Around 30% of individuals with schizophrenia will remain symptomatic and significantly impaired despite standard antipsychotic treatment and are considered to have treatment-resistant schizophrenia.Reference Suzuki, Remington, Mulsant, Uchida, Rajji and Graff-Guerrero1 Treatment resistance is usually defined as a failure to respond to two antipsychotic trials of sufficient dose and duration and is one of the most disabling forms of illness, thus presenting a major clinical challenge.Reference Kennedy, Altar, Taylor, Degtiar and Hornberger2 It is as yet unclear whether treatment resistance is better conceptualised as a form of illness at the severe end of a spectrum or as a more biologically homogeneous subgroup of those with schizophrenia, although recent evidence has supported the latter hypothesis.Reference Gillespie, Samanaite, Mill, Egerton and MacCabe3 Clozapine is the only medication with proven effectiveness for patients with treatment-resistant schizophrenia.Reference Leucht, Corves, Arbter, Engel, Li and Davis4 Nonetheless, substantial delays in receiving clozapine treatment are commonplace,Reference Howes, Vergunst, Gee, McGuire, Kapur and Taylor5 and these delays are associated with poorer outcomes.Reference Shah, Iwata, Plitman, Brown, Caravaggio and Kim6 Ensuring the right patients have timely access to clozapine is an important therapeutic goal in the management of this disorder.7
Although previous studies have identified clinical indicators of poor outcome in general, there have been relatively few studies investigating risk factors for treatment resistance specifically. The research that has been undertaken indicates that an early age at onset of psychosis, male gender, a longer duration of untreated psychosis and poor premorbid functioning may be associated with treatment resistance.Reference Wimberley, Stovring, Sorensen, Horsdal, MacCabe and Gasse8–Reference Frank, Lang, Witt, Strohmaier, Rujescu and Cichon10 Although one study reported an enrichment of schizophrenia polygenic risk scores (PRS) in clozapine-treated patients,Reference Frank, Lang, Witt, Strohmaier, Rujescu and Cichon10 this has not been replicated in larger subsequent studies.Reference Wimberley, Gasse, Meier, Agerbo, MacCabe and Horsdal11,Reference Martin and Mowry12 Other studies have reported an increased burden of genome-wide rare copy number duplications in treatment-resistant patientsReference Martin and Mowry12 and an excess of rare disruptive variants in gene targets of antipsychotics,Reference Ruderfer, Charney, Readhead, Kidd, Kahler and Kenny13 although both of these studies are yet to be independently replicated. The identification of reliable factors could serve to alert clinicians and help predict those at greater risk of developing treatment resistance when they first present with psychosis. This study aims to gain insights and identify factors measurable at illness onset that could be used predict treatment-resistant psychosis (TRP).
Method
Sample characteristics
Study individuals were from the CardiffCOGS (COGnition in Schizophrenia, n = 1070) sample, which has been previously describedReference Lynham, Hubbard, Tansey, Hamshere, Legge and Owen14,Reference Pardiñas, Holmans, Pocklington, Escott-Price, Ripke and Carrera15 and additional details are provided in Supplementary Methods available at https://doi.org/10.1192/bjp.2019.120. CardiffCOGS is a sample of patients with clinically diagnosed schizophrenia or related psychotic disorders recruited from community, in-patient and voluntary sector mental health services in the UK. Study individuals completed a comprehensive clinical interview based on the Schedules for Clinical Assessment in Neuropsychiatry (SCAN) instrument, donated a blood sample for genetic analysis and consented for access to their clinical case notes. The SCAN interview and clinical case notes were used to arrive at DSM-IV (1994) and ICD-10 (1992) lifetime diagnoses and to complete Operational Criteria Checklist for Psychotic Illness and Affective Illness (OPCRIT) ratings. All study individuals had a diagnosis of schizophrenia or related psychotic disorder (detailed in Supplementary Table 1). We included individuals with related psychotic disorders in addition to those with schizophrenia given that the study focuses on prediction at first presentation and diagnosis can be uncertain at this time. Thus we refer to all samples as either TRP or non-TRP (whether the diagnosis is schizophrenia or a related psychotic disorder) and we undertake sensitivity analyses for all results to examine whether effects are consistent when restricted to those with narrowly defined schizophrenia. The sample shows a degree of enrichment for TRP as a result of targeted recruitment from clozapine clinics (52.4% compared with a TRP prevalence of ~30%). The study had multi-site National Health Service ethics approval granted by South East Wales Research Ethics Committee Panel (reference number: 07/WSE03/110) and written informed consent was obtained for all study participants.
Outcome variable
Study individuals were diagnosed with TRP if they had either been rated negatively for OPCRIT item 89 (‘psychotic symptoms respond to neuroleptics’) or had received clozapine treatment. Individuals were diagnosed with non-TRP if they were rated positively for OPCRIT item 89 and had not received clozapine. OPCRIT item 89 was rated globally over the total period of illness based on interview and clinical notes data and scored positively if the illness appeared to respond to any type of antipsychotic or if relapse occurred when medication was stopped. Individuals were excluded from the analyses (n = 82) if (a) despite failure to respond to antipsychotics, they had not yet received two adequate antipsychotic trials at time of data collection; or (b) there was insufficient information to determine antipsychotic response.
Demographic, premorbid and illness onset clinical factors
To investigate characteristics that could – at illness onset – prospectively indicate a higher risk of TRP, we investigated variables related to demographics and family background, premorbid factors and characteristics related to illness onset. These variables were derived from self-report at interview, clinical case notes and OPCRIT ratings. Full definitions of these variables are given in Supplementary Table 2. The demographic factors assessed included (a) gender; (b) ‘urbanicity’ (city birth and upbringing); (c) family history of schizophrenia; (d) family history of a psychotic disorder, affective disorder or suicide; (e) mother's age at birth and (f) father's age at birth. The premorbid factors investigated included (a) birth complications, (b) complications during their mother's pregnancy, (c) developmental problems, (d) childhood abuse, (e) total years spent in education, (f) highest level of education, (g) premorbid IQ, (h) poor premorbid social adjustment and (i) poor premorbid work adjustment. Features related to the first illness presentation included (a) age at onset of psychosis, (b) duration of untreated psychosis, (c) regular cannabis use in the year before illness onset, (d) regular cigarette smoking in the year before illness onset, (e) a psychosocial stressor in the 6 months before onset of psychosis and (f) the mode of onset of psychosis.
Lifetime characteristics
In a secondary analysis, we investigated differences in post-onset symptom and outcome measures between individuals with TRP and non-TRP. These variables included demographics, lifetime clinical characteristics, clinical symptoms and substance use (full details in Supplementary Table 3).
Genetic liability for schizophrenia
Genotyping and quality control
The CardiffCOGS sample was genotyped on either the Illumina HumanOmniExpressExome-8 or the Illumina HumanOmniExpress-12 array as previously described.Reference Pardiñas, Holmans, Pocklington, Escott-Price, Ripke and Carrera15 After standard quality control procedures, imputation was performed by using IMPUTE2Reference Howie, Donnelly and Marchini16 and the 1000 Genomes (phase 3) and UK10K reference panels.Reference Huang, Howie, McCarthy, Memari, Walter and Min17 Genetic analyses were restricted to those of European ancestry, assessed by principal component analysis, and related individuals with π > 0.2 were identified and one member removed at random. Complete details of genotyping and quality control of the CardiffCOGS sample are provided in a prior publicationReference Pardiñas, Holmans, Pocklington, Escott-Price, Ripke and Carrera15 and Supplementary Methods.
Schizophrenia PRS
PRS were created based on the largest published schizophrenia genome-wide association study meta-analysis,Reference Pardiñas, Holmans, Pocklington, Escott-Price, Ripke and Carrera15 excluding individuals from CardiffCOGS. Scores were calculated following the method described by Wray et al Reference Wray, Lee, Mehta, Vinkhuyzen, Dudbridge and Middeldorp18 (Supplementary Methods). We selected nine P-value thresholds (5 × 10−8, 1 × 10−6, 1 × 10−4, 0.001, 0.01, 0.05, 0.1, 0.2 and 0.5) to compute PRS.
Copy number variation
The identification and quality control of copy number variations (CNVs) in the CardiffCOGS sample has been previously describedReference Rees, Walters, Georgieva, Isles, Chambert and Richards19 and detailed in Supplementary Methods. To compare the enrichment of rare, pathogenic CNVs in TRP with non-TRP, we analysed the presence of an intellectual disability-associated CNV,Reference Coe, Witherspoon, Rosenfeld, van Bon, Vulto-van Silfhout and Bosco20 the presence of a CNV previously associated with schizophreniaReference Rees, Walters, Georgieva, Isles, Chambert and Richards19 and the presence of any chromosomal deletions and duplications spanning 500 kb or 1 Mb in length.
Analysis
We conducted descriptive analyses to compare lifetime illness-related symptoms and outcome measures across TRP and non-TRP cases via univariate logistic regression. To assess the association of clinical predictive factors with TRP, we conducted univariate logistic regressions for each variable, adjusting for age at interview and method of recruitment (defined as recruitment from secondary mental healthcare services such as clinician referral or clozapine clinic, or from other sources such as opportunistic recruitment or via third sector organisations). To control for multiple testing of 21 variables, we applied a Bonferroni correction threshold of P ≤ 2.38 × 10−3.
Multivariate prediction modelling of TRP consisted of two approaches: (a) multivariate logistic regression including variables associated at P < 0.1 from univariate analyses and covarying for age at interview and recruitment method, and (b) a conditional inference random forests model. Machine-learning ensemble methods, such as the conditional inference random forests model, have been shown to have superior performance in detecting independent associations with health outcomes in comparison to logistic regression.Reference Mansiaux and Carrat21 The conditional inference random forest model was implemented via the ‘cforest’ function in the R ‘party’ package,Reference Hothorn, Kornik and Zeileis22 which is recommended for models that have variables of different types and that are correlated (Supplementary Figure 1).Reference Strobl, Hothorn and Zeileis23 The model was fitted based on 4000 trees (selected via grid search) and an unbiased variable selection to control for different variable types. To derive an importance value for each variable, conditional permutation was used to control for any correlated variables. This permuted importance value represents the decrease in classification accuracy after randomly permuting the values of that variable over all trees. The accuracy of the conditional inference forest model was derived using the R ‘caret’ package.Reference Kuhn24 The primary forest model could only be conducted in individuals with no missing data for the 21 variables analysed (n = 337), and so we repeated the analyses in the remaining sample (up to n = 733) for the five variables with the highest importance from the primary analysis. To make a direct comparison between the logistic regression and conditional inference forest model, we conducted a multivariate logistic regression in the 337 individuals with complete data. Rates of missing data for each variable are detailed in Supplementary Table 2. The differences between individuals with and without missing data were assessed via logistic regression and detailed in Supplementary Table 4; there was no difference in gender, age at interview or method of recruitment, but the participants with no missing data were less likely to have TRP (odds ratio 0.65, 95% CI = 0.50–0.84, P = 0.001).
To test the relationship between common variant genetic liability for schizophrenia and TRP, we regressed a model for each PRS created from various training P-value thresholds against a base model including the first five principal components and any additional principal components from the first 20 that were associated (P < 0.05) with TRP. To assess the proportion of variance explained we computed R2 on the liability scale,Reference Lee, Goddard, Wray and Visscher25 based on a TRP lifetime prevalence of 30% in schizophrenia, to account for ascertainment bias. The association of CNVs with TRP was tested via Firth's logistic regression.Reference Wang26
As this sample includes those with schizophrenia and related psychotic disorders, all analyses were replicated to restrict the sample to those with a schizophrenia or schizoaffective disorder, depressed type diagnosis.
Results
A total of 561 (52.4%) individuals included in the study were diagnosed with TRP and 509 (47.6%) with non-TRP. Because of our sampling methodology for this genetic study, the vast majority of participants (96.9%) were of White European ethnicity and a total of 662 (61.9%) were male. Study individuals with TRP had a younger age at interview compared with those with non-TRP (odds ratio 0.98, 95% CI = 0.97–0.99, P = 6.48 × 10−4) and were more likely to have been recruited from secondary mental healthcare services (odds ratio 2.32, 95% CI = 1.76–3.04, P = 1.52 × 10−9). As potential confounders of no experimental interest, both of these factors were included as covariates in subsequent regression analyses.
Lifetime characteristics
In a descriptive comparison of post-onset symptom and outcome measures, we found that individuals with TRP were more severely impaired than those with non-TRP across a range of measures (Supplementary Table 5). Individuals with TRP were significantly more likely to have a continuous course of disorder, poorer cognitive functioning at the time of the interview, a higher number of psychiatric in-patient admissions, a lower Global Assessment Scale (GAS) score, to have deteriorated from their premorbid level of functioning, to have been detained under the Mental Health Act and to have a schizophrenia diagnosis. Furthermore, study individuals with TRP had more severe lifetime positive and negative symptoms. The strengths of the associations were equivalent in analyses restricted to individuals with schizophrenia or schizoaffective depression (Supplementary Table 6).
Demographic, premorbid and illness onset clinical factors
Univariate logistic regression
The association of demographic, premorbid and illness onset clinical factors with TRP are listed in Table 1. Univariate analyses in the total sample (up to n = 1070) found significant associations with TRP for an earlier age at onset of psychosis (odds ratio 0.94, 95% CI = 0.92–0.96, P = 7.79 × 10−13) and poor premorbid social adjustment (odds ratio 1.64, 95% CI = 1.26–2.13, P = 2.41 × 10−4). Lower premorbid IQ, poor premorbid work adjustment and cannabis use in the year before illness onset were associated with TRP at P < 0.05 but did not survive correction for multiple testing.
Table 1 Demographic, premorbid and illness onset predictors of treatment-resistant psychosis

Association of demographic, premorbid and illness onset clinical factors with TRP. Columns represent clinical variables: TRP, non-TRP (reference group), odds ratio, 95% confidence intervals and P-value from univariate logistic regression adjusted for age at interview and method of recruitment, and adjusted multivariate logistic regression. For binary variables, numbers (N) and percentages (%) are provided and mean and s.d. for continuous variables, are provided.
TRP, treatment-resistant psychosis; s.d., standard deviation; CI, confidence interval.
For multivariate analyses * P < 0.05 and for univariate analyses * survive correction for multiple testing.
Multivariate logistic regression
Clinical factors associated at P < 0.1 with TRP from the univariate analysis were included in a multivariate logistic regression (Table 1, n = 621). We found that an earlier age at onset of psychosis (odds ratio 0.95, 95% CI = 0.92–0.97, P = 1.60−5), poor premorbid social adjustment (odds ratio 1.88, 95% CI = 1.27–2.78, P = 1.49 × 10−3), lower premorbid IQ (odds ratio 0.98, 95% CI = 0.96–0.99, P = 7.76 × 10−3), younger father's age at birth (odds ratio 0.97, 95% CI = 0.95–0.99, P = 0.015) and cannabis use in the year before onset of psychosis (odds ratio 1.60, 95% CI = 1.06–2.41, P = 0.025) predicted TRP. The multivariate model explained 16.3% of the variance (Nagelkerke R2 of multivariate model minus that explained by covariates alone) of TRP. These associations remained consistent in regression analyses that restricted the sample to individuals with schizophrenia and schizoaffective depression (Supplementary Table 7).
Conditional inference forest model
A conditional inference forest model was fitted to predict TRP using all 21 clinical factors previously described for 337 individuals with no missing data. The accuracy of the predictive model in this training data-set was 0.59. By using conditional permutation for importance factors (which represents the decrease in classification accuracy if the values of that variable are randomly permuted), we found that a younger age at onset of psychosis was the most important factor in the prediction of TRP, followed by poor premorbid social adjustment, family history of schizophrenia, lower premorbid IQ and poor premorbid work adjustment (Fig. 1). These findings were consistent in a model restricted to individuals with a diagnosis of schizophrenia (Supplementary Figure 2). The importance of these top 5 variables was replicated in 428 individuals that were excluded from the primary forest model analysis on the basis of having 1 or more of the 21 clinical factors missing. Age at onset of psychosis was again the most important factor for TRP prediction, followed by premorbid IQ and poor premorbid social adjustment. A logistic regression model for the 337 individuals included in the primary model is provided in Supplementary Table 8 for a direct comparison between the methods, although the findings from this restricted set are consistent with the primary regression analysis in any case.

Fig. 1 Variable importance plots from conditional inference forests models predicting treatment-resistant psychosis. (a) Permuted importance from primary analysis (n = 337 with complete data for all 21 variables assessed). (b) Permuted importance from replication analysis for top five variables in remaining sample (n = 428).
Genetic liability for schizophrenia
Schizophrenia PRS
Although individuals with TRP had higher schizophrenia PRS on average across the P-value thresholds from the discovery cohort, this difference was only associated at a single threshold of P < 0.001 (Table 2; R2 = 0.011, odds ratio 1.20, 95% CI = 1.03–1.39, P = 0.016). These findings were consistent in analyses restricted to those with schizophrenia or schizoaffective depression (Supplementary Table 9). This study had 80% power to detect an association at P < 0.05 if the correlation between genetic effects on schizophrenia and TRP was 49%.Reference Dudbridge27
Table 2 Association of genetic liability for schizophrenia with treatment-resistant psychosis

Association of genetic liability for schizophrenia with TRP. Columns for schizophrenia polygenic risk scores represent the P-value threshold used in discovery cohort to derive scores, odds ratio and 95% confidence intervals, R2 calculated on the liability scale,Reference Lee, Goddard, Wray and Visscher25 area under the curve, standard error and P-value of association of each score of with TRP. Columns for CNV analysis represent CNVs assessed, frequencies of each CNV in non-TRP (reference group), TRP, odds ratio, 95% confidence intervals and P-value from Firth's logistic regression.
s.e., standard error; TRP, treatment-resistant psychosis; CI, confidence interval; CNV, copy number variation.
CNVs
We found that there was no difference in the burden of rare, pathogenic CNVs previously associated with schizophrenia or intellectual disability in individuals with TRP compared with those with non-TRP (Table 2). Furthermore, we found no enrichment of 500 kb or 1 Mb deletions or duplications in individuals with TRP. These findings were consistent in analyses restricted to those with schizophrenia or schizoaffective depression (Supplementary Table 10). Our sample size had 80% power to detect an odds ratio >1.7 for a burden of CNVs with a frequency of 2.5%Reference Rees, Kendall, Pardiñas, Legge, Pocklington and Escott-Price28 at a significance level of P < 0.05.Reference Purcell, Cherny and Sham29
As genetic liability for schizophrenia (CNVs or PRS) was not associated with TRP, they were not combined in multivariate analyses with clinical factors.
Post hoc analyses related to age at onset of psychosis
Given the significant association of age at onset of psychosis with TRP, we conducted additional exploratory analyses. The association between TRP and age at onset of psychosis was consistent for males (odds ratio 0.95, 95% CI = 0.93–0.97, P = 3 × 10−6) and females (odds ratio 0.93, 95% CI = 0.90–0.95, P = 7.9 × 10−8). To investigate whether clozapine-prescribing practice could be influencing these results, we restricted the analysis to TRP patients who had not received clozapine treatment (n = 87) and found the association between age at onset of psychosis and TRP remained (odds ratio 0.95, 95% CI = 0.92–0.98, P = 5.88 × 10−4).
Age at onset of psychosis alone explained 7.3% of the variance (Nagelkerke R2) of TRP and had an area under the curve of 0.65. Figure 2 displays the relationship between age at onset and the proportion with TRP in our sample (data used given in Supplementary Table 11). Assuming a TRP prevalence of 30%, we found that the positive predictive value for non-TRP was 0.51 for those with an age at onset of less than 16 years. This increased to 0.60, 0.66, 0.73, 0.79 and 0.92 for ages at onset of 16–20, 21–25, 26–30, 31–40, and 41 years and over, respectively (Supplementary Table 12).
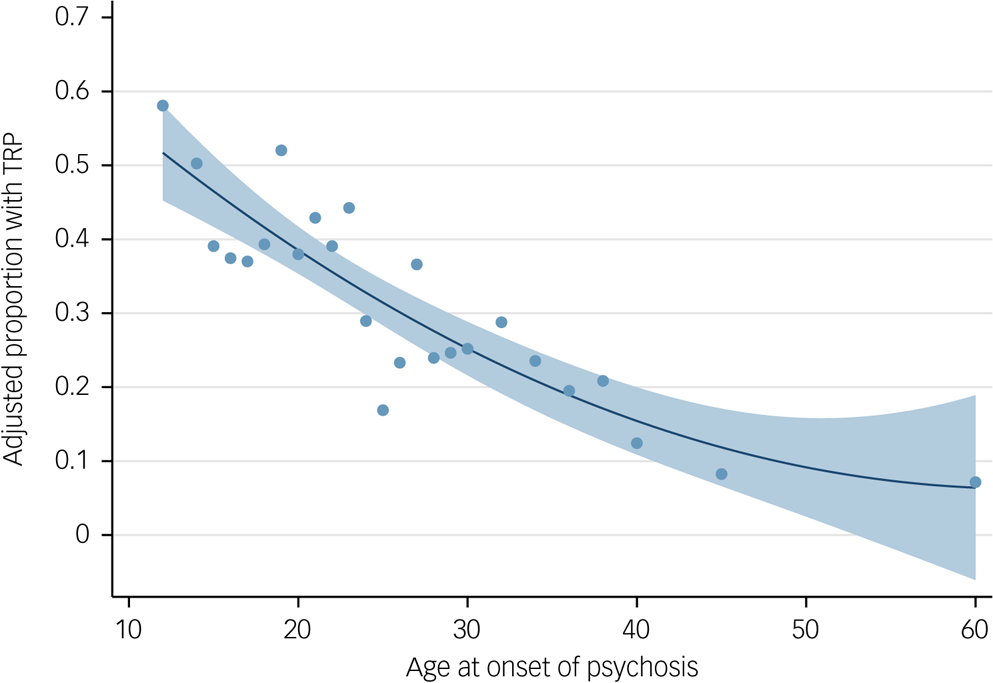
Fig. 2 Quadratic fit plot of proportion of sample that have treatment-resistant psychosis (TRP) by age of onset of psychosis. Blue dots represent proportion of sample with TRP for each age at onset. Shaded area represents 95% confidence intervals. Data used to produce plot are provided in Supplementary Table 11; the minimum number per group was 23 individuals and the mean average number per group was 40 individuals.
We investigated whether the relationship between age at onset of psychosis and TRP could be explained by genetic liability to schizophrenia indicated by PRS and found that schizophrenia PRS were associated with the age at onset of psychosis (Supplementary Tables 13 and 14; at single nucleotide polymorphism [SNP] threshold P < 0.01: R2 = 0.006, Beta = −0.86, 95% CI = −1.62–0.10, P = 0.027). The association between age at onset at psychosis and TRP (odds ratio 0.94, 95% CI = 0.92–0.96, P = 4.60 × 10−11) was not attenuated when conditioning on schizophrenia PRS (odds ratio 0.94, 95% CI = 0.92–0.96, P = 6.38 × 10−11).
Discussion
In this study, we used regression and machine-learning analyses to determine whether genetic liability for schizophrenia and/or clinical characteristics measurable at illness onset can prospectively indicate a higher risk of TRP. We found age of onset of psychosis to be a significant and important clinical indicator in TRP. In addition, we found evidence across models that poor premorbid social functioning and lower premorbid IQ increased the risk of TRP. Genetic liability for schizophrenia indicated by polygenic risk scores and rare, pathogenic CNVs were not associated with TRP and did not explain the relationship with age of onset of psychosis.
Main findings
Age at onset of psychosis, defined as the age at which treatment was first sought or when symptoms first caused significant impairment (if earlier), was the most important indicator of TRP in this study; it was significant in univariate and multivariate regression analyses and had the highest importance value in the conditional inference forest model. These findings were consistent in analyses restricting the sample to individuals with a schizophrenia diagnosis and were not influenced by clozapine-prescribing practice. An early age at onset of psychosis has been identified in several previous studies as a predictor of TRP.Reference Wimberley, Stovring, Sorensen, Horsdal, MacCabe and Gasse8,Reference Kim, Park, Choi, Park, Tchoe and Choi9,Reference Martin and Mowry12 This study provides evidence that the increased risk for TRP is not restricted to those with a very early onset, as suggested by previous studies,Reference Lally, Ajnakina, Di Forti, Trotta, Demjaha and Kolliakou30,Reference Meltzer31 but rather that the risk of TRP continues to reduce throughout adulthood: the positive predictive value for treatment response to standard antipsychotics increased from 0.51 for individuals with an age at onset of below 16 years to 0.92 for those with an age at onset of over 41 years. Given the continued reduction in the risk of TRP throughout adulthood, it seems unlikely that the relationship is explained by differences in provision of health services or related factors across different age groups. Nonetheless, these findings do suggest the importance of engagement and proactive management of patients with an early age at onset of psychosis.
We also found evidence for the role of premorbid factors in TRP. Poor premorbid social functioning – defined as a difficulty entering or maintaining social relationships, isolation or social withdrawal before the onset of psychotic symptoms – was significantly associated with TRP in univariate and multiple regression analyses (odds ratio 1.88, 95% CI = 1.27–2.78) and was rated as important in the conditional inference forest model. This finding is consistent with previous studies that have identified premorbid social functioning as predictive of poor outcomes in schizophrenia and also TRP.Reference Kanahara, Yamanaka, Suzuki, Takase and Iyo32 Furthermore, lower premorbid IQ (estimated from the National Adult Reading Test) was associated with an increased risk of TRP in multivariate regression analysis (odds ratio 0.98, 95% CI = 0.96–0.99) and was identified by the conditional inference forest model as predictive. These premorbid factors have been implicated in other studies of poor outcomes in schizophreniaReference Frank, Lang, Witt, Strohmaier, Rujescu and Cichon10,Reference Carbon and Correll33 and warrant further investigation for their role in treatment resistance. The association of premorbid factors with TRP also suggests that the neurodevelopmental hypothesis of schizophrenia may have relevance to treatment outcomes. The multivariate logistic regression also found some evidence for the role of father's age at birth and cannabis use in the year before onset of psychosis in TRP. However, these factors were not identified in the forest model and thus replication is required to investigate their role in TRP.
In this study we did not find evidence of an association between TRP and several established risk factors for schizophrenia such as male gender, childhood abuse, duration of untreated psychosis, family history of psychosis or urbanicity. A lack of association with these variables has also been reported in other studies of TRPReference Wimberley, Stovring, Sorensen, Horsdal, MacCabe and Gasse8,Reference Martin and Mowry12 and provides support against a psychosis spectrum theory, within which you would expect to find increased rates of risk factors for schizophrenia in TRP. However, in the case of early adversity, a previous study reported a cumulative effect of lifetime adversity in TRP;Reference Hassan and De Luca34 this suggests that although we did not find an independent association with childhood abuse, it is possible that it could be contributing to TRP in a cumulative manner.
We found no evidence for the association of genetic liability for schizophrenia indicated by PRS or rare, pathogenic CNVs with TRP. These findings are consistent with other studies investigating the association of schizophrenia PRS with TRP,Reference Frank, Lang, Witt, Strohmaier, Rujescu and Cichon10–Reference Martin and Mowry12 suggesting that genetic liability to TRP is not strongly influenced by liability to schizophrenia (apart from the requirement to have schizophrenia). However, larger samples are required to provide definitive answers in this regard. Within the power limitations of the study design, our findings add support to other evidenceReference Gillespie, Samanaite, Mill, Egerton and MacCabe3 suggesting that treatment resistance may not be best conceptualised as a form of illness at the severe end of a psychosis spectrum, in which case you would expect a higher genetic loading of generic schizophrenia-associated SNPs in TRP patients. The use of PRS in the personalised prediction of TRP may be better informed by more specific training sets, for example from genetic studies investigating TRP directly, as opposed to PRS derived from those with a broad schizophrenia diagnosis.
Schizophrenia PRS were weakly associated with age at onset of psychosis in this study. However, the relationship between TRP and age at onset of psychosis in this study was not confounded or explained by differences in genetic liability to schizophrenia, suggesting that genetic factors influencing the age at onset of psychosis are distinct from those that increase liability for the disorder and may have particular relevance to treatment resistance.
Lifetime characteristics
Many of our findings regarding lifetime characteristics (e.g. greater impairments with TRP, continuous course of disorder, poorer cognitive function) have been previously documented,Reference Kennedy, Altar, Taylor, Degtiar and Hornberger2 but few studies have looked at so many variables in a single cohort of this size. These findings reinforce the importance of efforts to identify early indicators of TRP and thus improve the ability of clinicians to identify and appropriately treat those with an increased risk of TRP.
Strengths and limitations
A strength of this study is the use of a single large sample consisting of people with schizophrenia and related psychotic disorders, with detailed clinical phenotypes derived from both interview and clinical case notes. Consequently, the outcome variable of TRP is of a high quality, including people confirmed to respond to treatment as controls, which is not the case for many studies in this field. We did not have objective evidence (such as medication serum levels) to confirm treatment resistance, but our TRP definition takes non-adherence into account by relying upon either clinician diagnosis of treatment resistance to prescribe clozapine or on ratings from interview and clinical note review which reported non-adherence. A further strength is the use of both regression and machine-learning approaches. Machine-learning models are increasingly being applied in prediction models for disease and to inform the personalised prevention of disease.Reference Koutsouleris, Kambeitz-Ilankovic, Ruhrmann, Rosen, Ruef and Dwyer35
The primary limitation of this study is the use of retrospective reports for the premorbid and illness-onset variables, although the use of contemporaneous clinical records will have increased the reliability of the key clinical variables such as age at onset of psychosis. The study sample is enriched for individuals with TRP (52% v. estimated 30% prevalence) as a result of targeted recruitment from clozapine clinics. Recruitment strategy was controlled for in regression analyses, which did not alter the results, and all predictive values were corrected for prevalence and thus we do not believe that this enrichment biases the results of the study. We found minor but consistent levels of missing data across clinical variables, which significantly reduced the sample size in multivariate analyses. However, we were able to replicate our findings in these excluded individuals for smaller subsets of associated variables. We were not able to incorporate some factors into the present study that may have affected the likelihood of treatment resistance such as type of individual antipsychotics prescribed and other treatments received. Finally, like many genetic studies our analyses primarily consisted of individuals of White European ethnicity and thus further studies are required to establish the generalisability of these findings to all people with TRP.
Future research and clinical implications
The results in this study indicate that genetic studies investigating TRP directly, rather than a broad schizophrenia diagnosis, will be needed to gain insights into the nature of treatment resistance in schizophrenia and related psychotic disorders. Age at onset of psychosis, poor premorbid social functioning and premorbid IQ may serve as useful indicators, along with other factors, in a predictive algorithm for TRP. From a clinical perspective, the results indicate that patients with these characteristics are less likely to respond to standard antipsychotic treatment and thus require additional monitoring, support and perhaps clozapine treatment should be considered earlier.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2019.120.
Funding
This project was supported by the following grants: Medical Research Council Centre (MR/L010305/1), Programme (G0800509), Project (MR/L011794/1) grants to Cardiff University; and The National Centre for Mental Health, funded by the Welsh Government through Health and Care Research Wales.
Acknowledgements
We thank the participants, clinicians, field team and Medical Research Council Centre for Neuropsychiatric Genetics and Genomics laboratory staff for their help with the CardiffCOGS study. We thank the staff at deCODE Genetics for sample genotyping.







eLetters
No eLetters have been published for this article.