Obesity in children and adolescents is a serious public health problem which is associated with an enhanced risk of chronic diseases such as diabetes, hypertension and cardiovascular failure( Reference Lobstein, Baur and Uauy 1 ). Furthermore, obesity in childhood increases the likelihood of obesity and its associated complications in adulthood( Reference Lobstein, Baur and Uauy 1 , Reference Guo, Wu and Chumlea 2 ). Different methods have been used to identify obesity in childhood and adolescence, the most common being BMI, which is significantly associated with relative fatness in this age group( Reference Lobstein, Baur and Uauy 1 ). On the other hand, the use of other simple measures to evaluate adiposity, such as waist circumference (WC) or waist-to-height ratio (WHtR), has also been suggested( Reference de Koning, Merchant and Pogue 3 , Reference Ashwell and Hsieh 4 ).
Although obesity is a multifactorial disease, dietary intake and physical activity play an important role in the development of this condition in children and adolescents. Evidence suggests that overweight and obese adolescents often have low physical activity levels and excessive or inadequate consumption of specific food groups( Reference Page, Cooper and Stamatakis 5 – Reference Newby 7 ). Although the findings are inconsistent, it has been seen that low-nutrient, energy-dense foods (i.e. fast food, sweets and pastries) and beverages (i.e. soda, juice and soft drinks) are associated with enhanced risk of children and adolescents being overweight or obese( 8 , Reference Fraser, Edwards and Cade 9 ). On the other hand, some studies have identified food groups whose intake has an inverse association with obesity, such as fruits, vegetables, ready-to-eat cereals and dairy products( Reference Spence, Cifelli and Miller 10 – Reference Kelishadi, Pour and Sarraf-Zadegan 12 ). However, the results regarding the role of specified food groups in the development of overweight/obesity remain controversial in paediatric populations.
Socio-economic status (SES) has also been associated with childhood obesity. It has been suggested that in developed countries low SES is associated with increased obesity prevalence, whereas in developing countries the opposite seems to be true( Reference Brisbois, Farmer and McCargar 13 ).
In light of the fact that childhood obesity is a public health problem worldwide, there is a critical need to identify related risk factors by evaluating adolescents’ food group intakes, physical activity levels and SES, as well as the measures of obesity used with this age group. Thus, the aim of the present study was to examine the associations among the consumption of certain food groups, physical activity, socio-economic factors and different measures of obesity in adolescents.
Materials and methods
Sampling
Data for the present cross-sectional study were derived from a school-based study, the Azorean Physical Activity and Health Study II, which aimed to evaluate physical activity, physical fitness, overweight/obesity prevalence, dietary intake, health-related quality of life and other factors in 15- to- 18-year-old adolescents, in 2008. The study was carried out in six of the nine Azorean Islands (São Miguel, Terceira, Faial, Pico, São Jorge and Graciosa), where 95 % of the Azorean population lives( 14 ). The Azorean Archipelago had a population of 246 772 habitants in 2011( 15 ), holds European ancestry and the main economic activities include services, agriculture and fishing. It is classified as one of the outermost territories of the European Union, and consequently is supported by European Union funds for social and economic development( 16 ). Furthermore, the Azores has some unique social, geographical and urban design features that differ from the mainland. All of the islands are of volcanic origin, hosting numerous landscapes of virgin forest and green fields. Most of the urban areas are small and located on the coast.
All participants in the study were informed of its goals, and the parent or guardian of each participant provided written informed consent for his/her child to participate. The study was approved by the Faculty of Sport, University of Porto and the Portuguese Foundation for Science and Technology Ethics Committee; it was conducted in accordance with the World Medical Association's Helsinki Declaration for Human Studies.
The population was selected by means of proportionate stratified random sampling, taking into account location (island) and number of students, by age and sex, in each school. The estimated number of students for representativeness of the adolescent population was 1422, but in order to prevent the collection of incomplete information, data were collected for 1515 adolescents. Some adolescents were not included in our analysis (n 306) as information was missing on their dietary intake (n 286), BMI or WC (n 20). Thus, the final sampling resulted in a total of 1209 participants (503 boys). The adolescents who were excluded from the study did not differ significantly from those who were included regarding age (16·2 (sd 1·0) years v. 16·1 (sd 1·0) years, P = 0·158), parental education (9·1 (sd 4·5) years v. 9·1 (sd 4·4) years, P = 0·890) and gender (girls: 61·1 % v. 58·4 %, boys: 38·9 % v. 41·6 %, P = 0·388). Finally, the sample was weighted in accordance with the distribution of the Azorean population in schools so as to guarantee the real representativeness of each group (by age and gender).
Anthropometric measures
Body height and body weight
Body height and body weight were determined using standard anthropometric methods. Height was measured to the nearest millimetre in bare or stocking feet, with adolescents standing upright against a Holtain portable stadiometer (Crymych, UK). Weight was measured to the nearest 0·10 kg, with participants lightly dressed (in underwear and T shirts), with the use of a portable digital beam scale (Tanita Inner Scan BC 532, Tokyo, Japan).
BMI was calculated using the ratio of weight/height2 (kg/m2). Participants were classified as normal weight, overweight or obese, according to age- and sex-specific cut-off points specified by Cole et al.( Reference Cole, Bellizzi and Flegal 17 , Reference Cole, Flegal and Nicholls 18 ). Underweight participants (2·6 %) were combined with participants in the normal weight category, due to the fact that the former represented a small proportion of the overall sample.
Waist circumference and waist-to-height ratio
For the present study, WC and WHtR were both used as proxy measures of abdominal obesity. WC measurements were taken midway between the tenth rib and the iliac crest and recorded to 0·1 cm. A non-elastic flexible tape measure was used, with participants standing erect – arms by sides, feet together and abdomen relaxed – as well as without clothing covering the waist area. Participants were divided into two categories, <90th percentile (<P90) and ≥90th percentile (≥P90), according to age- and sex-specific cut-off points specified by Moreno et al.( Reference Moreno, Mesana and Gonzalez-Gross 19 ). Participants who had WC ≥ P90 were considered to have abdominal obesity( Reference Zimmet, Alberti and Kaufman 20 ). WHtR was calculated as the ratio between WC (in centimetres) and height (in centimetres). A WHtR cut-off point of 0·500 was used to define abdominal obesity in males and females( Reference Al-Hazzaa, Abahussain and Al-Sobayel 21 – Reference Taylor, Jones and Williams 23 ).
Pubertal stage
To determine pubertal stage (which ranged from 1 to 5), each participant was asked to self-assess his/her stage of development of secondary sexual characteristics. Breast development in girls and genital development in boys was evaluated according to criteria outlined by Tanner and Whitehouse( Reference Tanner and Whitehouse 24 ).
Sociodemographic and lifestyle variables
Participants answered a questionnaire that assessed several sociodemographic and lifestyle variables.
Parental education and employment status
For the present study, mothers’ and fathers’ education and employment status were both used as proxy measures of SES. Participants were divided into three categories, reflecting divisions within the Portuguese educational system: mandatory or less (≤9 school years), secondary (10–12 school years) and college/university (>12 school years). Employment status was divided into four categories, according to the standard Portuguese method of classifying occupations: (i) high employment status (which included armed forces members, representatives of legislative branch offices and agencies, officers, directors and executive officers, and specialists in intellectual and scientific occupations); (ii) medium employment status (which included mid-level technicians and professionals, administrative staff, personal service workers, safety and protective service providers and sellers, and qualified workers in agriculture, fishery and forestry); (iii) low employment status (which included qualified workers in industry and construction, artisans, operators of machinery and equipment, and unqualified workers); and (iv) no relationship with employment (which included pensioners, students and the unemployed)( 25 , 26 ).
Dietary intake
Dietary intake was measured via a self-administered semi-quantitative FFQ, validated for the Portuguese population( 27 ). This semi-quantitative FFQ was designed in accordance with criteria laid out by Willett et al.( Reference Willett 28 ) and adapted to include a variety of typical Portuguese food items. The FFQ was adapted for adolescents by including foods more frequently eaten by this age group( Reference Silva, Rego and Guerra 29 ); the adolescent version covered the previous 12 months and comprised ninety-one food items and beverage categories. For each item, the questionnaire offered nine frequency response options, ranging from ‘never’ to ‘six or more times per day’, and solicited information on standard portion size and seasonality. Any foods not listed in the questionnaire could be listed by participants in a free-response section. Energy and nutritional intakes were estimated with regard to participants’ ratings of the frequency, portion and seasonality of each food and beverage item consumed using the software Food Processor Plus. This program uses nutritional information from the USA that has been adapted for use with typical Portuguese foods and beverages.
For the present study, we defined eleven food groups: (i) dairy (milk, yoghurt and cheese); (ii) milk (whole, semi-skimmed and skimmed); (iii) yoghurt; (iv) cheese; (v) ready-to-eat cereals; (vi) fruits (fresh fruits, including tropical fruits); (vii) vegetables (cabbage, spinach, broccoli, lettuce, peppers, tomatoes, cucumbers, onions, carrots, etc.); (viii) vegetable soup; (ix) sweets and pastries (other biscuits apart from simple ones, croissants, doughnuts, cakes, chocolates, chocolate snacks, quince jam, compote, jelly, honey, sugar, candy); (x) fast food (pizza, hamburgers, mayonnaise, salted snacks); and (xi) sugar-sweetened beverages (soda, juice, fruit juice). Then, participants were categorized using sex-specific tertiles of each food group amount.
Physical activity
Physical activity was assessed via a self-report questionnaire that evaluated leisure-time physical activities( Reference Telama, Yang and Laakso 30 ). This questionnaire has been shown to have good test–retest reliability among Portuguese adolescents (intra-class correlation coefficient: 0·92–0·96)( Reference Mota and Esculcas 31 ). It consists of five questions with four answer choices (each rated on a 4-point scale): (i) ‘Outside school, do you take part in organized sports/physical activities?’; (ii) ‘Outside school, do you take part in non-organized sports/physical activities?’; (iii) ‘Outside school hours, how many times a week do you take part in sports or physical activities for at least 20 min?’; (iv) ‘Outside school hours, how many hours a week do you usually take part in physical activities, so much that you get out of breath or sweat?’; and (v) ‘Do you take part in competitive sports?’ The maximum number of points possible was 20. A physical activity index was obtained for each participant by totalling his/her points, which corresponded to activity level rankings that ranged from ‘sedentary’ to ‘vigorous’. Participants whose physical activity indices were greater than 10 points were classified as ‘active’, while those whose physical activity indices were 10 points or less were classified as ‘low-active’( Reference Mota and Esculcas 31 , Reference Raitakari, Porkka and Taimela 32 ).
Statistical analysis
The information concerning boys and girls was analysed separately. The Kolmogorov–Smirnov test was used to verify the variables’ normality. The independent-samples t test or the Mann–Whitney test was performed to compare continuous variables between groups, while the χ 2 test was used for categorical variables. In this report descriptive analysis is presented in terms of means and standard deviations, unless otherwise stated.
A univariate logistic regression model was used to verify the relationship among overweight/obesity or abdominal obesity (WC ≥ P90 or WHtR ≥ 0·500) and each food group consumption, SES and physical activity (Model 1). Variables from the univariate analysis with P ≤ 0·25 were considered potential independent variables and entered into the logistic regression model as candidate variables for inclusion( Reference Hosmer and Lemeshow 33 , Reference Mu, Edwards and Horan 34 ). Then we used a conditional stepwise logistic regression model to identify significant variables associated with overweight/obesity or abdominal obesity. A cut-off value of P < 0·05 was used to include the variables in the multivariate model. The final model was adjusted for age (in years), pubertal stage, energy intake (in kJ/kcal) and dietary fibre (in g/4184 kJ (1000 kcal); Model 2). Age, energy intake and dietary fibre were entered as continuous variables. Furthermore, we adjusted the final logistical model for under-reporting of energy intake, which was estimated using the ratio between reported energy intake and predicted BMR (EI:BMR)( Reference Goldberg, Black and Jebb 35 – Reference Black 37 ). The thresholds that defined low-energy reporters (under-reporters) were EI:BMR ≤1·70 and ≤1·71 for girls and boys between 15 and 17 years old and EI:BMR ≤1·67 and ≤1·81 for girls and boys aged 18 years. ‘Low-energy reporter’ (a categorical variable) was included in the final model as a confounding factor.
Odds ratios and 95 % confidence intervals were computed for overweight/obesity and abdominal obesity (WC ≥ P90 or WHtR ≥ 0·500), according to food group intakes, physical activity and SES. A P value of <0·05 was regarded as significant. All analyses were performed using the statistical software package IBM SPSS Statistics Version 20.
Results
Descriptive characteristics of the adolescents in the sample, according to their BMI, WC and WHtR status, are shown in Tables 1 and 2. Within both genders, adolescents who were classified as overweight/obese or abdominally obese presented higher weight, BMI, WC and WHtR values (P < 0·001, for all). Girls with abdominal obesity had a higher proportion of fathers with low education level and were more likely to have two parents with low employment status (P < 0·05, for all). Concerning physical activity, boys with abdominal obesity were less active than their lean counterparts (P < 0·05, for all). Concerning girls, no significant differences were seen in physical activity between the groups, regardless of their BMI, WC or WHtR status. Our data showed that 16·7 % (boys: 17·1 % v. girls: 19·3 %) of adolescents reported being in Tanner stage 3 or lower, while 59·1 % reported being in stage 4 (boys: 59·0 % v. girls: 59·1 %) and 24·2 % in stage 5 (boys: 21·7 % v. girls: 27·8 %).
Table 1 Characteristics of the boys’ sample, according to BMI, waist circumference and waist-to-height ratio status: adolescent boys aged 15–18 years, Azorean Archipelago, Portugal, 2008
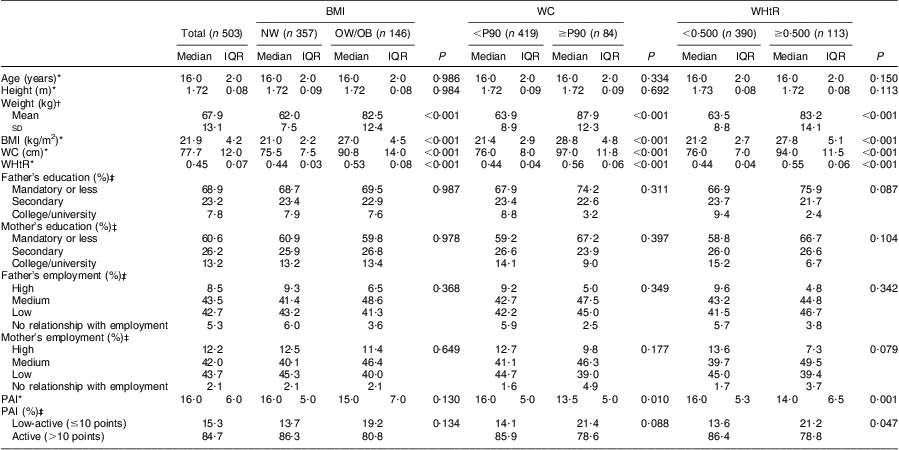
WC, waist circumference; WHtR, waist-to-height ratio; NW, normal weight; OW/OB, overweight and obese; P90, 90th percentile; IQR, interquartile range; PAI, physical activity index.
*Analysis by Mann–Whitney's test for continuous variables.
†Analysis by t test for continuous variables.
‡Analysis by χ 2 test for categorical variables.
Table 2 Characteristics of the girls’ sample, according to BMI, waist circumference and waist-to-height ratio status: adolescent girls aged 15–18 years, Azorean Archipelago, Portugal, 2008
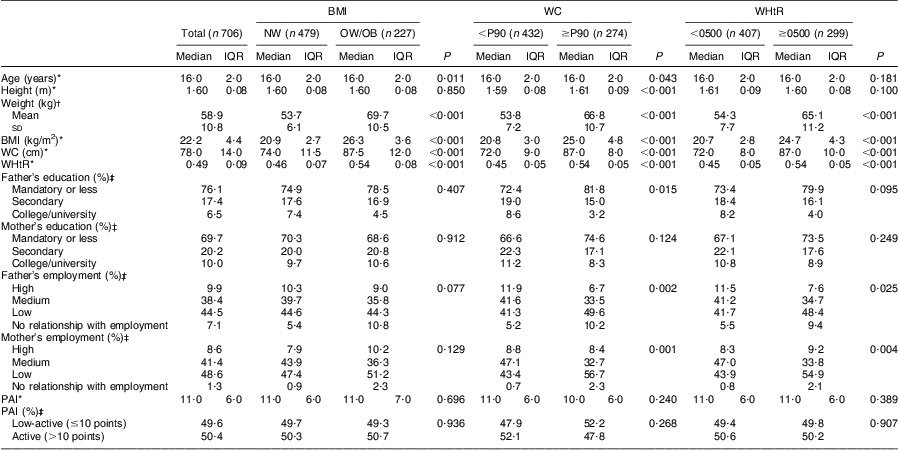
WC, waist circumference; WHtR, waist-to-height ratio; NW, normal weight; OW/OB, overweight and obese; P90, 90th percentile; IQR, interquartile range; PAI, physical activity index.
*Analysis by Mann–Whitney's test for continuous variables.
†Analysis by t test for continuous variables.
‡Analysis by χ 2 test for categorical variables.
The adolescents’ nutritional and dietary characteristics, and their food group consumption, according to BMI, WC and WHtR status, are presented in Tables 3 and 4. In both genders, adolescents who were overweight/obese or abdominally obese had lower intakes of ready-to-eat cereals, sweets and pastries (P < 0·05, for all). Overweight/obese or abdominally obese boys had also lower intakes of vegetables (P < 0·05, for all). Girls who were overweight/obese or abdominally obese had lower energy and sugar-sweetened beverage intakes (P < 0·05, for all). Lower dairy and milk consumption was seen in girls with abdominal obesity, compared with those classified as without abdominal obesity (P < 0·05, for all). In both genders there was no significant difference across groups concerning yoghurt and fruit intakes.
Table 3 Dietary and nutritional characteristics of the boys’ sample, according to BMI, waist circumference and waist-to-height ratio status: adolescent boys aged 15–18 years, Azorean Archipelago, Portugal, 2008

WC, waist circumference; WHtR, waist-to-height ratio; NW, normal weight; OW/OB, overweight and obese; P90, 90th percentile; IQR, interquartile range; RTEC, ready-to-eat cereals; SSB, sugar-sweetened beverages.
*Analysis by Mann–Whitney's test.
†Analysis by Student's t test.
‡Analysis by χ 2 test for categorical variables.
Dairy: tertile 1, ≤297·57 g/d; tertile 2, 297·58–676·43 g/d; tertile 3, ≥676·44 g/d. Milk: tertile 1, ≤244·00 g/d; tertile 2, 244·01–610·00 g/d; tertile 3, ≥610·01 g/d. Yoghurt: tertile 1, ≤17·85 g/d; tertile 2, 17·86–98·21 g/d; tertile 3, ≥98·22 g/d. Cheese: tertile 1, ≤4·28 g/d; tertile 2, 4·29–23·57 g/d; tertile 3, ≥23·58 g/d. RTEC: tertile 1, ≤17·14 g/d; tertile 2, 17·15–40·00 g/d; tertile 3, ≥40·01 g/d. Fruits: tertile 1, ≤127·09 g/d; tertile 2, 127·10–301·93 g/d; tertile 3, ≥301·94 g/d. Vegetables: tertile 1, ≤38·99 g/d; tertile 2, 39·00–113·91 g/d; tertile 3, ≥113·92 g/d. Vegetable soup: tertile 1, ≤42·12 g/d; tertile 2, 42·13–126·43 g/d; tertile 3, ≥126·44 g/d. Sweets and pastries: tertile 1, ≤28·26 g/d; tertile 2, 28·27–66·54 g/d; tertile 3, ≥66·55 g/d. Fast food: tertile 1, ≤31·62 g/d; tertile 2, 31·63–66·47 g/d; tertile 3, ≥66·48 g/d. SSB: tertile 1, ≤158·84 ml/d; tertile 2, 158·85–418·26 ml/d; tertile 3, ≥418·29 ml/d.
Table 4 Dietary and nutritional characteristics of the girls’ sample, according to BMI, waist circumference and waist-to-height ratio status: adolescent girls aged 15–18 years, Azorean Archipelago, Portugal, 2008
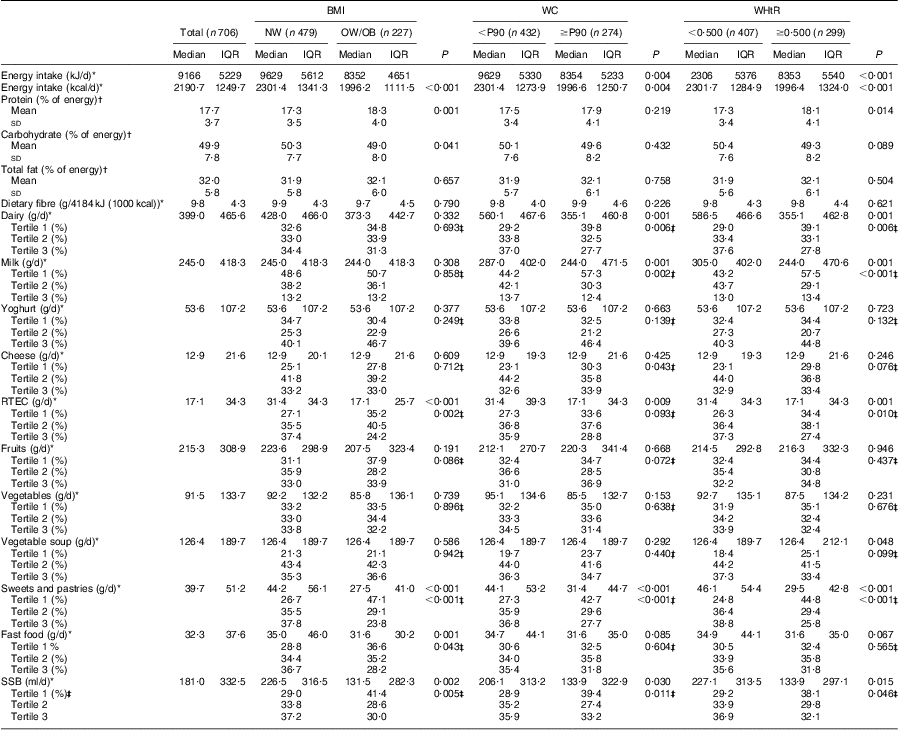
WC, waist circumference; WHtR, waist-to-height ratio; NW, normal weight; OW/OB, overweight and obese; P90, 90th percentile; IQR, interquartile range; RTEC, ready-to-eat cereals; SSB, sugar-sweetened beverages.
*Analysis by Mann–Whitney's test.
†Analysis by Student's t test.
‡Analysis by χ 2 test for categorical variables.
Dairy: tertile 1, ≤293·92 g/d; tertile 2, 293·93–667·66 g/d; tertile 3, ≥667·67 g/d. Milk: tertile 1, ≤244·00 g/d; tertile 2, 244·01–610·00 g/d; tertile 3, ≥610·01 g/d. Yoghurt: tertile 1,: ≤22·37 g/d; tertile 2, 22·38–98·21 g/d; tertile 3, ≥98·22 g/d. Cheese: tertile 1, ≤4·28 g/d; tertile 2, 4·29–13·00 g/d; tertile 3, ≥13·01 g/d. RTEC: tertile 1, ≤17·14 g/d; tertile 2, 17·15–31·43 g/d; tertile 3, ≥31·44 g/d. Fruits: tertile 1, ≤141·05 g/d; tertile 2, 141·06–321·30 g/d; tertile 3, ≥321·31 g/d. Vegetables: tertile 1, ≤54·25 g/d; tertile 2, 54·26–130·01 g/d; tertile 3, ≥130·02 g/d. Vegetable soup: tertile 1, ≤42·12 g/d; tertile 2, 42·13–231·78 g/d; tertile 3, ≥231·79 g/d. Sweets and pastries: tertile 1, ≤24·76 g/d; tertile 2, 24·77–56·62 g/d; tertile 3, ≥56·63 g/d. Fast food: tertile 1, ≤31·01 g/d; tertile 2, 31·02–49·45 g/d; tertile 3, ≥49·46 g/d. SSB: tertile 1, ≤94·24 ml/d; tertile 2, 94·25–300·42 ml/d; tertile 3, ≥300·43 ml/d.
Univariate associations of overweight/obesity and abdominal obesity (WC ≥ P90 or WHtR ≥ 0·500), according to consumption of the eleven food groups measured, physical activity and SES, are shown in Table 5. The variables that remained in the model after the conditional stepwise method are presented in Table 6. After adjustment, in boys, higher intake of ready-to-eat cereals was a negative predictor and higher intake of vegetables was a positive predictor of overweight/obesity and abdominal obesity. Active boys had lower odds of abdominal obesity (WHtR ≥ 0·500) compared with inactive boys (OR = 0·454; 95 % CI 0·234, 0·880). Boys whose mother had a low education level had higher odds of abdominal obesity (WHtR ≥ 0·500) compared with boys whose mother presented a high education level (secondary: OR = 3·054; 95 % CI 1·085, 8·596; mandatory or less: OR = 3·172; 95 % CI 1·200, 8·382). In girls, higher intake of sweets and pastries was a negative predictor of overweight/obesity and abdominal obesity. Girls in tertile 2 of milk intake had lower odds of abdominal obesity (WC ≥ P90 and WHtR ≥ 0·500) than those in tertile 1. Girls whose father had no relationship with employment had higher odds of abdominal obesity (defined by WC ≥ P90) compared with girls whose father presented a high employment status (OR = 2·672; 95 % CI 1·062, 6·726).
Table 5 Univariate associations between overweight/obesity and abdominal obesity (WC ≥ P90 or WHtR ≥ 0·500) according to food group intakes, physical activity and socio-economic status: adolescent boys and girls aged 15–18 years, Azorean Archipelago, Portugal, 2008
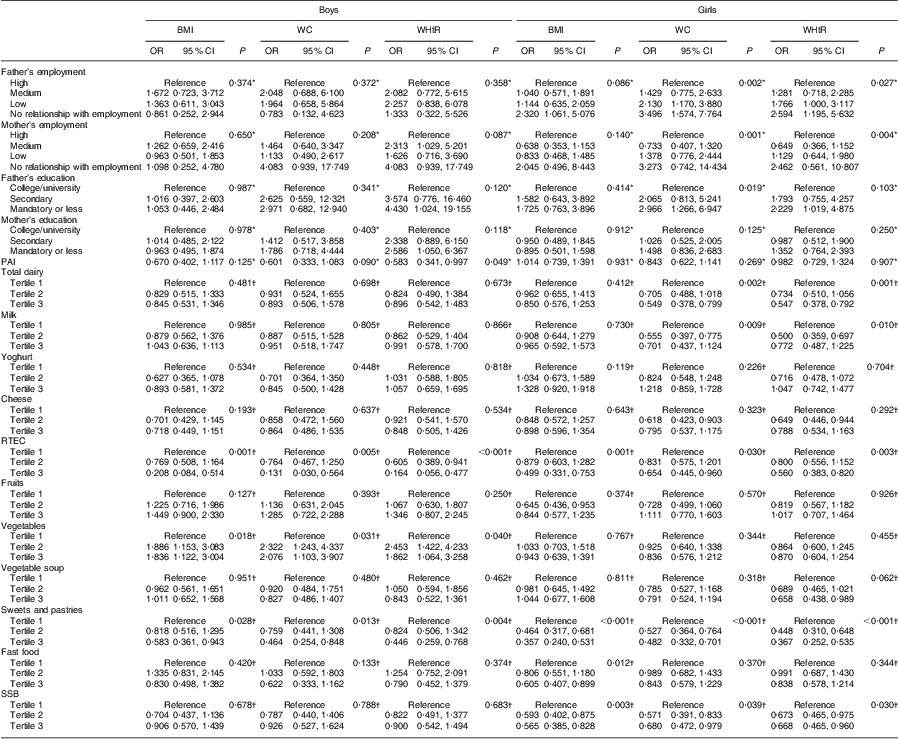
WC, waist circumference; P90, 90th percentile; WHtR, waist-to-height ratio; PAI, physical activity index; RTEC, ready-to-eat cereals; SSB, sugar-sweetened beverages.
Each variable enters in the model separately; the reference group in all food group items is tertile 1 while for PAI the reference is low activity.
*P value for heterogeneity.
†P value for trend.
Boys. Dairy: tertile 1, ≤297·57 g/d; tertile 2, 297·58–676·43 g/d; tertile 3, ≥676·44 g/d. Milk: tertile 1, ≤244·00 g/d; tertile 2, 244·01–610·00 g/d; tertile 3, ≥610·01 g/d. Yoghurt: tertile 1, ≤17·85 g/d; tertile 2, 17·86–98·21 g/d; tertile 3, ≥98·22 g/d. Cheese: tertile 1, ≤4·28 g/d; tertile 2, 4·29–23·57 g/d; tertile 3, ≥23·58 g/d. RTEC: tertile 1, ≤17·14 g/d; tertile 2, 17·15–40·00 g/d; tertile 3, ≥40·01 g/d. Fruits: tertile 1, ≤127·09 g/d; tertile 2, 127·10–301·93 g/d; tertile 3, ≥301·94 g/d. Vegetables: tertile 1, ≤38·99 g/d; tertile 2, 39·00–113·91 g/d; tertile 3, ≥113·92 g/d. Vegetable soup: tertile 1, ≤42·12 g/d; tertile 2, 42·13–126·43 g/d; tertile 3, ≥126·44 g/d. Sweets and pastries: tertile 1, ≤28·26 g/d; tertile 2, 28·27–66·54 g/d; tertile 3, ≥66·55 g/d. Fast food: tertile 1, ≤31·62 g/d; tertile 2, 31·63–66·47 g/d; tertile 3, ≥66·48 g/d. SSB: tertile 1, ≤158·84 ml/d; tertile 2, 158·85–418·26 ml/d; tertile 3, ≥418·29 ml/d.
Girls. Dairy: tertile 1, ≤293·92 g/d; tertile 2, 293·93–667·66 g/d; tertile 3, ≥667·67 g/d. Milk: tertile 1, ≤244·00 g/d; tertile 2, 244·01–610·00 g/d; tertile 3, ≥610·01 g/d. Yoghurt: tertile 1, ≤22·37 g/d; tertile 2, 22·38–98·21 g/d; tertile 3, ≥98·22 g/d. Cheese: tertile 1, ≤4·28 g/d; tertile 2, 4·29–13·00 g/d; tertile 3, ≥13·01 g/d. RTEC: tertile 1, ≤17·14 g/d; tertile 2, 17·15–31·43 g/d; tertile 3, ≥31·44 g/d. Fruits: tertile 1, ≤141·05 g/d; tertile 2, 141·06–321·30 g/d; tertile 3, ≥321·31 g/d. Vegetables: tertile 1, ≤54·25 g/d; tertile 2, 54·26–130·01 g/d; tertile 3, ≥130·02 g/d. Vegetable soup: tertile 1, ≤42·12 g/d; tertile 2, 42·13–231·78 g/d; tertile 3, ≥231·79 g/d. Sweets and pastries: tertile 1, ≤24·76 g/d; tertile 2, 24·77–56·62 g/d; tertile 3, ≥56·63 g/d. Fast food: tertile 1, ≤31·01 g/d; tertile 2, 31·02–49·45 g/d; tertile 3, ≥49·46 g/d. SSB: tertile 1, ≤94·24 ml/d; tertile 2, 94·25–300·42 ml/d; tertile 3, ≥300·43 ml/d.
Table 6 Adjusted odds ratios for overweight/obesity and abdominal obesity (WC ≥ P90 or WHtR ≥ 0·500) according to food group intakes, physical activity and socio-economic status that remained after the conditional stepwise method: adolescent boys and girls aged 15–18 years, Azorean Archipelago, Portugal, 2008

WC, waist circumference; P90, 90th percentile; WHtR, waist-to-height ratio; PAI, physical activity index; RTEC, ready-to-eat cereals.
Odds ratios adjusted for age, maturation, total energy intake (kJ/kcal), low-energy reporters and dietary fibre (g/4184 kJ (1000 kcal)). For total dairy products, milk, yoghurt and cheese do not enter in the model. Significant results are shown in bold font.
*P value for heterogeneity.
†P value for trend.
Boys. RTEC: tertile 1, ≤17·14 g/d; tertile 2, 17·15–40·00 g/d; tertile 3, ≥40·01 g/d. Vegetables: tertile 1, ≤38·99 g/d; tertile 2, 39·00–113·91 g/d; tertile 3, ≥113·92 g/d. Sweets and pastries: tertile 1, ≤28·26 g/d; tertile 2, 28·27–66·54 g/d; tertile 3, ≥66·55 g/d.
Girls. Dairy: tertile 1, ≤293·92 g/d; tertile 2, 293·93–667·66 g/d; tertile 3, ≥667·67 g/d. Milk: tertile 1, ≤244·00 g/d; tertile 2, 244·01–610·00 g/d; tertile 3, ≥610·01 g/d. RTEC: tertile 1, ≤17·14 g/d; tertile 2, 17·15–31·43 g/d; tertile 3, ≥31·44 g/d. Sweets and pastries: tertile 1, ≤24·76 g/d; tertile 2, 24·77–56·62 g/d; tertile 3, ≥56·63 g/d.
Discussion
The present study explored the relationship among food group intakes, physical activity and SES and obesity, as well as different measures of abdominal obesity (i.e. WC and WHtR). After adjustments the results suggested that the intake of ready-to-eat cereals in boys, and intakes of milk and sweets and pastries in girls, were negative predictors of overweight/obesity or abdominal obesity. It was also seen that higher vegetable intake was a positive predictor of overweight/obesity and abdominal obesity only in boys. On the other hand, physical activity seems to be negatively associated with abdominal obesity in boys. In addition, mother's education level in boys and father's employment status in girls were positive predictors of abdominal obesity.
Few studies have examined the relationship between specific types of dairy intake and measures of abdominal obesity in children and adolescents. Data from the Third National Health and Nutrition Examination Survey showed that mean dairy intake was inversely associated with central obesity in adolescents( Reference Bradlee, Singer and Qureshi 38 ). In a cross-sectional study with children aged 10 years, increased milk consumption was also associated with lower WC, whereas no significant association was seen with BMI( Reference Hirschler, Oestreicher and Beccaria 39 ), as in our study. It has been described that BMI is the most convenient way of indirectly measuring body fat( Reference Lobstein, Baur and Uauy 1 ). However, the same BMI percentile does not represent the same percentage body fatness at different ages for boys and girls( Reference Flegal and Ogden 40 ), and this may contribute to the specific difference in the findings. In the present study we found a significant inverse association between milk intake and abdominal obesity only in girls. Evidence suggests that gender may influence body composition, with girls having greater body fat( Reference Cowell, Briody and Lloyd-Jones 41 , Reference Taylor, Jones and Williams 42 ), and it is possible that the interaction between milk (and its components) and body fat may differ across different body fat thresholds( Reference Moreira, Padez and Mourao 43 ). In accordance with this, Vergnaud et al.( Reference Vergnaud, Peneau and Chat-Yung 44 ) reported that milk and yoghurt intakes were protective against 6-year changes in body weight only in adults who were initially overweight. Furthermore, although in our study no inverse association was found between the higher and lower tertile, higher consumption of milk did not increase the probability of being obese, as other studies had reported( Reference Huh, Rifas-Shiman and Rich-Edwards 45 , Reference Noel, Ness and Northstone 46 ). A number of possible explanations have been suggested for the protective effect of milk intake on obesity. Milk is an important source of Ca, which appears to play a significant role in the regulation of energy metabolism by reducing the levels of lipogenesis in adipocytes and increasing both faecal fat excretion and fat oxidation( Reference Dougkas, Reynolds and Givens 47 ). Moreover, milk proteins, especially whey proteins, have been positively associated with satiety( Reference Dougkas, Reynolds and Givens 47 ). On the other hand, milk compounds may also be involved in body fat distribution. It has described that visceral adipose tissue has greater amounts of 11-β-hydroxysteroid dehydrogenase type 1( Reference Morris and Zemel 48 ), which is over-expressed in vitro in those with central adiposity( Reference Masuzaki, Paterson and Shinyama 49 ). It has been suggested that a high-Ca and high-dairy diet down-regulates 11-β-hydroxysteroid dehydrogenase type 1 expression and decreases the concentration of glucocorticoid, which consequently decreases the size of adipose fat deposits( Reference Morris and Zemel 48 ).
Concerning ready-to-eat cereal intake, our results are in accordance with evidence suggesting that ready-to-eat cereal consumption protects against childhood obesity. Kafatos et al.( Reference Kafatos, Linardakis and Bertsias 11 ) found that adolescents who are daily consumers of ready-to-eat cereals had lower mean BMI, WC and WHtR values, compared with non-consumers and occasional consumers. Other cross-sectional( Reference Kosti, Panagiotakos and Zampelas 50 – Reference Albertson, Anderson and Crockett 52 ) and prospective studies( Reference Albertson, Thompson and Franko 53 , Reference Barton, Eldridge and Thompson 54 ) have shown similar results. According to the latter studies, the ‘antiobesity’ effect of ready-to-eat cereals may be due to the association of these cereals with higher breakfast consumption and milk and dietary fibre intake, which have been associated with lower risk of obesity.
It has been suggested that SES in childhood may influence health behaviours and, consequently, the predisposition to obesity( Reference Hardy, Wadsworth and Kuh 55 ). In the present study SES was assessed by measuring parental education and employment. After adjustment, only mother's education level in boys and father's employment status in girls remained significant predictors of abdominal obesity. A recent study with data from different countries found that higher maternal education was associated with more favourable growth in young children; that is, lower obesity and overweight in the UK and Sweden, and lower stunting and underweight in rural China( Reference Lakshman, Zhang and Koch 56 ). Likewise, Koupil and Toivanen( Reference Koupil and Toivanen 57 ) reported in a sample of 18-year-old Swedish men that prevalence of overweight and obesity decreased with higher maternal education. On the other hand, as recently proposed in a review, father's employment is also a probable early marker of the development of obesity in adulthood( Reference Brisbois, Farmer and McCargar 13 ). In particular, the employment of fathers in ‘low status’, ‘blue collar’, ‘unskilled’ and ‘manual’ jobs is classically associated with increased risk of being obese as an adult( Reference Brisbois, Farmer and McCargar 13 ).
In our sample, boys with abdominal obesity were less active than their lean counterparts and physical activity seemed to be a negative predictor of abdominal obesity. Indeed, evidence suggests that low physical activity levels may play a role in the development of abdominal obesity in youth( Reference Kim and Lee 58 ). A cross-sectional study with adolescents showed that WC was inversely associated with structured physical activity (outside school, >140 min/week), independently of time spent on sedentary activities( Reference Klein-Platat, Oujaa and Wagner 59 ). Likewise, Ortega et al.( Reference Ortega, Ruiz and Sjostrom 60 ) found that children and adolescents with low levels of vigorous physical activity had higher odds of having high WC, when compared with those with high levels of vigorous physical activity.
Overweight and obese adolescents, as well as adolescents with abdominal obesity, presented lower intake of sweets and pastries. In addition, vegetable intake was positively associated with abdominal obesity in boys, whereas sweets and pastries intake was negatively associated with the same in girls. There is no evidence that a high intake of vegetables is associated with higher risk of obesity in children and adolescents. It is described that the wide variability in methods of cooking and preparing vegetables may contribute to differences in energy density and macronutrient composition, which may modify the effects of vegetables on body weight( Reference Matthews, Wien and Sabate 61 , Reference Tohill, Seymour and Serdula 62 ). On one hand, these findings could be related to the effect described earlier, that obese adolescents under-report their food intake more than their lean counterparts( Reference Bandini, Schoeller and Cyr 63 , Reference Araujo, Severo and Lopes 64 ). Furthermore, foods that are more socially desirable and approved may be overestimated, and the opposite may also occur( Reference Collins, Watson and Burrows 65 ). On the other hand, these results may also be due to the cross-sectional design of the present study, which might have distorted the temporal relationship between diet and weight. Adolescents who are overweight and obese probably decrease their intake of highly energy-dense foods, in order to lose weight( Reference Araujo, Severo and Lopes 64 ). For instance, girls are more likely to avoid high sugar and fat intakes since they usually demonstrate a greater concern for their body image and weight control than boys( Reference Sweeting and West 66 ).
In the current study, various measures of obesity had differential associations with food group intakes, physical activity and SES. Therefore, it may be important to use different measures of obesity, because the association of each measurement with health risks seems to be distinct. BMI is correlated with body fat, which, in excessive amounts, is related to metabolic complications, but BMI is also associated with lean mass, which may lead to some misclassification( Reference Maynard, Wisemandle and Roche 67 ). There is evidence that WC is associated with visceral adipose tissue and is an independent risk factor for insulin resistance, hyperinsulinaemia, dyslipidaemia and hypertension in youth( Reference Taylor, Jones and Williams 23 , Reference Bacha, Saad and Gungor 68 , Reference Wajchenberg 69 ). On the other hand, WHtR has been shown to be superior in its ability to predict CVD risk factors, compared with either BMI or percentage body fat, in children( Reference McCarthy and Ashwell 22 , Reference Savva, Tornaritis and Savva 70 , Reference Hara, Saitou and Iwata 71 ).
Some limitations of the present study should be acknowledged. As in every cross-sectional study, conclusions related to cause and effect cannot be drawn. The abdominal obesity measures used in the study are indirect estimates of visceral fat, and there are some sophisticated methods of accurately measuring visceral fat, such as MRI and dual-energy X-ray absorptiometric densitometry. However, such techniques cannot feasibly be applied in large epidemiological studies due to their complexity, time-consuming nature and expense. Finally, with self-reported physical activity and dietary intake data, one cannot rule out some reporting bias, although both questionnaires have been tested previously( 27 , Reference Mota and Esculcas 31 ).
Conclusion
We found that ready-to-eat cereals in boys and milk in girls were negative predictors of overweight/obesity or abdominal obesity. It was also seen physical activity seems to be negatively associated with abdominal obesity in boys. Moreover, mother's education level in boys and father's employment status in girls were positive predictors of abdominal obesity. In addition, we also reported that different measures of obesity have distinct associations with food group intakes and SES. Thus, prospective and randomized clinical investigations are needed to examine the roles of food group intakes, physical activity and SES in the development of obesity, as assessed by different measures.
Acknowledgements
Sources of funding: This study was supported by Fundação para a Ciência e Tecnologia (FCT) – Ministério da Ciência, Tecnologia e Ensino Superior (MCTES; grant numbers BPD/65180/2009, BD/44422/2008, PEst-OE/SAU/UI0617/2011 and SFRH/BPD/50002/2009) and by the Azorean Government. All funders had no role in the design, analysis or writing of this article. Conflicts of interest: The authors declare no conflicts of interest. Authors’ contributions: S.A., R.S. and P.M. made substantial contributions to conception and design of data. S.A. and R.S. carried out the data collection, statistical analysis, interpretation of the data, and wrote the manuscript. C.A., P.C.S. and J.M. were involved in drafting the manuscript. All authors read and approved the final manuscript.








