A group of risk factors, including high levels of blood pressure, triglycerides (TG) and glucose, along with low levels of high-density lipoprotein cholesterol (HDL-C) and central obesity, are highly associated with insulin resistance and they are often clustered among individuals with chronic diseases such as coronary heart disease (CHD)Reference Grundy1, type 2 diabetesReference Grundy, Benjamin, Burke, Chait, Eckel and Howard2 and certain type of cancers in adultsReference Trevisan, Liu, Muti, Misciagna, Menotti and Fucci3. Results from recent research suggest that environmental factors in utero or early life may have a profound influence on the initiation of insulin resistanceReference Oken and Gillman4. It has been observed that individuals with low birth weight (BW) are at higher risk for cardiovascular disease (CVD), type 2 diabetes and some other chronic diseasesReference Barker5–Reference Gluckman and Hanson9. These epidemiological observations plus evidence from experimental studies has led to the developmental plasticity hypothesis that the risk of developing some chronic non-communicable diseases in adulthood is influenced not only by genetic and adult lifestyle factors but also by environmental factors acting in early life; metabolic changes among those individuals with low BW may be the result of foetal adaptations to inadequate intrauterine nutrition; and individuals developmentally adapted to one environment may be at risk when exposed to another when they are olderReference Bateson, Barker, Clutton-Brock, Deb, D'Udine and Foley10, Reference Gluckman and Hanson11.
Although BW is the most commonly used index of foetal growth, it may not be the most biologically pertinent outcome in reflecting foetal nutritional status and its impact on human healthReference Beattie and Johnson12, Reference Wilcox13. In contrast to the link between low BW and a group of chronic diseases, a positive association has also been persistently observed between BW and attained body mass index (BMI), a measurement of body fat distributionReference Oken and Gillman4, Reference Singhal, Wells, Cole, Fewtrell and Lucas14, and persons who are overweight or obese, measured as high value of BMI, are at increased risk for CVD and type 2 diabetesReference Grundy1.
The nutrition available to the foetus represents a balance between the interests of the mother and of the foetusReference Haig15; foetal nutritional status is determined by both pregnancy needs (foetal growth and maternal metabolism) and nutritional supplyReference Kuzawa16. In general, food or nutritional supply is not a major concern in the developed countries. Therefore, BW can usually be simplified as a result of a balance between foetal growth and maternal maintenance metabolism during pregnancy. Hence, it would be expected that taller mothers, generally, are more likely to have relatively large babies compared with shorter mothers; however, it may mean that tall mothers and their babies are at a relatively higher risk of undernutrition as well, because they need more food supply for foetal growth and the maintenance of normal maternal metabolism. On another hand, it would also suggest that short mothers and their babies are at relatively higher risk of overnutrition because they demand less food to supply their needs. To describe this phenomenon, we propose the Foetal Nutritional Status Index (FNSI), calculated by dividing the child's BW (kg) by the mother's height (m2). We hypothesise that both foetal over- and undernutrition may be associated with a poorer cardiovascular risk profile among children; this may help explain the growing prevalence of insulin resistance in modern society.
We tested our hypothesis using data from the US Third National Health and Nutrition Examination Survey (NHANES III) and applied the FNSI to examine its relationship with cardiovascular risk factors among the NHANES III children aged 5–11 years who had the necessary information needed for this analysis.
Methods
Study population
Data for this analysis were obtained from the NHANES III conducted between 1988 and 1994 on a nationwide multi-stage probability sample of about 40 000 persons including 11 728 children, 2 months to 11 years age, from the civilian, non-institutionalised population of the USA excluding reservation lands of American Indians. Of these, 3109 children (1576 males and 1533 females) aged 5–11 years old had information on BW and mother's height collected during household interview as well as a non-fasting blood sample collected at a mobile examination centre (MEC). Among these 3109 children, 985 children aged 5 and 6 years had birth certificate information, which contains the official record of BW, gestational age and other birth-related information. Details of the planning, sampling, operation, informed consent procedures and measures taken to maintain confidentiality of information have been given previously17.
Measures
Birth weight and mother's height
The information on BW and mother's height was obtained from the questions asked in a household interview of the parents or guardians of children aged 2 months to 11 years: ‘How much did the child weigh at birth?’ and ‘How tall is the child's mother/are you?’ For children aged 2 months to 6 years, their parents or guardians were asked for permission to obtain copies of the children's birth certificates from the natal dataset prepared by the Division of Vital Statistics, National Center for Health Statistics; it provides accurate information on BW, gestational age and birth order. Other interview variables are described elsewhere17.
Blood lipids
Non-fasting blood samples were obtained at the MEC for children aged 5–11 years. Serum total cholesterol values were determined at the Centers for Disease Control and Prevention using a modified ferric chloride technique (GFAA/Perkin–Elmer Model 3030 and 5100)17. HDL-C was measured in serum following the precipitation of other lipoproteins with a polyanion/divalent action mixture and triglycerides (TG) were measured enzymatically using a Hitachi 704 autoanalyser (Boehringer-Mannheim Diagnostics).
Anthropometric measurements
A standardised protocol was used for all anthropometric measurements and all examiners were trained in the standard procedures for obtaining measurementsReference Lohman, Roche and Martorell18. During the physical examination, participants wore only under-shorts and disposable paper shirts, pants and foam slippers. Body weight was measured with an electronic load cell scale to the nearest 0.01 kg. Standing height was measured without shoes in the Frankort horizontal plane to the nearest 0.1 cm using a fixed stadiometer. BMI (kg/m2) was derived from weight and height. Waist circumference, buttocks circumference, hip circumference and thigh circumference were measured by trained technicians. Waist-to-hip ratio was calculated as waist circumference over hip circumference. Skinfolds were measured using Holtain skinfold callipers to the nearest 0.1 mm at four different anatomic body sites (supraspinal, subscapular, triceps and biceps). Independent measures were taken at each body site by two technicians, resulting in a minimum of two skinfold observations for each site when the difference between the two measurements at a given site was within a pre-specified tolerance limit. The subscapular-to-triceps skinfold ratio was calculated as subscapular skinfold over triceps skinfold.
Blood pressure
Blood pressure (BP) was measured three times during the household interview (trained technologists) and three more times during the MEC examination (physicians) using mercury sphygmomanometers and a standard protocolReference Perloff, Grim, Flack, Frohlich, Hill and McDonald19. The available measurements (six or fewer) of the first Korotkoff sound were used to calculate the average of systolic BP (SBP)17.
Criterion of metabolic syndrome
The metabolic syndrome (MS) in children is evolving and there is no general agreement about the overall assessment of this syndrome. However, research indicates that adulthood insulin resistance is initiated at childhoodReference Ten and Maclaren20. For instance, childhood BP is a predictor of adulthood BPReference Klumbiene, Sileikiene, Milasauskiene, Zaborskis and Shatchkute21; those who had higher SBP levels in childhood were more likely to have arterial stiffness in adulthoodReference Li, Chen, Srinivasan and Berenson22; and the patterning of the risk clustering seen in adults is present in healthy adolescents and obesity is the predominant correlate of cumulative riskReference Goodman, Dolan, Morrison and Daniels23. Therefore, in this study, we used a cluster of cardiovascular risk factors that are associated with insulin resistance to define MS. Abnormal levels of cardiovascular risk factors were defined using the sex-specific third quartile of SBP, TG, subscapular-to-triceps skinfold ratio (an indicator of central obesity), and the first quartile of HDL-C (Table 1). The MS was defined as the presence of two or more positive components.
Table 1 Sex-specific cut points for metabolic syndrome components*

* The sex-specific third quartile of systolic blood pressure (SBP), triglycerides (TG) and subscapular-to-triceps skinfold ratio were used to define high blood pressure, high TG level and central obesity, and the first quartile of high-density lipoprotein cholesterol (HDL-C) was used to define low HDL-C level.
Confounding variables
Other variables in the analysis included the child's age at interview (years), race (1 = white, 0 = non-white), mother's age at the child's birth (years), mother's smoking status during the pregnancy (1 = yes, 0 = no) and sex (when necessary).
Statistical analyses
All analyses were conducted using Stata/SE 8.2 for Windows (StataCorp). All analyses incorporated the sampling weights to calculate means, percentages and regression coefficients and the complex survey design to calculate standard errors. To examine if the reported BW is reliable, we used the sub-sample of 985 children who were 5–6 years old and had BW information from both birth certificate and house interview to compare the differences in BW between the two sources. Using Cronbach's αReference Bland and Altman24, we found that the reported BW (mean = 3341.4 g) and the recorded BW (mean = 3339.2 g) were highly consistent with one another (Cronbach's α = 0.99). Therefore, we developed the FNSI using the reported BW (kg) over mother's height (m2) for the entire sample and the recorded BW (kg) over mother's height (m2) for the sub-sample. The association between the FNSI and BW and mother's height was examined by sex in two ways. First, the three variables were treated as continuous variables and Pearson correlation coefficients were calculated. Then the Pearson χ2 statistic was computed between the FNSI (in quintiles) and mother's height (in quartiles) and BW (in quintiles); this statistic was corrected for the survey design using the second-order correction of Rao and Scott and converted into an F statisticReference Rao and Scott25.
The means of cardiovascular risk-related variables were compared and the trends of the means of cardiovascular risk-related variables were tested by sex among children within different quintiles of the FNSI after adjustment for the child's age, mother's age at the child's birth, maternal smoking status and race. Multiple logistic regression models were used to assess the association between these cardiovascular risk factors and the FNSI after adjustment for the confounding variables listed above. The cardiovascular risk factors, including central obesity (1 = third quartile of subscapular-to-triceps skinfold ratio, 0 = else), high BP (1 = third quartile of SBP, 0 = else), high TG (1 = third quartile of TG, 0 = else) and low HDL-C (1 = first quartile of HDL-C, 0 = else), and MS (1 = any two or more of the cardiovascular risk factors, 0 = else) were regressed on four indicator variables created to represent the FNSI quintiles (with the third quintile as the reference group). Odds ratios (ORs) and 95% confidence intervals (CIs) were computed. Logistic regression analyses were also repeated on examining the association of these cardiovascular risk factors and BW in quintiles (the third quintile as the reference group).
Since the sub-sample of 985 children (486 males, 499 females) aged 5–6 years had additional information from the birth certificate including measured BW, gestational age and birth order, we also conducted a similar analysis to assess the ORs for these cardiovascular risk factors and MS among this sub-sample with adjustment for gestational age and birth order with sex combined, as there were not enough children in this age group to conduct analyses by sex. In addition, an interaction term between sex and foetal nutritional status was created and its relationship with cardiovascular risk factors examined.
Results
The FNSI was highly and positively associated with BW (Pearson correlation coefficients in the entire sample: 0.89 in males vs. 0.87 in females, P < 0.0001; in the sub-sample: 0.89 in males vs. 0.85 in females, P < 0.0001) and negatively associated with mother's height (in the entire sample: − 0.34 in males vs. − 0.33 in females, P < 0.0001; in the sub-sample: − 0.42 in males vs. − 0.40 in females, P < 0.0001). The distributions of BW in quintiles and of mother's height in quartiles according to the FNSI in quintiles are shown in Figs 1 and 2 respectively. The Pearson χ2 statistics suggest these variables are not independent from each other (P < 0.001). It is clear that the majority within the first quintile of the FNSI are those babies with a relatively smaller BW (67.7% of males and 66.6% of females with BW < 2.9 kg) and with a relatively taller mother (70.5% of males and 67.5% of females with mother's height >1.63 m); while within the fifth quintile of the FNSI, most subjects are those babies with a relatively larger BW (72.4% of males with BW >3.8 kg and 75.9% of females with BW >3.7 kg) and with a relatively shorter mother (72.4% of males and 81.2% of females with mother's height < 1.63 m). The means (ranges in parentheses) of the FNSI, BW and mother's height, and means or percentages of the confounding variables, by quintiles of the index for the entire sample are shown in Table 2. Compared with those in the higher quintiles, those in the lower quintiles have a lower proportion of white mothers, younger average mother's age at birth and higher proportion of reported maternal smoking (P-values for trends < 0.05). There are similar results for the sub-sample (data not shown).

Fig. 1 Distribution (%) of birth weight (BW) in quintiles according to the Foetal Nutritional Status Index (FNSI) in quintiles

Fig. 2 Distribution (%) of maternal height (MHT) in quartiles according to the Foetal Nutritional Status Index (FNSI) in quintiles
Table 2 Current age and birth-related variables by quintiles of the Foetal Nutritional Status Index (FNSI) and sex: the Third National Health and Nutrition Examination Survey (1988–94)

*P < 0.05, **P ≤ 0.0001.
Table 3 summarises the adjusted means for two groups of cardiovascular risk factors, the variables related to body fat distribution and the variables related to lipids and blood pressure. For males, except for the mean level of HDL-C in the first quintile, there were no statistical differences in variables related to body fat distribution or lipids and blood pressure. For females, the mean levels of most measurements related to body fat distribution increased with increasing value of the FNSI (P-values for trends < 0.05). Those in the fifth quintile had the highest levels of the anthropometric measurements, highest levels of TG and the lowest levels of HDL-C. However, in comparison to the reference group, the mean levels of subscapular-to-triceps skinfold ratio were higher in those within the first (P < 0.05) and second quintiles (P = 0.07).
Table 3 The means* of cardiovascular risk factors among children by sex and quintile of the Foetal Nutritional Status Index (FNSI): the Third National Health and Nutrition Examination Survey (1988–94)

BMI – body mass index; TC – total cholesterol; HDL-C – high-density lipoprotein cholesterol; TG – triglycerides; SBP – systolic blood pressure.
* Adjusted for the child's age, mother's age at the child's birth, maternal smoking status and race.
† Referent category.
‡ P < 0.05, §P = 0.07, ¶P < 0.01; **P < 0.05 for trends.
The prevalence and adjusted OR of the cardiovascular risk factors are shown in Table 4. In males, the odds for those in the first FNSI quintile was 2.4 times higher in the low HDL-C category (P < 0.01) and 2.1 times higher in the MS category (P = 0.01) in comparison to those in the reference group. In females, approximately 24% of those in the first and fifth quintiles of the FNSI had central obesity (OR (95% CI): 2.1 (1.2–4.0) for the first quintile, 2.7 (1.5–4.8) for the fifth quintile). In addition, over one-third of those in the fifth quintile of the index had higher TG (OR (95% CI): 2.0 (1.1–3.7)) and lower HDL-C (OR (95% CI): 2.0 (1.1–3.5)). Overall, the odds of having MS was approximately two times higher for those in the first and fifth quintiles compared with those in the reference group (P < 0.05). When the analyses were conducted using BW in quintiles (Table 5), the results from males were almost identical to those using the FNSI. In females, however, no associations between the cardiovascular risk factors and BW were observed.
Table 4 Relationship between cardiovascular risk factors* and the Foetal Nutritional Status Index (FNSI) among children by sex and quintile of FNSI: the Third National Health and Nutrition Examination Survey (1988–94)

TG – triglycerides; HDL-C – high-density lipoprotein cholesterol; OR – odds ratio; CI – confidence interval.
* Central obesity – subscapular-to-triceps skinfold ratio >75th percentile (males: 0.8206, females: 0.8714); high blood pressure – systolic blood pressure >75th percentile (males: 103 mmHg, females: 103 mmHg); high TG level – TG >75th percentile (males: 116.5 mg dl− 1, females: 124 mg dl− 1); low HDL-C level – HDL-C < 25th percentile (males: 45 mg dl− 1, females: 44 mg dl− 1); insulin resistance syndrome – any two or more of the four components.
† Defined as the presence of two or more of the components of the metabolic syndrome.
‡ Adjusted for the child's age, mother's age at the child's birth, maternal smoking status and race.
Table 5 Relationship between cardiovascular risk factors* and birth weight (BW) among children by sex and quintile of BW: the Third National Health and Nutrition Examination Survey (1988–94)

TG – triglycerides; HDL-C – high-density lipoprotein cholesterol; OR – odds ratio; CI – confidence interval.
* Central obesity – subscapular-to-triceps skinfold ratio >75th percentile (males: 0.8206, females: 0.8714); high blood pressure – systolic blood pressure >75th percentile (males: 103 mmHg, females: 103 mmHg); high TG level – TG >75th percentile (males: 116.5 mg dl− 1, females: 124 mg dl− 1); low HDL-C level – HDL-C < 25th percentile (males: 45 mg dl− 1, females: 44 mg dl− 1); insulin resistance syndrome – any two or more of the four components.
† Defined as the presence of two or more of the components of the metabolic syndrome.
‡ Adjusted for the child's age, mother's age at the child's birth, maternal smoking status and race.
To examine the impact of gestational age and birth order on the relationship between foetal nutritional status and cardiovascular risk factors, we repeated the logistic regression analyses among the sub-sample with additional adjustment for gestational age and birth order (Table 6). The results are very similar to those of the entire sample except for low HDL-C level. There were no interactions between sex and cardiovascular risk factors (data not shown) except for the lower level of HDL-C; it indicates that females in the fifth quintile were more likely to have lower HDL-C level (OR (95% CI): 2.9 (1.2–7.3)).
Table 6 Relationship between cardiovascular risk factors* and Foetal Nutritional Status Index (FNSI) among children 5–6 years of age by quintile of FNSI: the Third National Health and Nutrition Examination Survey (1988–94)
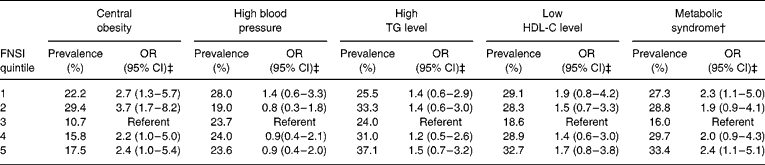
TG – triglycerides; HDL-C – high-density lipoprotein cholesterol; OR – odds ratio; CI – confidence interval.
* Central obesity – subscapular-to-triceps skinfold ratio >75th percentile (males: 0.8206, females: 0.8714); high blood pressure – systolic blood pressure >75th percentile (males: 103 mmHg, females: 103 mmHg); high TG level – TG >75th percentile (males: 116.5 mg dl− 1, females: 124 mg dl− 1); low HDL-C level – HDL-C < 25th percentile (males: 45 mg dl− 1, females: 44 mg dl− 1); insulin resistance syndrome – any two or more of the four components.
† Defined as the presence of two or more of the components of the metabolic syndrome.
‡ Adjusted for the child's age, mother's age at the child's birth, maternal smoking status, gestational age, birth order and race.
Discussion
To overcome the limitation of using BW alone to reflect foetal nutritional status, we propose using the child's BW and mother's height – two easily obtained and relatively accurate measurements – to create the Foetal Nutrition Status Index (FNSI): an index reflecting the relationship between maternal maintenance needs (mother's height) and foetal growth (birth weight). We argue that although many factors may affect foetal growth, maternal maintenance needs will have a tremendous modifying effect on foetal growth when other factors are relatively stable. It is already noticed that the neonatal phenotype is highly associated with maternal size and body composition26. For instance, maternal height is often used as a predictor of birth outcomeReference Leary, Fall, Osmond, Lovel, Campbell and Eriksson27 as well as a rational proxy of maternal nutritional needsReference Kuzawa16. It is expected to have relatively larger babies from taller mothers and to have relatively smaller babies from shorter mothers; this means that the taller mothers need relatively more food supply and shorter mothers need relatively less. As observed from Figs 1 and 2, the subjects within the lowest quintile of the FNSI are more likely to be lighter babies with taller mothers; in contrast, the subjects within the highest quintile of the FNSI are more likely to be heavier babies with shorter mothers. These results suggest that babies from taller mothers are relatively more susceptible to undernutrition compared with those from shorter mothers; while foetal growth among these shorter mothers is relatively more susceptible to overnutrition compared with that among taller mothers.
We reasoned that if both foetal nutritional insufficiency and foetal overnutrition elevate cardiovascular risk, there should be evidence that these circumstances are related to the components of MS. We hypothesised that both foetal nutritional insufficiency and foetal overnutrition may be associated with elevated cardiovascular risk among children. In comparison with the reference group, the results from the entire sample suggested that the FNSI has a similar cardiovascular risk pattern as BW in males; the subjects in the lowest quintile of either the FNSI or BW have higher risk of MS and abnormal HDL-C. In females, BW was not associated with MS and cardiovascular risk factors; however, those within the highest quintile of the FNSI have higher risk of abnormal cardiovascular risk factors and MS. These results indicate that foetal nutritional status has an impact on cardiovascular risk profiles among children and that BW may not be a good indicator of foetal nutritional status, at least among female children.
Our analyses suggest that those children who were subjected to a nutritionally insufficient or an overly nutritious environment in the foetal stage may have higher cardiovascular risk factors levels, although the impact may be slightly different between the sexes. In general, among full-term singletons, male infants weigh approximately 125–135 g more than female onesReference Chase28, Reference Cogswell and Yip29. Because male infants are expected genetically to be heavier than their female counterparts, mothers bearing males consumed more energy during pregnancy than mothers bearing femalesReference Tamimi, Lagiou, Mucci, Hsieh, Adami and Trichopoulos30; consequently, taller mothers with male infants are relatively more easily affected by foetal nutritional insufficiency. Thus, if the metabolic pattern is programmed during foetal life as suggested by the developmental plasticity hypothesisReference Bateson, Barker, Clutton-Brock, Deb, D'Udine and Foley10, Reference Gluckman and Hanson11, then males would be expected to have a higher level of risk for an abnormal metabolism pattern among those subjected to a nutritionally insufficient environment in the foetal period than females similarly exposed. On the other hand, if overnutrition also affects the metabolic pattern in later life then females would be more likely to have a higher level of risk for an abnormal metabolic pattern among those shorter mothers with heavier babies than would their male counterparts. Our findings support the hypothesis that both foetal under- and overnutrition may contribute the development of insulin resistance, which may help to explain the epidemic of obesity and its related chronic diseases.
Several studies have noted the impact of maternal factors on cardiovascular risk profiles in later life. In a study of the relationship between BW and cardiovascular risk factors in 477 Indian children aged 8 years, it was found that the most insulin-resistant individuals were from those whose parents were short, but who had grown the tallest by 8 years of ageReference Bavdekar, Yajnik, Fall, Bapat, Pandit and Deshpande31. A follow-up study on young adults who were born during a longitudinal nutritional supplementation trial in Guatemala was conducted to examine the relationship between fasting glucose level and maternal nutritional status, and an inverse association was found with birth size among women born to fatter mothers and men born to short mothersReference Conlisk, Barnhart, Martorell, Grajeda and Stein32. Results from the Cebu Longitudinal Health and Nutrition Survey also indicated that males born small to tall mothers who were relatively poorly nourished during pregnancy tended to have the highest low-density lipoprotein cholesterol levels in adolescenceReference Kuzawa16. In addition, an inverse relationship between maternal height and childhood BP was observed among UK adolescentsReference Whincup, Cook, Papacosta, Walker and Perry33. However, using the FNSI to reflect foetal nutritional status and examine its cardiovascular risk association is novel. Although our findings support the hypothesis that both foetal under- and overnutrition may be associated with abnormal cardiovascular risk factors, it is important that additional studies be performed to confirm these results and determine the impact of using the FNSI to examine foetal nutritional status on cardiovascular risk factors among children.
There are several specific limitations in our study that need to be addressed. First, the mother's height used for deriving the FNSI was self-reported height from household interview. However, mothers from the current analyses were relatively young and the average age of mothers at the child's birth was approximately 25 years; the results from studies of the NHANES III suggest that the self-reported heights are reliable among younger adultsReference Kuczmarski, Kuczmarski and Najjar34, especially among younger female adultsReference Ezzati, Martin, Skjold, Vander Hoorn and Murray35. Second, the measurement of TG level was from a non-fasting blood sample because of the participants' young age. This may affect the relationship between foetal nutritional status and cardiovascular risk factors in the analyses; however, results from some studies indicated that non-fasting TG levels were positively associated with central obesity among pre-school childrenReference Cowin and Emmett36 and were a good predictor of CHD risk among adultsReference Iso, Naito, Sato, Kitamura, Okamura and Sankai37. Third, there were not enough samples to examine the ethnic differences observed among the different quintiles of the FNSI.
Notwithstanding these limitations, the strength of this study is that it is based on the NHANES III sample design with the rigorously standardised medical examination including the measurement of anthropometric characteristics. In summary, our study indicates that the FNSI derived from birth weight (kg) divided by mother's height (m2) may be a useful proxy indicator of foetal nutritional status that can be used to test specific hypotheses of whether foetal nutritional restriction or overnutrition programmes CVD risk in adulthood.
Acknowledgements
Sources of funding: This study was partially funded by Brock University (BROCKAC 335-737-014).
Conflict of interest declaration: The authors have no conflicts of interest.
Authorship responsibilities: Study concept and design, acquisition of data, analysis and interpretation of data ( J.L. and C.S.); statistical analysis, drafting the manuscript ( J.L.); critical revision of the manuscript for intellectual content ( J.L. and C.S.); obtaining funding, administrative technical/material support and study supervision ( J.L.).










