Introduction
Protozoa of the order Coccidia [infraphylum Apicomplexa, class Coccidomorphea, subclass Coccidia (Ruggiero et al., Reference Ruggiero, Gordon, Orrell, Bailly, Bourgoin, Brusca and Kirk2015)] are a large group of parasites that contains some of the most important parasitic pathogens of humans and animals, including members of the genera Cryptosporidium, Toxoplasma, Neospora, Eimeria and Cystoisospora (Current et al., Reference Current, Upton, Long and Long2019). Their life cycle is complex, as it includes a change from asexual to the sexual stage, and the latter displays a characteristic sexual dimorphism (Smith et al., Reference Smith, Walliker and Ranford-Cartwright2002). Generally, sporozoites infect a host and undergo asexual proliferation inside host cells to form merozoites, which infect further host cells. Subsequently, merozoites undergo several rounds of cellular division and develop into sexual stages, which then fuse to form a zygote and eventually oocysts. In most Eimeria and in Toxoplasma gondii (Barta et al., Reference Barta, Schrenzel, Carreno and Rideout2005; Jonscher et al., Reference Jonscher, Erdbeer, Günther and Kurth2015), sporozoites penetrate cells of the gut, form an intermediate stage and start merogony. Newly formed merozoites egress from the host cell and spread the infection to neighbouring epithelial cells. Once merogony is finished (after a genus-specific number of cycles or generations) merozoites develop into micro- and macrogametes which mature, fuse and form a zygote that subsequently develops into an infectious oocyst (Smith et al., Reference Smith, Walliker and Ranford-Cartwright2002; Feix et al., Reference Feix, Cruz-Bustos, Ruttkowski and Joachim2020).
The coccidian parasite Cystoisospora suis [syn. Isospora suis (Biester and Murray, Reference Biester and Murray1934)] is an important entero-pathogen of swine in their first weeks of live (Joachim and Shrestha, Reference Joachim, Shrestha and Dubey2020) considerably impairing animal health. It is a member of the family Sarcocystidae (Barta et al., Reference Barta, Schrenzel, Carreno and Rideout2005) and thus closely related to T. gondii (Smith et al., Reference Smith, Walliker and Ranford-Cartwright2002; Palmieri et al., Reference Palmieri, Shrestha, Ruttkowski, Beck, Vogl, Tomley, Blake and Joachim2017). As with all Coccidia, the life cycle of C. suis is characterized by a switch from asexual to sexual development with micro- and macrogametes. The microgametes form as a consequence of the division of microgamonts and develop into small uninucleate bodies with flagella which they use for their quick movement. Macrogametes do not divide but constitute a large, usually immobile cell. Presumably, micro- and macrogametes fuse to a zygote and complete their life cycle with the formation of an oocyst (Lindsay et al., Reference Lindsay, Quick, Steger, Toivio-Kinnucan and Blagburn1998; Smith et al., Reference Smith, Walliker and Ranford-Cartwright2002).
The development of these parasites is presumably regulated by genes linked to cell cycle progression which are, amongst others, involved in meiosis and gamete fusion (Walker et al., Reference Walker, Ferguson, Miller and Smith2013; Shibuya and Watanabe, Reference Shibuya and Watanabe2018; Tandel et al., Reference Tandel, English, Sateriale, Gullicksrud, Beiting, Sullivan, Pinkston and Striepen2019). The class II gamete fusogen HAP2 is important for the function of male sexual stages in many eukaryotic kingdoms (Fédry et al., Reference Fédry, Liu, Péhau-Arnaudet, Pei, Li, Tortorici, Traincard, Meola, Bricogne, Grishin, Snell, Rey and Krey2017). In apicomplexan parasites (including the Coccidia), it appears to be essential for fusion of the gamete membranes and consequently for fertilization (Wong and Johnson, Reference Wong and Johnson2010). The oocyst is the characteristic stage of the Coccidia and maintains parasite transmission between individual hosts which is supported by its environmental resistance and longevity (Belli et al., Reference Belli, Smith and Ferguson2006). For the formation of the oocyst wall, oocyst-wall forming bodies (WFBs) are assembled in the macrogamete and later translocated (Scholtyseck et al., Reference Scholtyseck, Mehlhorn and Hammond1972). As described for the genus Eimeria, WFB 1 gives rise to the outer layer of the oocyst wall, WFB 2 forms the inner layer (Scholtyseck et al., Reference Scholtyseck, Mehlhorn and Hammond1972; Belli et al., Reference Belli, Smith and Ferguson2006; Mai et al., Reference Mai, Sharman, Walker, Katrib, De Souza, McConville, Wallach, Belli, Ferguson and Smith2009). WFBs are present in female sexual stages, macrogamonts and macrogametes, as well as in early unsporulated oocysts (Templeton et al., Reference Templeton, Lancto, Vigdorovich, Liu, London, Hadsall and Abrahamsen2004; Possenti et al., Reference Possenti, Cherchi, Bertuccini, Pozio, Dubey and Spano2010). In addition, oocyst wall formation requires tyrosine-rich proteins (TRPs). Studies on Eimeria suggest that tyrosine-rich precursor glycoproteins, i.e. gam 56 and gam 82, are processed and incorporated into the oocyst wall by TRPs crosslinking via tyrosine residues through peroxidase activity followed by translocation (Belli et al., Reference Belli, Wallach, Luxford, Davies and Smith2003).
Of the coccidia, only Cryptosporidium species have been propagated in a host cell-free environment (Boxell et al., Reference Boxell, Hijjawi, Monis and Ryan2008). In Cryptosporidium hominis, all asexual stages as well as gamonts were detected over a 9-day incubation period (Hijjawi et al., Reference Hijjawi, Estcourt, Yang, Monis and Ryan2010) and the production of newly formed oocysts was possible. The complete development of Cryptosporidium parvum in a host cell-free culture could also be demonstrated, with both unstained and stained life cycle stages (Hijjawi et al., Reference Hijjawi, Meloni, Ng'anzo, Ryan, Olson, Cox, Monis and Thompson2004; Boxell et al., Reference Boxell, Hijjawi, Monis and Ryan2008). During its development in culture, the parasite underwent changes in its DNA levels, indicating cell division and multiplication (Zhang et al., Reference Zhang, Sheoran and Widmer2009).
The current study describes a novel host cell-free culture system for C. suis as a representative of the intestinal Coccidia and compares the parasite's morphological development with the transcription profile of selected sexual genes previously identified. A C. suis in vitro model employing intestinal host cell monolayer cultures is already established and is suitable for several applications (Worliczek et al., Reference Worliczek, Ruttkowski, Schwarz, Witter, Tschulenk and Joachim2013; Feix et al., Reference Feix, Cruz-Bustos, Ruttkowski and Joachim2020; Joachim and Ruttkowski, Reference Joachim and Ruttkowski2021). We hypothesized that (i) after the intracellular propagation of merozoites, C. suis can complete its life cycle in a host cell-free environment which can be demonstrated by the development of gamonts, gametes, unsporulated and sporulated oocysts; and that (ii) the expression of genes related to the sexual development of C. suis is comparable to the in vitro expression in cell cultures infected with C. suis.
Materials and methods
Seeding of a host cell-free in vitro culture with sporozoites
To check whether the C. suis life cycle can exclusively occur outside of a host cell, 5 × 103 sporozoites were released into fresh Advanced® DMEM/F-12 culture medium (Gibco) supplemented with 5% fetal calf serum (Gibco) and penicillin/streptomycin plus l-glutamine 100x (Gibco) onto a new uncoated ibidi 8-well ibiTreat® μ-slide (ibidi, Gräfelfing, Germany) and were incubated at 40°C under 5% CO2. The development of parasite stages was monitored daily.
Infection of a host cell-free in vitro culture with merozoites
To start the host cell-free culture, free merozoites were obtained from monolayer culture supernatant of intestinal porcine epithelial cells 6 days after infection with sporozoites (Feix et al., Reference Feix, Cruz-Bustos, Ruttkowski and Joachim2020). Merozoites were washed with phosphate-buffered saline (PBS; Gibco, Thermo Fisher Science, Waltham, USA) and purified by density gradient centrifugation on a Percoll® (GE Healthcare, Uppsala, Sweden) gradient (60, 40 and 20%, topped up with the merozoite suspension in PBS) at 600 × g for 10 min at 20°C in a MegaStar 3.0R swing bucket centrifuge (VWR International, Leuven, Belgium). Both acceleration and deceleration were at the lowest possible setting. Purified merozoites were transferred to fresh Advanced® DMEM/F-12 culture medium (Gibco) supplemented with 5% fetal calf serum (Gibco) and penicillin/streptomycin plus l-glutamine 100x (Gibco) onto a new uncoated ibidi 8-well ibiTreat® μ-slide (ibidi, Gräfelfing, Germany) at a concentration of 1.2 × 105 merozoites per mL medium and were incubated at 40°C under 5% CO2. The development of parasite stages was monitored daily, and stages were harvested at 3 and 4 dpt (days post transfer), pelleted and stored at −20°C.
Parasite stage evaluation, harvest and microphotography
The numbers of merozoites, sexual stages and oocysts were monitored from the first dpt onwards. The numbers of gamonts and oocysts were estimated in the host cell-free culture chambers and 10 μL of each well was counted in a Neubauer chamber at each given time point for calculation of the average numbers of sexual stages and oocysts, and checked for regular morphology. To show significance between the average number of gamonts and oocysts on different culture days an unpaired Student's t-test was performed.
Digital microphotographs of all C. suis stages in culture were taken with an Olympus IX71 inverse microscope (Olympus, Shinjuku, Japan) at 400× and 600× magnification. Autofluorescence of oocysts was detected at UV 385 nm.
Flow cytometry
Purified parasite stages harvested 4 dpt were stained with 5 μg/mL DAPI (4′,6-diamidino-2-phenylindol) working solution, washed with PBS and transferred to 1 mL of PBS solution to be immediately processed. The different parasite stages were subjected to fluorescence analysis in an Amnis® FlowSight® imaging flow cytometer (Luminex, Austin, USA) and were evaluated by size, shape and fluorescence intensity. A total of 10 000 recordings were measured for every replicate (n = 5) and gated with software IDEAS® 6.2.64.0 (Luminex) based on size, which we used for previous morphological studies, and intensity, which made it possible to differentiate debris from different parasite cells.
Transcription levels of C. suis sexual stages in a host cell-free culture
To quantify the transcript levels of three genes related to sexual stages, quantitative real-time polymerase chain reaction (qPCR) was used. Total RNA was isolated from pelleted host cell-free cultures using an RNeasy mini kit (Qiagen, Hilden, Germany), and treated with RNase-free DNase (Qiagen) according to the manufacturer's instructions. Total RNA was quantified using a NanoDrop® 2000 (Thermo Fischer Science, Waltham, USA). For reverse transcription, an iScript® cDNA synthesis kit (Bio-Rad, Hercules, USA) was used.
Transcription levels were analysed 3 and 4 dpt for HAP2, OWP and TyRP, when sexual stages could be detected. The primers for gene amplification used in this study are described in previous studies (Feix et al., Reference Feix, Cruz-Bustos, Ruttkowski and Joachim2020). Quantitative PCR amplification of cDNA was carried out on an Mx3000P thermal cycler. The reaction mixtures each contained 2.5 μL of sample DNA (100 ng/μL), 5 μL of SsoAdvanced™ Universal Probes Supermix (Bio-Rad) and 1.3 μL of nuclease-free water with primers and probes at a final concentration of 500 and 200 nm. Activation of polymerase was performed at 95°C for 2 min, followed by 50 cycles of 95°C for 15 s and 60°C for 30 s. The qPCR results were normalized against each of the two reference genes, glyceraldehyde-3-phosphate and actin, and the complete experiment was performed in five separate biological replicates. The samples were quantified according to the percent threshold cycle (CT) method, and all the assays were technically performed at least three times.
Results and discussion
Morphology
In order to confirm the presence of various developmental stages of C. suis in a host cell-free environment, microphotographs of sexual stages and oocysts were captured at different time points of cultivation. An increase of gamonts (2 dpt) and oocysts (4 dpt), followed by a decrease of gamonts, could be shown in the host cell-free environment (Fig. 1). Although the total number of parasite stages at each time point was lower than in in vitro cultures with host cell monolayers (Feix et al., Reference Feix, Cruz-Bustos, Ruttkowski and Joachim2020), a significant difference between the days was noted (P < 0.0001). From an initial amount of 1.2 × 105 merozoites (a mixture of types 1 and 2) up to 200 gamonts developed. Approximately 400 oocysts (unsporulated and sporulated) were detected by 4 dpt. The number of developed gamonts might be related to the numbers of the different merozoite types seeded into the host cell-free culture, since we assume that only type II-merozoites develop to viable sexual stages and consequently to oocysts. This hints at a sexual commitment of C. suis merozoites, which has not yet been investigated. For Plasmodium falciparum (Poran et al., Reference Poran, Nötzel, Aly, Mencia-Trinchant, Harris, Guzman, Hassane, Elemento and Kafsack2017; Brancucci et al., Reference Brancucci, De Niz, Straub, Ravel, Sollelis, Birren, Voss, Neafsey and Marti2018; Neveu et al., Reference Neveu, Beri and Kafsack2020) and T. gondii (Ramakrishnan et al., Reference Ramakrishnan, Maier, Walker, Rehrauer, Joekel, Winiger, Basso, Grigg, Hehl, Deplazes and Smith2019), sexual commitment of merozoites preceding sexual differentiation has been shown and represents an important developmental switch in the life cycle of apicomplexan parasites (Poran et al., Reference Poran, Nötzel, Aly, Mencia-Trinchant, Harris, Guzman, Hassane, Elemento and Kafsack2017; Ramakrishnan et al., Reference Ramakrishnan, Maier, Walker, Rehrauer, Joekel, Winiger, Basso, Grigg, Hehl, Deplazes and Smith2019; Neveu et al., Reference Neveu, Beri and Kafsack2020).

Fig. 1. Total amount of gamonts and oocysts of Cystoisospora suis in a host cell-free culture. Values represent the mean from five independent experiments. ****P < 0.0001.
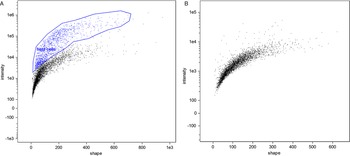
In flow cytometry, a difference between unstained cells and DAPI-stained cells was evident; however, microgamonts, macrogamonts and macrogametes have a similar shape and fluorescence intensity, making differentiation by flow cytometry not possible (Fig. 2). Microgametes are distinguishable by this technique, due to their significantly smaller size, their flagella are highly breakable, decreasing the amount of complete microgametes found by flow cytometry. Similar to previous studies on C. parvum using COWP1-tdTomato staining (Tandel et al., Reference Tandel, English, Sateriale, Gullicksrud, Beiting, Sullivan, Pinkston and Striepen2019), sexual stages were only visible at an increased intensity of UV-light, whereas uninfected cells were still visible at a lower intensity. Nevertheless, cell sorting clearly differentiated between debris, dead cells and living parasite stages, and will be an improvement for the isolation of different C. suis stages in the future. Further experiments with antibodies against stage-specific proteins should make it possible to sort out single parasite cells of a specific developmental stage and will be conducted in the future; as such antibodies do not exist for C. suis yet.

Fig. 2. Comparison of flow cytometry of an in vitro cell culture and the cell-free in vitro culture of parasite cells. (A) Microgamonts, macrogamonts and macrogametes (black) and host cells and few intact microgametes in the top right corner (blue), in vitro culture, 10 dpi. (B) Exclusively parasite stages (black) are found in the host cell-free culture 4 dpt.
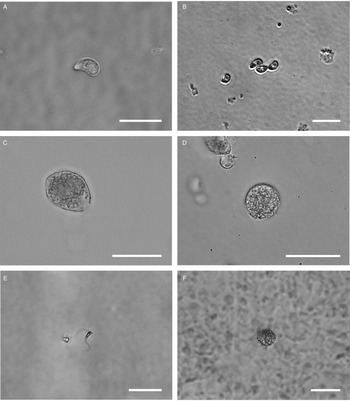
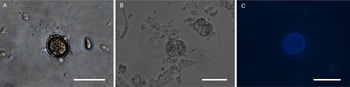
All life cycle stages were morphologically comparable to in vitro cell cultures (Fig. 3). Second-generation merozoites (Fig. 3A and B) could be detected 4 h after the start of the host cell-free culture. Previous studies showed male and female sexual stages and first oocysts from 120 h post infection in vivo (Matuschka and Heydorn, Reference Matuschka and Heydorn1980). Previous studies in vitro first detected gamonts at 216 h post infection of cell cultures and first oocysts after 240 h, so that the in vitro life cycle of C. suis takes roughly twice as long as in vivo (Feix et al., Reference Feix, Cruz-Bustos, Ruttkowski and Joachim2020). The sexual development of C. suis in a host cell-free environment takes as long as that in in vitro cell culture. Lindsay et al. (Reference Lindsay, Quick, Steger, Toivio-Kinnucan and Blagburn1998) demonstrated the complete development of C. suis in a swine testicle cell line, and detected oocysts at 12 dpi. Therefore, the developmental delay of both sexual stages and oocysts in vitro might be influenced by the culture conditions and the used cell lines. The numbers of newly developed micro- and macrogamonts increased until 3 dpt (Fig. 3C and D). At that time point, microgamonts contained motile microgametes (Supplementary material file V1). Free, motile microgametes (Fig. 3E) and macrogametes (Fig. 3F) could be detected from 3 dpt onwards. Microgametes from host cell-free culture had a central body and two opposing flagella and showed no obvious morphological differences compared to the cell culture, and they also displayed their typical extremely fast movement (Supplementary material file V2). Macrogametes were spherical and appeared to have a thinner outer layer in comparison with in vivo and previous in vitro studies (Fig. 3F). Micro- and macrogametes could frequently be found in close proximity to each other, as observed previously (Feix et al., Reference Feix, Cruz-Bustos, Ruttkowski and Joachim2020). Microgametes appeared to adhere to the macrogamete before (presumably) fusing with it, and newly formed oocysts were found in the same area approximately 24 h later. Both unsporulated and sporulated oocysts were present by 4 dpt. Characteristic, clear oocyst walls were seen in unsporulated oocysts (Fig. 4A). Unsporulated oocysts later matured and developed two sporocysts (Fig. 4B). Sporulated oocysts appeared to have thinner oocyst walls, in comparison with oocysts sampled from in vitro cell cultures. Harleman and Meyer (Reference Harleman and Meyer1984) demonstrated that merozoites collected at different time points in their development still conclude their life cycle in their specific time frame. Second-generation merozoites were shown at 3–4 days in vivo, which then develop into third-generation meronts at 4–5 days and further into sexual stages at 5–6 dpi. In this study, we could show that C. suis still follows its time line, as merozoites become sexual stages despite the absence of host cells.

Fig. 3. Light microscopy of different stages of C. suis in host cell-free culture, captured with differential interference contrast (DIC) or brightfield microscopy. Scale bars: 20 μm unless indicated otherwise. (A) First-generation merozoite, 0 dpt, DIC. (B) Second-generation merozoite, 0 dpt, brightfield microscopy. (C) Microgamont with an opening on one end and moving microgametes in the centre, 3 dpt, DIC. (D) Macrogamont, 3 dpt, DIC. (E) Microgamete, 4 dpt, scale bar: 5 μm. DIC. (F) Macrogamete, 4 dpt, DIC.

Fig. 4. Light microscopy of oocysts of C. suis in a host cell-free culture. (A) Unsporulated oocyst, 4 dpt. (B) Sporulated oocysts, 4 dpt. (C) Autofluorescent, unsporulated oocyst, 4 dpt. Scale bars: 20 μm.
In vivo the life cycle of C. suis takes place in a slightly shorter time frame (Harleman and Meyer, Reference Harleman and Meyer1983), as seen in vitro. The life cycle progression in the host cell-free in vitro culture, however, continues at the same pace as in other in vitro cultures (Table 1). However, the morphology of C. suis sexual stages and oocysts does not differ between in vivo (Joachim et al., Reference Joachim, Ruttkowski and Sperling2018; Lindsay et al., Reference Lindsay, Houk, Mitchell and Dubey2014) or in vitro systems.
Table 1. Comparison of the life cycle progression and morphology of some stages in all three systems used in Cystoisospora suis, i.e. in vivo, in vitro (cell culture) and the novel in vitro cell-free culture

a Sporulated oocysts were used to infect piglets in vivo (Harleman and Meyer, Reference Harleman and Meyer1984), sporozoites (derived from oocysts after excystation in vitro) were used to infect IPEC cell cultures (Feix et al., Reference Feix, Cruz-Bustos, Ruttkowski and Joachim2020).
b In the current study, mixed type 1 and 2 merozoites were used to seed the in vitro cell-free culture.
Genes linked to sexual stages
In addition to the morphological description of all sexual stages, we analysed three genes with upregulated transcripts in microgametes, macrogametes and oocysts, based on previous studies on C. suis (Feix et al., Reference Feix, Cruz-Bustos, Ruttkowski and Joachim2020).
To evaluate the transcription level of genes related to sexual stages in C. suis (HAP2, OWP and TyRP) the time points 3 and 4 dpt were chosen as the total amount of sexual stages was highest on these days, and compared to 0 dpt (i.e. to merozoites from cell cultures 6 days after infection of cells, before transfer to cell-free culture). The transcription levels of all three examined genes increased during the cell-free cultivation, and significant differences between all days were shown. The transcription of HAP2 had its peak 4 dpt with a 40-fold increase (Fig. 5A). It is usually only found in microgamonts, microgametes and unsporulated oocysts (Fritz et al., Reference Fritz, Bowyer, Bogyo, Conrad and Boothroyd2012a; Jonscher et al., Reference Jonscher, Erdbeer, Günther and Kurth2015; Fédry et al., Reference Fédry, Liu, Péhau-Arnaudet, Pei, Li, Tortorici, Traincard, Meola, Bricogne, Grishin, Snell, Rey and Krey2017), which correlates with the present and previous results for C. suis in vitro (Feix et al., Reference Feix, Cruz-Bustos, Ruttkowski and Joachim2020). Enhanced transcription of HAP2 in a host cell-free culture system supports the hypothesis that sexual stages can develop outside the host cell and that fertilization and oocyst production can occur extracellularly.

Fig. 5. Relative normalized expression levels of genes related to sexual development in a host cell-free culture evaluated by qRT-PCR 0, 3 and 4 dpt. (A) Microgamete-related gene HAP2. (B) Macrogamete related gene OWP1. (C) Macrogamete related gene TyRP. Values represent the mean ± standard error (s.e.) from five independent experiments. ****P < 0.0001.
As newly formed oocysts could be detected at 4 dpt, the oocyst wall formation must have taken place at that time point already. We could show a continuous transcriptional increase of OWP until 4 dpt when it was 9-fold increased (Fig. 5B). Cryptosporidium parvum also showed increased levels of OWP after 72 h of cultivation (Tandel et al., Reference Tandel, English, Sateriale, Gullicksrud, Beiting, Sullivan, Pinkston and Striepen2019). As the expression of OWP in the current study increased during the days in which macrogametes and oocysts were present, the slight morphological deviations of C. suis oocyst morphology in the host cell-free culture did not seem to be correlated with changes in the expression of OWPs.
Upregulation could also be detected for TyRP (Fig. 5C) which is characteristic for macrogamonts and –gametes as well as oocysts. Autofluorescence of C. suis oocysts occurs due to dityrosine bonds between TRPs in the oocyst wall (Fritz et al., Reference Fritz, Bowyer, Bogyo, Conrad and Boothroyd2012a, Reference Fritz, Buchholz, Chen, Durbin-Johnson, Rocke, Conrad and Boothroydb). Oocysts derived from the host cell-free culture system could be detected by autofluorescence microscopy (Fig. 4C) and TyRP was distinctly upregulated on dpt 3 and 4 similar to the development in in vitro cell cultures (Feix et al., Reference Feix, Cruz-Bustos, Ruttkowski and Joachim2020), oocyst wall formation can be assumed to be possible also in a cell-free environment. TyRP already seems to be present in macrogametes, but the crosslinking of TRPs only occurs in oocysts (Silvestrini et al., Reference Silvestrini, Bozdech, Lanfrancotti, Di Giulio, Bultrini, Picci, deRisi, Pizzi and Alano2005). This specific oocyst wall structure seems to be responsible for the resistance to environmental stress oocysts have to deal with (Silvestrini et al., Reference Silvestrini, Bozdech, Lanfrancotti, Di Giulio, Bultrini, Picci, deRisi, Pizzi and Alano2005; Jonscher et al., Reference Jonscher, Erdbeer, Günther and Kurth2015). The oocyst-related gene transcript of TyRP reached its peaks on dpt 4, with a 3-fold increase compared to the reference time point (Fig. 5C), which is comparable with our previous results for cell culture, where we could show the same increase in transcription. This upregulation can also be found in T. gondii in vitro and in vivo, demonstrating a key function of TyRP in oocyst development (Silvestrini et al., Reference Silvestrini, Bozdech, Lanfrancotti, Di Giulio, Bultrini, Picci, deRisi, Pizzi and Alano2005; Jonscher et al., Reference Jonscher, Erdbeer, Günther and Kurth2015).
Both male and female sexual stages (and their respective specific genes) could be detected simultaneously, hinting at a mandatory combination (and fusion) of both gametes to continue the parasite life cycle, as described in previous studies (Tandel et al., Reference Tandel, English, Sateriale, Gullicksrud, Beiting, Sullivan, Pinkston and Striepen2019; Feix et al., Reference Feix, Cruz-Bustos, Ruttkowski and Joachim2020). However, the relative quantitative expression of the analysed male sexual stage specific protein was almost 45-fold higher than those for female sexual stage specific proteins.
In the current study, we could demonstrate that merozoites derived from cell cultures infected with sporozoites 6 days earlier can complete their development to sporulated oocysts in a cell-free environment. It appears that the early events of the endogenous development of C. suis require an intracellular niche because initial experiments with cell-free cultivation of sporozoites were unsuccessful. Sporozoites from freshly excysted oocysts could only survive for a few hours and did not develop to merozoites (data not shown).
From these findings we draw three conclusions: (1) host cells are necessary for the asexual development but not for sexual development; (2) fertilization takes place outside the host cells (in vitro, but also in vivo, in the lumen of the gut) and (3) merozoites seeded into cell-free cultures are already sexually committed.
The sexual commitment has long been considered a crucial step in the development of Apicomplexan parasites (Kaushal et al., Reference Kaushal, Carter, Miller and Krishna1980; Cornelissen, Reference Cornelissen1988; Bruce et al., Reference Bruce, Alano, Duthie and Carter1990); however, the underlying mechanisms are still mainly unclear (Smith et al., Reference Smith, Walliker and Ranford-Cartwright2002; Bechtsi and Waters, Reference Bechtsi and Waters2017). Sexually committed merozoites undergo a developmental determination to develop either into male or female gametocytes prior to their actual sexual development (Jeninga et al., Reference Jeninga, Quinn and Petter2019). During schizont segregation in Plasmodium sp. sexual commitment (based on the increase in their transcript abundance) could be shown, similar to what we observed in the current study. Sexual commitment could be key in the development of transmission-blocking agents (Bechtsi and Waters, Reference Bechtsi and Waters2017). Cystoisospora suis life cycle progression in a host cell-free environment hints at sexual commitment also in this protozoan species. We hope to be able to analyse this phenomenon in future studies, since we assume that C. suis exhibits sexual commitment in second-generation merozoites which determines the life cycle progression of the parasite independent of its environment and could also be a target for intervention.
Previous studies have shown that blocking the fusion of micro- and macrogametes could also serve as a novel tool for intervention in the control of coccidial infections. In T. gondii HAP2 knockout parasites failed to produce sporulated oocysts in vivo (Ramakrishnan et al., Reference Ramakrishnan, Maier, Walker, Rehrauer, Joekel, Winiger, Basso, Grigg, Hehl, Deplazes and Smith2019), which would support the hypothesis that fertilization (and, more broadly, sexual development) is a potential bottleneck in the life cycle progression of coccidian parasites, and interfering with these crucial events to block further development will preclude transmission of the parasite via infectious oocysts.
Taken together, with the newly developed host cell-free culture system it would be possible to evaluate the effects of targeting sexual stages of C. suis in vitro, opening new avenues for the control of this and possibly related parasites by vaccination or selective drugs.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021001074.
Data
All data are accessible in the manuscript.
Author contributions
AJ designed the study. BR provided oocysts, sporozoites and merozoites and was responsible for the maintenance of the cell culture. ASF drafted the manuscript, was responsible for the host cell-free culture system and processed and analysed all samples. TCB provided the necessary expertise for the molecular analysis. TR and MM were involved in the establishment and conduction of the flow cytometry. All authors read and approved of the final version of the manuscript.
Financial support
ASF is funded through the Graduate School ‘Pig and Poultry Medicine’ of the University of Veterinary Medicine Vienna. TCB is funded by the Austrian Science fund (FWF; project no.: P 33123).
Conflict of interest
The authors declare that they have no competing interests.