by the Micronutrient Initiative, Borlaug LEAP, the German Academic Exchange Service, and Addis Ababa University. The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: K.B. and G.S.M. developed the study design. D.G. and K.B. coordinated and supervised the field work. D.G. conducted the biochemical analysis. D.G. and B.J.S. analysed and interpreted the data. All authors contributed to manuscript preparation. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the National Health Research Ethics Review Committee at the Ethiopian Science and Technology Commission, and the Institutional Review Boards at McGill University, Canada and Oklahoma State University, USA. Written informed consent was obtained from all parents or guardians of the study children.









