It is possible that Ca intake has an impact on cancer risk, with some observational studies suggesting that high intakes of Ca and/or vitamin D are associated with a reduced risk of colorectal(Reference Garland, Shekelle and Barrett-Connor1–Reference Wu, Willett and Fuchs3) and breast cancer(Reference Shin, Holmes and Hankinson4, Reference McCullough, Rodriguez and Diver5), and an increased risk of prostate cancer(Reference Giovannucci, Rimm and Wolk6–Reference Chan, Stampfer and Ma8); however, these results are not consistent(Reference Kearney, Giovannucci and Rimm9–Reference Anderson, Cotterchio and Vieth14). To date, few randomised, placebo-controlled trials of Ca supplements with or without vitamin D have reported cancer outcomes. In a 4-year fracture prevention trial, Lappe et al. (Reference Lappe, Travers-Gustafson and Davies15) reported that Ca monotherapy decreased total cancer risk by 47 % (P= 0·06), and Ca with vitamin D by 60 % (P= 0·01).
In contrast, three randomised, placebo-controlled trials(Reference Avenell, Maclennan and Jenkinson16–Reference Bolland and Reid19) of Ca with or without vitamin D have found no evidence of an effect on cancer risk. We found no effect of Ca monotherapy on total cancer incidence in a 5-year trial in postmenopausal women(Reference Bolland and Reid19). The Randomised Evaluation of Calcium Or Vitamin D (RECORD) investigators found no effect of Ca with or without vitamin D on cancer mortality or incidence in older people treated for a median of 45 months(Reference Avenell, Maclennan and Jenkinson16). The Women's Health Initiative (WHI) investigators reported no effect of Ca plus vitamin D on the risk of colorectal(Reference Wactawski-Wende, Kotchen and Anderson18) or breast(Reference Chlebowski, Johnson and Kooperberg17) cancer in >36 000 postmenopausal women treated for an average of 7 years. However, we recently reanalysed the publicly available WHI dataset and found significant interactions between treatment allocation, personal Ca or vitamin D supplement use and the risk of total, breast and colorectal cancers, suggesting that widespread personal (non-protocol) supplement use may have obscured a therapeutic effect of Ca and vitamin D on cancer endpoints(Reference Bolland, Grey and Gamble20). When analyses were restricted to participants not taking personal Ca or vitamin D at baseline, Ca and vitamin D significantly reduced the risk of total and breast cancer by 14–20 %, and non-significantly reduced the risk of colorectal cancer by 17 %.
Thus, there is some evidence that Ca and vitamin D supplements might lower cancer risk; however, it remains uncertain whether these possible effects are related to Ca, vitamin D or the combination of both agents. We have updated a large database of randomised clinical trials of Ca supplements in older adults, originally assembled to assess the effect of Ca supplements on cardiovascular risk, to determine whether Ca used as a monotherapy has an impact on cancer risk.
Methods
In November 2007, Medline, Embase and Cochrane Central were searched for randomised, placebo-controlled trials of Ca supplements, using the terms ‘calcium’, ‘randomised controlled trial’ and ‘placebo’ as text words, and corresponding Medical Subject Headings (MeSH) terms. The reference lists of meta-analyses published between 1990 and 2007 on the effect of Ca supplements on fracture, bone density, colorectal neoplasia and blood pressure, and two clinical trial registries (ClinicalTrials.gov and Australian New Zealand Clinical Trial Registry) were searched. No language restrictions were applied(Reference Bolland, Avenell and Baron21). In February 2012, searches of the electronic databases were updated (Medline: 1966–February 2012; Embase: 1980–February 2012; Cochrane Central: January 2012).
Study selection
Studies were included if they were randomised, double-blind, placebo-controlled trials; the mean age of participants at baseline was >40 years; a dose of ≥ 500 mg/d of elemental Ca was administered; ≥ 100 participants were randomised; participants of either sex were studied; and the duration of the trial was >1 year. Studies were excluded if Ca and vitamin D were co-administered and compared with placebo (studies were eligible if vitamin D was given to both intervention and control groups); if Ca was administered in the form of a complex nutritional supplement or as a dietary modification; and if most participants had a major systemic disease other than osteoporosis or colorectal adenoma.
Search results
For the present review, two investigators carried out the search (M. J. B. and S. M. B.) and two investigators independently reviewed all potential studies (M. J. B. and A. G.) to determine the adequacy of randomisation, the concealment of allocation, the blinding of participants and investigators and the extent of loss to follow-up.
Data description
The lead author of each eligible trial was invited to provide patient-level data for cancer outcomes for their study. When such data were not available, we requested trial-level summary data. Complete trial-level data were available on total cancer events for seven studies (9447 participants)(Reference Lappe, Travers-Gustafson and Davies15, Reference Reid, Ames and Evans22–Reference Reid, Ames and Mason27), on colorectal cancer for eight studies (9863 participants)(Reference Lappe, Travers-Gustafson and Davies15, Reference Reid, Ames and Evans22–Reference Bonithon-Kopp, Kronborg and Giacosa28), on breast cancer for six studies (7641 participants)(Reference Lappe, Travers-Gustafson and Davies15, Reference Reid, Ames and Evans22–Reference Reid, Mason and Horne26), on prostate cancer for three studies (1806 participants)(Reference Baron, Beach and Mandel23, Reference Grant, Avenell and Campbell25, Reference Reid, Ames and Mason27) and on cancer-related mortality for six studies (8109 participants)(Reference Reid, Ames and Evans22, Reference Baron, Beach and Mandel23, Reference Grant, Avenell and Campbell25–Reference Bonithon-Kopp, Kronborg and Giacosa28). Partially complete trial-level data were available on total cancer for a further three studies (1049 participants)(Reference Bonithon-Kopp, Kronborg and Giacosa28–Reference Riggs, O'Fallon and Muhs30), and on colorectal and breast cancer for a further two studies (633 participants)(Reference Chailurkit, Saetung and Thakkinstian29, Reference Riggs, O'Fallon and Muhs30). Of the studies that supplied complete trial-level data, patient-level data were also available on total cancer and colorectal cancer events for four studies (7221 participants)(Reference Reid, Ames and Evans22, Reference Grant, Avenell and Campbell25–Reference Reid, Ames and Mason27), on breast cancer events for three studies (6087 participants)(Reference Reid, Ames and Evans22, Reference Grant, Avenell and Campbell25, Reference Reid, Mason and Horne26) and on prostate cancer events for two studies (1134 participants)(Reference Grant, Avenell and Campbell25, Reference Reid, Ames and Mason27). No data on cancer events were available for six studies (2743 participants)(Reference Peacock, Liu and Carey31–Reference Dawson-Hughes, Dallal and Krall36).
We therefore had complete trial-level data on total cancer events for 71 % of participants, at least partially complete trial-level data for 79 % of participants, patient-level data for 54 % of participants and no data for 21 % of participants, from sixteen eligible trials.
Ascertainment of cancer events
For seven studies, data on cancer events were supplied by the lead authors. Data were obtained from a combination of self-reports, hospital discharge data and death certificates(Reference Reid, Ames and Evans22, Reference Bonnick, Broy and Kaiser24–Reference Reid, Ames and Mason27, Reference Chailurkit, Saetung and Thakkinstian29, Reference Riggs, O'Fallon and Muhs30) and cancer registries(Reference Grant, Avenell and Campbell25). For one study, data on prostate cancer(Reference Baron, Beach and Wallace37) and colorectal cancer(Reference Baron, Beach and Mandel23) were obtained from published data, and data on total cancer, breast cancer and cancer mortality were supplied by the lead author and derived from hospital discharge data and death certificates. For another study, data on colorectal cancer were obtained from published data(Reference Bonithon-Kopp, Kronborg and Giacosa28), and data on cancer-related mortality were supplied by the lead author and derived from physician-reported cause of death. For the final study, data on total, breast and colorectal cancers were obtained from published data(Reference Lappe, Travers-Gustafson and Davies15). The lead authors provided a description of each type of cancer events, and cancers described as ‘skin’, ‘epidermal/epidermoid’ or ‘basal cell’ or the International Classification of Diseases (ICD) code C44 were not considered in these analyses. For the one study for which total cancer events were obtained from published data(Reference Lappe, Travers-Gustafson and Davies15), the authors reported only total non-skin cancers. When data on cancer events were obtained from published data, data were extracted independently by two investigators (M. J. B. and S. M. B.).
Endpoints
The pre-specified primary endpoint was the incidence of first total cancer, excluding non-melanoma skin cancers. Secondary endpoints were the incidence of colorectal cancer, breast cancer, prostate cancer and cancer-related mortality.
Statistical analysis
The primary analysis was of trial-level data. Statistical heterogeneity was assessed using Cochran's Q statistic (P< 0·10) and the I 2 statistic (I 2>50 %). No significant statistical heterogeneity existed between trials in any of the analyses. Trial-level summary data were pooled using DerSimonian and Laird random-effects meta-analyses. Publication bias was assessed using funnel plots. Analyses were carried out using SAS version 9.2 or Review Manager 5.1 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2011). All tests were two-tailed and P< 0·05 was considered as significant.
Studies that also provided patient-level data were included in a secondary patient-level analysis. Each endpoint was analysed using a Cox proportional hazards model stratified by study, and the hazard ratio (HR) and 95 % CI reported. The assumption of proportional hazards was explored graphically and by carrying out a test for proportionality of the interaction between variables included in the model and the logarithm of time. Assessment of the effect-modifying influence of covariates on outcomes was done by repeating the models including the following covariates potentially associated with cancer incidence: age, sex, smoking status, BMI and weight. This was also done by undertaking subgroup analyses using interaction terms between treatment allocation and the following pre-specified subgroups: sex, age ( ≥ 75 or < 75 years), dietary Ca (above or below the median for all studies), serum 25-hydroxyvitamin D ( ≥ 50 or < 50 nmol/l) and supplement type (citrate, carbonate or lactogluconate–carbonate), where data were available for >80 % of participants.
Based on the assumption that cancers diagnosed early on in the trials may have been present, but undetected, at baseline, we repeated these models including latent periods of 1 and 2 years. As a sensitivity analysis, the models were repeated including only the trials in which data were obtained from unverified sources (self-reports) or verified sources (cancer registries).
Results
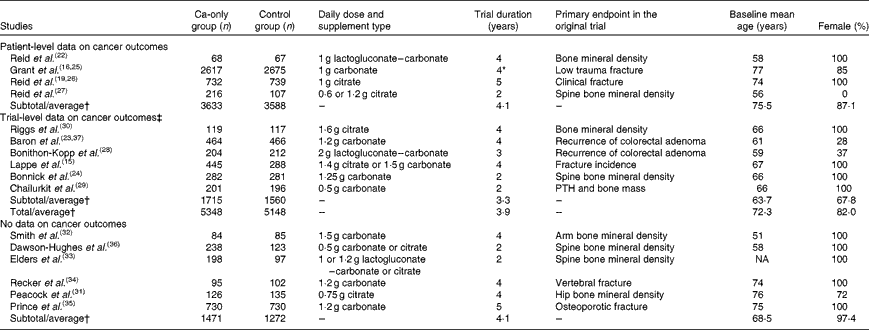
The results of the literature search are shown in Fig. 1, and the characteristics of the eligible studies in Table 1. All ten eligible studies providing data were randomised, double-blind, placebo-controlled trials. The method of randomisation was stated in six trials: one used a centralised randomisation service and five used computer-generated random numbers. Allocation concealment was explicitly stated by four trials. Details of participants who were lost to follow-up or withdrew were reported by nine trials. All ten trials reported compliance; however, the definitions of compliance differed and were not always comparable. Table 2 shows the selected baseline characteristics of participants.

Fig. 1 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart of studies. The initial search was in November 2007 with 9358 reports identified, 173 reports of potentially relevant studies retrieved, 150 reports excluded and twenty-three reports of fifteen individual studies identified. The search was updated in March 2010 and February 2012: a further 3374 reports were identified, thirty-three reports retrieved and one new study identified.
Table 1 Characteristics of the sixteen studies eligible for inclusion in the meta-analysis

PTH, parathyroid hormone; NA, not available.
* Mean duration was 45 months, with all participants followed for at least 2 years during the trial.
† Weighted by person-years of follow-up.
‡ Complete trial-level data on total cancer events were available for seven studies(Reference Lappe, Travers-Gustafson and Davies15, Reference Reid, Ames and Evans22–Reference Reid, Ames and Mason27) and partially complete trial-level data were available for three studies(Reference Bonithon-Kopp, Kronborg and Giacosa28–Reference Riggs, O'Fallon and Muhs30).
Table 2 Baseline variables in the trials with patient- or trial-level data available for cancer outcomes (Mean values and standard deviations)

NA, not available.
* 25-Hydroxyvitamin D measured in a sample of sixty participants.
† Value is median.
Trial-level analysis
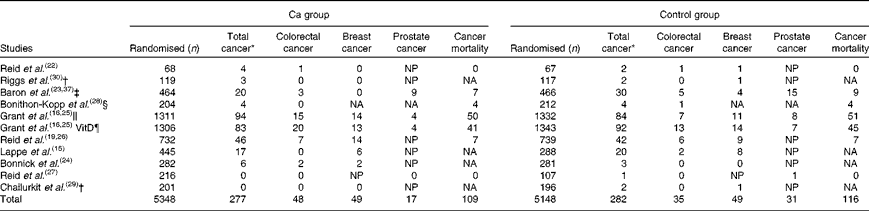
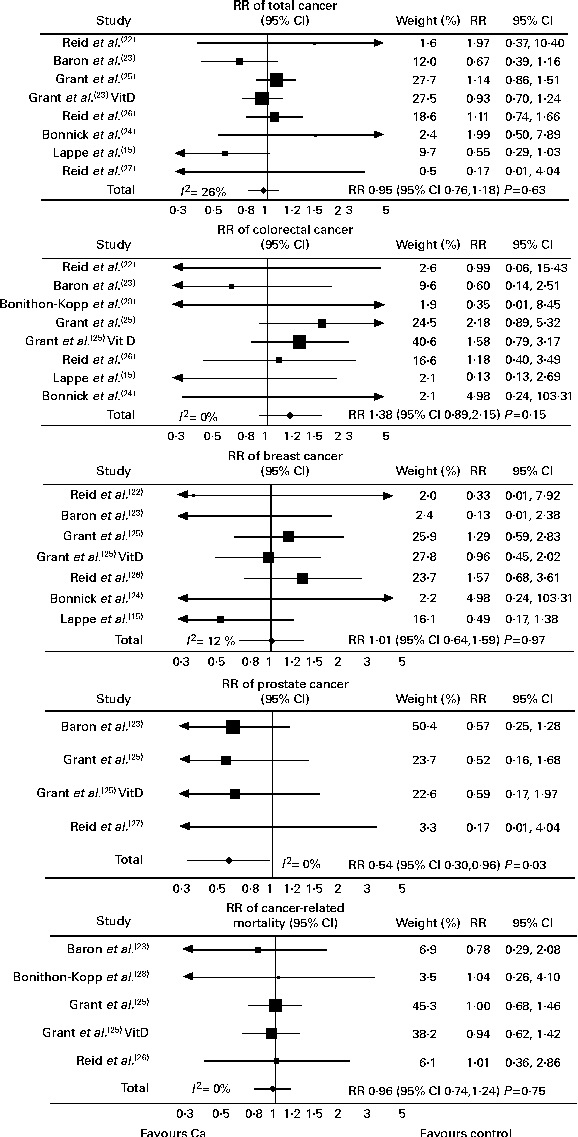
There were seven studies that provided complete trial-level data on total cancer events and were included in the primary analysis. The number of people with cancer events in each study by treatment allocation is shown in Table 3, and the results of the trial-level analysis are shown in Fig. 2. Allocation to Ca supplements had no effect on the risk of total cancer, colorectal cancer, breast cancer or cancer-related mortality. Allocation to Ca supplements was associated with a significant decrease in the risk of prostate cancer. A further three trials(Reference Bonithon-Kopp, Kronborg and Giacosa28–Reference Riggs, O'Fallon and Muhs30) for total cancer and two trials(Reference Chailurkit, Saetung and Thakkinstian29, Reference Riggs, O'Fallon and Muhs30) for colorectal and breast cancer had data only for subgroups of participants, and were included in a sensitivity analysis that included data from all ten trials. Including data from these further trials did not change the results for any endpoint. Publication bias was not evident on inspection of funnel plots in any analysis.
Table 3 Number of people with cancer and cancer-related mortality by treatment group

NP, not applicable for breast cancer as all subjects were male, or for prostate cancer as all subjects were female; NA, not available.
* Total cancer excluding non-melanoma skin cancers.
† Unpublished trial-level data on reasons for study withdrawals provided by author.
‡ Unpublished trial-level data on total cancers, breast cancers and cancer-related mortality provided by the author, data on colorectal cancers and prostate cancers from published data.
§ Unpublished trial-level data on cancer deaths provided by the author, data on colorectal cancers from published data.
∥ Ca v. placebo arms in the Randomised Evaluation of Calcium Or Vitamin D (RECORD) study.
¶ Ca plus vitamin D and placebo plus vitamin D arms in the RECORD study.

Fig. 2 Random-effects models of calcium supplementation on cancer events and cancer mortality. Full trial-level data were available for eight studies for colorectal cancer and six studies for cancer mortality. However, some studies are not shown as no events occurred: there were no colorectal cancer events in the study by Reid et al. (Reference Reid, Ames and Mason27) and was no cancer-related mortality in the study by Reid et al. (Reference Reid, Ames and Evans22) or Reid et al. (Reference Reid, Ames and Mason27). Grant et al. (Reference Grant, Avenell and Campbell25) is the calcium v. placebo arms of this study, and Grant et al. (Reference Grant, Avenell and Campbell25) vitamin D (VitD) is the calcium plus VitD v. VitD-only arms. RR, relative risk.
Patient-level analysis
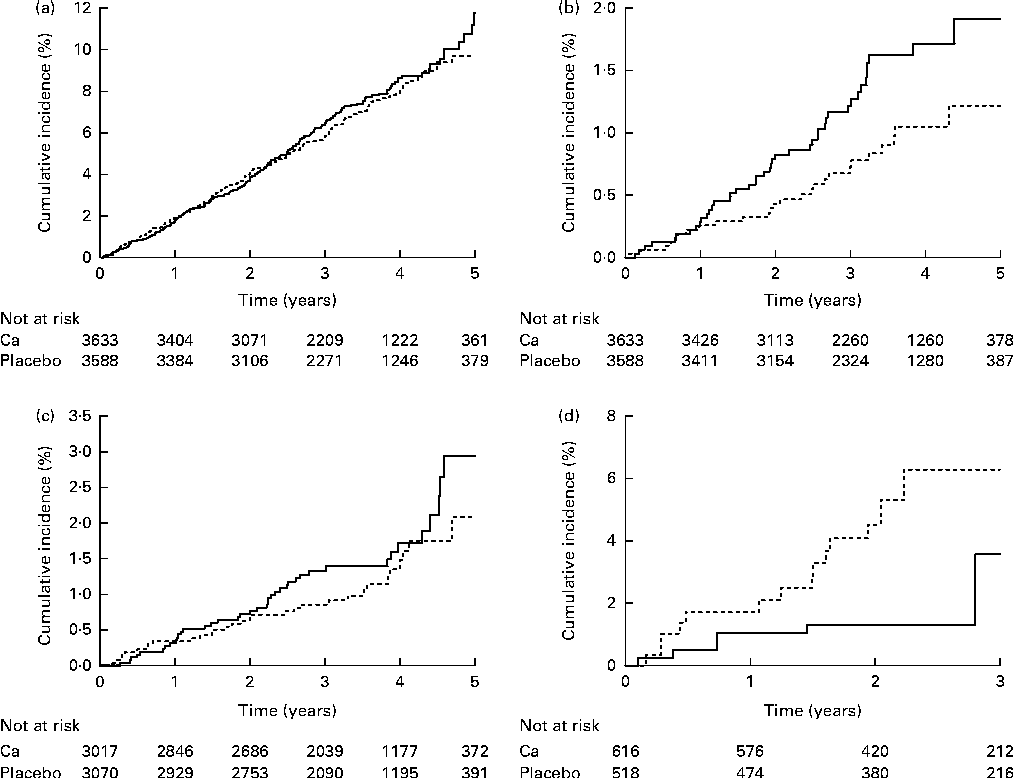
Among the eligible trials, four studies that provided trial-level data also provided patient-level data and were included in a secondary patient-level analysis. The selected baseline characteristics are shown in Table 4, and the results in Table 5 and Fig. 3. The median duration of follow-up in both groups was 3·5 (interquartile range 2·6–4·4) years. Allocation to Ca supplements had no effect on the risk of total cancer, breast cancer or cancer-related mortality. Allocation to Ca supplements significantly increased the risk of colorectal cancer, and non-significantly decreased the risk of prostate cancer. Adjusting for pre-specified covariates likely to be related to cancer outcomes, with data available for more than 80 % of participants (age, sex, smoking status and weight), did not change these results, nor did including trials in which data were obtained only from verified sources. Repeating the models including a latent period of 1 or 2 years to attempt to adjust for cancers that were present, but undetected at baseline, moved the results for colorectal cancer and prostate cancer towards significance, but did not alter the results in any other way (Table 5). In pre-specified subgroup analyses, no interactions were identified between treatment allocation and age, sex, vitamin D status, smoking status, dietary Ca intake and supplement type for any cancer endpoint.
Table 4 Baseline characteristics of participants in four studies included in the patient-level analysis by treatment allocation (Mean values and standard deviations; medians and interquartile ranges; percentages)

* Proportion of women was significantly higher in the placebo group because one study that only involved men had a 2:1 ratio of allocation to Ca or placebo. No other differences existed between groups.
† Data available from four studies for 1050 participants in the Ca group and 952 participants in the placebo group.
Table 5 Results of the patient-level analysis† (Hazard ratios and 95 % confidence intervals)

* P< 0·05.
† Number of people with cancer events.
‡ Events during the first year censored.
§ Events during the first and second year censored.

Fig. 3 Cumulative incidence of (a) total cancer (hazard ratio (HR) 1·07, 95 % CI 0·89, 1·28, P= 0·50), (b) colorectal cancer (HR 1·63, 95 % CI 1·01, 2·64, P= 0·046), (c) breast cancer (HR 1·27, 95 % CI 0·81, 2·02, P= 0·30) and (d) prostate cancer (HR 0·49, 95 % CI 0·21, 1·14, P= 0·098) in four studies that contributed patient-level data. ![]() , Ca;
, Ca; ![]() , placebo.
, placebo.
Discussion
In the present meta-analysis of 10 500 participants from ten trials, Ca supplements without co-administered vitamin D did not alter the risk of total cancer, breast cancer or cancer-related mortality over 4 years. Ca supplements significantly reduced prostate cancer risk; however, this was based on a small number of events. Ca supplements did not alter colorectal cancer risk in the trial-level analysis, while there was an increased risk in the patient-level analysis; however, this was based on a small number of events. Including a latent period of 1 or 2 years did not meaningfully alter the results.
While the association between Ca intake and cancer risk has been the subject of numerous observational studies, few randomised, placebo-controlled trials of Ca supplements have examined cancer incidence as an outcome and none as a primary endpoint. In a 4-year trial of 1179 postmenopausal women, Lappe et al. (Reference Lappe, Travers-Gustafson and Davies15) reported a non-significant 47 % reduction in total cancer risk with Ca monotherapy, and a significant 60 % reduction with Ca plus vitamin D. There was no reduction in risk with Ca plus vitamin D compared with Ca monotherapy (P= 0·46), suggesting that Ca supplements were responsible for the protective effects. We were unable to confirm reductions in cancer risk with Ca monotherapy over an equivalent 4-year period in the present meta-analysis, with consistent findings in both trial- and patient-level analyses. Compared with the Lappe trial, the population in the present meta-analysis was older (72 v. 67 years), but had a lower annualised incidence of cancer in the control group (1·4 v. 1·7 %), suggesting that the positive findings in the Lappe trial might have resulted from an unexplained high rate of cancer in the control group(Reference Schabas38), and a small number of total cancer events (n 50, compared with 550 in the present meta-analysis). It is also possible that the Lappe trial results are an outlying result that might have arisen by chance.
Consistent with the present meta-analysis, a recent analysis of the RECORD study(Reference Avenell, Maclennan and Jenkinson16), a trial of 5292 older people randomised to Ca, vitamin D, Ca with vitamin D or placebo for a median duration of 45 months and followed for a further 3 years, found no effect of Ca (with or without vitamin D) on the risk of total cancer incidence or cancer-related mortality.
A protective effect of Ca with vitamin D supplements against total cancer, breast cancer and possibly colorectal cancer was suggested in a recent reanalysis of WHI data(Reference Bolland, Grey and Gamble20). As Ca and vitamin D were administered together in that trial, it was not possible to determine which agent was responsible for the protective effects. The results of the present meta-analysis suggest that Ca supplements for 4 years without co-administered vitamin D have no effect on the risk of total cancer, which might indicate that vitamin D, or a combination of both agents, was responsible for the observed reduction in risk in the WHI reanalysis. However, it is also possible that the present meta-analysis lacked sufficient power and/or was of too short duration to detect a small effect of Ca on total cancer risk, though the beneficial trends in the WHI were apparent from year 2. The total cancer endpoint in the WHI reanalysis was based on 1300 cancer events over 7 years among 15 600 women, whereas in the present meta-analysis, there were 550 cancer events over 4 years among 10 500 men and women. The lack of an effect of Ca on total cancer in the present meta-analysis suggests that if Ca does have an effect on total cancer risk, it is small. Future trials should use estimates from these meta-analyses to calculate sample sizes: such trials will need to be large and of a long duration. The design of such trials is discussed in detail in the description of a large trial of vitamin D and n-3 for cancer and cardiovascular prevention(Reference Manson, Bassuk and Lee39).
In contrast with earlier observational studies(Reference Garland, Shekelle and Barrett-Connor1–Reference Wu, Willett and Fuchs3) and colorectal adenoma chemoprevention trials(Reference Baron, Beach and Mandel23, Reference Hofstad, Almendingen and Vatn40), we found no evidence that Ca monotherapy reduces colorectal cancer risk. This may have been due to the duration of the trials: if colorectal cancer has a latency period of 10–20 years, participants diagnosed during each trial may have had early-stage cancer that was present but asymptomatic and undetected at baseline. While Ca may inhibit colorectal cancer initiation, such as adenoma formation(Reference Baron, Beach and Mandel23), little research has examined its effects on cancer progression. In polyp-bearing participants, Ca and antioxidants had no effect on existing adenoma growth, but significantly reduced new adenoma development over 3 years(Reference Hofstad, Almendingen and Vatn40). The present meta-analysis included two trials that had participants with a history of colorectal adenoma. Because of the small number of colorectal cancer events in these trials (three in the combined Ca groups and six in the combined control groups), we were unable to investigate whether the effect of Ca on colorectal cancer was different in these participants.
We found some evidence of increased colorectal cancer risk with Ca, although this was only statistically significant in the patient-level analysis. The increased risk may have resulted from increased screening for colorectal cancer in the group allocated to Ca, as a change in bowel habits is an early symptom of colorectal cancer(Reference Majumdar, Fletcher and Evans41), and Ca supplements cause gastrointestinal side effects(Reference Reid, Mason and Horne26, Reference Lewis, Zhu and Prince42). While the increased risk could reflect an effect of Ca on colorectal cancer progression, the small number of colorectal cancer events and the lack of statistical significance in the primary trial-level analysis suggest that it might be a chance finding.
We observed a reduction in prostate cancer risk with Ca in the primary trial-level analysis, largely based on two trials(Reference Grant, Avenell and Campbell25, Reference Baron, Beach and Wallace37) (in a third trial, only one prostate cancer event occurred(Reference Reid, Ames and Mason27)). The risk estimate derived from the patient-level analysis was similar, although not statistically significant, probably because there were 50 % fewer events than in the trial-level analysis. In a more detailed analysis of one of these trials(Reference Baron, Beach and Wallace37), Ca had no effect on prostate cancer risk over 10 years (4 years of treatment and 6 years of post-treatment follow-up), but reduced risk by 48 % during the first 6 years. These results suggest that the positive association between Ca intake and increased prostate cancer risk, suggested by some(Reference Giovannucci, Rimm and Wolk6–Reference Chan, Stampfer and Ma8) but not all(Reference Park, Murphy and Wilkens12, Reference Park, Mitrou and Kipnis13) observational studies, may be unrelated to Ca, and instead due to other factors correlated with Ca intake, such as dairy product intake, or may have occurred from residual confounding. The small number of prostate cancer events means that the present findings should be interpreted cautiously, but if Ca does protect against prostate cancer, this could be mediated through the Ca-sensing receptor, found on prostate cells(Reference Sanders, Chattopadhyay and Kifor43), or indirectly through parathyroid hormone, which has been implicated in prostate carcinogenesis(Reference Ritchie, Thomas and Andrews44).
The present meta-analysis has some limitations. Cancer was not a primary outcome of any included study, and cancer events were not collected in a standardised manner. However, unless there was a differential misclassification or misreporting of cancer events in people allocated to Ca (as we suggested for colorectal cancer), this should not have affected the present results. No data for cancer events were available for six trials comprising 20 % of participants. With one exception, these trials were small, and the consistency of the findings suggests that the addition of these trials would not have affected the present results.
In summary, we found no evidence that Ca supplements without co-administered vitamin D influence total cancer risk over 4 years. The differences between the findings of the present meta-analysis and those of a recent reanalysis of the WHI, suggesting the benefits of co-administered Ca and vitamin D on cancer incidence, might be attributable to the co-administration of vitamin D in that trial. However, we cannot rule out that the present meta-analysis lacked sufficient power or that the duration of trials was too short to detect a small effect of Ca supplements. Any future trials of Ca supplements on cancer incidence should base sample size calculations on the effect sizes observed here. Such trials will need to be very large and of a long duration.
Acknowledgements
The present meta-analysis was supported by the Health Research Council of New Zealand and the University of Auckland School of Medicine Foundation. S. M. B. is the recipient of a University of Auckland Doctoral Scholarship. M. J. B. is the recipient of a Sir Charles Hercus Health Research Fellowship. The Health Services Research Unit, University of Aberdeen, is funded by the Chief Scientist Office of the Scottish Government Health Directorates. The UK Medical Research Council funded the central organisation of the RECORD trial (grant no. G9706483).
The authors' responsibilities were as follows: S. M. B., M. J. B., A. A. and I. R. R. designed the research; S. M. B. and G. D. G. analysed the data; S. M. B., M. J. B., G. S. M., A. A., A. G. and I. R. R. wrote the manuscript; S. M. B. had primary responsibility for the content. All of the authors had access to the data and read and approved the final manuscript.
I. R. R. had study medications for clinical trials of Ca supplementation supplied by Mission Pharmacal and received research support from and acted as a consultant for Fonterra. A. A. and G. S. M. had study medications for the RECORD trial supplied by Shire Pharmaceuticals Group and Nycomed AS. None of the other authors has any conflicts of interest to declare.