Introduction
Rhabdias Stiles and Hassall, 1905 are lung-dwelling parasites of amphibians and some reptiles (Kuzmin et al. Reference Kuzmin, Tkach and Brooks2007, Reference Kuzmin, Vasconcelos Melo, Filho HF da and Nascimento dos Santos2016), distributed worldwide, except for Antarctica (Kuzmin et al. Reference Kuzmin, Tkach and Snyder2003, Reference Kuzmin, du Preez, Nel and Svitin2022). The genus is composed of 93 species, with 23 found parasitising Neotropical hosts (Marcaida et al. Reference Marcaida, Nakao, Fukutani, Nishikawa and Urabe2022; Müller et al. Reference Müller, Morais, da Costa, de Vasconcelos Melo, Giese, Ávila and da Silva2023; Alcantara et al. Reference Alcantara, Müller, Úngari, Ferreira-Silva, Emmerich, Giese, Morais, Santos, O’Dwyer and Silva2023). In Brazil, 13 species of Rhabdias have been reported (da Silva et al. Reference de Cássia Silva do Nascimento, Gonçalves, de Vasconcelos Melo, Giese, Furtado and dos Santos2013; Alcantara et al. Reference Alcantara, Müller, Úngari, Ferreira-Silva, Emmerich, Giese, Morais, Santos, O’Dwyer and Silva2023), which is a low number when compared with the diversity of known anurans for the region (Santos-Pereira et al. Reference Santos-Pereira, Pombal and Rocha2018).
Rhabdias species are very similar morphologically, and most species’ descriptions are based mainly on morphological characteristics. Thus, the current identification and delimitation of species is still an issue, making taxonomic resolution difficult in most species inventories and ecological studies (Kuzmin Reference Kuzmin2013; Tavares-Costa et al. Reference Tavares-Costa, Rebêlo, Müller, Jesus, Nandyara, Silva, Costa-Campos, dos Santos and Melo FT de2022). However, different approaches, combining morphological and molecular analyses, have contributed to the recognition and identification of new species (Langford & Janovy Reference Langford and Janovy2013; Tkach et al. Reference Tkach, Kuzmin and Snyder2014; Müller et al. Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and da Silva2018). These studies point to the taxonomy of Rhabdias lungworms as a rising venue for research that may preclude understanding of the diversification and phylogeography of such a fascinating group of organisms.
Proceratophrys boiei Wied-Neuwied, 1824 is a medium-sized, nocturnal, and terrestrial anuran, found in the leaf litter of primary and secondary forests and also in degraded areas (Prado & Pombal Jr. Reference Prado and Pombal2008). This species is endemic in the Brazilian Atlantic Forest, occurring from southern Espírito Santo, southern and western Rio de Janeiro state into south São Paulo, and eastern Paraná to eastern Santa Catarina (Frost Reference Frost2024). Until now, six species of helminth parasites are known for Proceratophrys boiei: Aplectana delirae (Fabio, 1971); Cosmocerca parva Travassos 1925; Oxyascaris oxyascaris Travassos, 1920; Physaloptera sp.; Centrorhynchidae gen. sp.; and Physalopteridae gen. sp.1 (Klaion et al. Reference Klaion, Almeida-gomes, Tavares, Rocha and Sluys2011; Campião et al. Reference Campião, Morais, Dias, Aguiar, Toledo, Tavares and Da Silva2014; Euclydes et al. Reference Euclydes, De La Torre, Dudczak, Melo FT de and Campião2022).
Euclydes et al. (Reference Euclydes, Dudczak and Campião2021) reported a species of Rhabdias parasitising P. boiei that did not correspond to any known congeneric species. Thus, based on this material, we describe a new species of Rhabdias found in Proceratophrys boiei based on morphological, molecular, and phylogenetic data using the cytochrome oxidase subunit I (COI) DNA sequences of the mitochondrial DNA.
Material and methods
Host sampling, parasite collection, and identification
Between October 2018 and January 2019, 27 individuals of Proceratophrys boiei were collected in Marumbi State Park, municipality of Piraquara, Paraná State, Brazil (Mananciais da Serra - 25°30’22 “S; 45°01’41”W) (Euclydes et al. Reference Euclydes, Dudczak and Campião2021). The Marumbi State Park features a subtropical climate composed of forests with typical Atlantic Forest formations, consisting mainly of Araucaria angustifolia, the dominant tree species that characterises this type of forest (Reginato & Goldenberg Reference Reginato and Goldenberg2007; Bergamini & Thomas Reference Bergamini and Thomas2011). The anurans were euthanised with lidocaine (4%) topically applied, and then the specimens were necropsied by a longitudinal incision in the anteroposterior axis on the ventral region of the body.
The hosts’ lungs were examined, and nematodes found were rinsed in saline solution, heat-killed, and stored in 70% alcohol for morphological identification. Some specimens were preserved in 100% alcohol for molecular analysis. For morphological analysis, the nematodes were rehydrated in distilled water, cleared in lactophenol, mounted on temporary slides, and examined under an Olympus BX41 microscope coupled with a drawing tube. We also analysed the morphology of the apical region of the anterior end, by manual sections using a razor blade, and posterior end face observations. Taxonomic illustrations were made using line drawings handmade and posteriorly prepared in CorelDraw 2018 (Corel Corporation, Ottawa, Ontario, Canada) and processed with Photoshop Version 21.0.2 (Adobe Systems Incorporated, San Jose, California, USA).
Specimen measurements are presented as the values of the holotype followed by the mean and range for the entire type series, with both values in parentheses (reported in micrometers unless otherwise indicated). We deposited the type series of the new species in the invertebrate collection of the Federal University of Paraná (Accession numbers: DZUP 541909–541913).
Molecular and phylogenetic analysis
The specimens preserved in absolute alcohol were sectioned in the anterior end (close to the esophagus–intestinal junction) and just after the posterior portion of the female reproductive system. The anterior and posterior parts were stored in absolute alcohol and deposited as the hologenophore (see Pleijel et al. Reference Pleijel, Jondelius, Norlinder, Nygren, Oxelman, Schander, Sundberg and Thollesson2008) in the parasitological section of the invertebrate collection of the Federal University of Paraná.
We performed DNA extraction from the middle part of the parasite body using the Wizard® Genomic DNA extraction kit (Promega, Madison, Wisconsin, USA), following the manufacturer’s instructions. Then, the extracted DNA was submitted to a conventional polymer chain reaction (PCR) with previously designed primers (LCO1490/Foward - 5’ GGTCAACAAATCATAAAGATATTGG 3’ and HC02198/Reverse - 5’ TAAACTTCAGGGTGACCAAAA 3’) (described by Folmer et al. 1994), and analysis of these amplified fragments was performed using 0.75% agarose gel electrophoresis. The fragments were visualised with ultraviolet light, and the corresponding fragments were cut and purified with the PureLink™ Quick Gel Extraction and PCR Purification Combo Kit (Thermofisher, Waltham, Massachusetts, USA). After purification, the samples were submitted to PCR for sequencing, using BigDye™ Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Warrington, UK). These samples were analysed by an ABI PRISM 3500 Genetic Analyser sequencer (Thermo Fisher Scientific [Applied Biosystems], Waltham, Massachusetts, USA).
The obtained sequences were analysed and assembled in FinchTV Version 1.4.0 software (Geospiza [agora parte da PerkinElmer], Waltham, Massachusetts, USA). A search was carried out in Nucleotide Basic Local Alignment Search Tool (BLASTn) using the sequence obtained to investigate the existence of similar sequences in the National Center for Biotechnology Information (NCBI) database, considering only sequences with more than 90% similarity. Using the default parameters, we aligned the sequences in MAFFT v. 7 (Katoh & Standley Reference Katoh and Standley2013). We cut the alignment ends and checked the stop codons in Geneious V.4.7 (Biomatters Ltd. [agora parte da Dotmatics], Auckland, New Zealand). We evaluated the substitution saturation with the Iss index by testing the alignment data in DAMBE 7.3.32 software (Xia Reference Xia2018). We calculated the number of base substitutions between sequences. For analyses calculating estimates and standard errors, we used the Kimura 2-parameter model with the MEGA 11 software package (Kimura Reference Kimura1980; Tamura et al. Reference Tamura, Stecher and Kumar2021).
For phylogenetic analysis, we ran our aligned matrix using jModelTest 2.1.10 software (Posada Reference Posada2008). The evolution model selected was GTR + I + G according to the Akaike information criterion (AIC). We performed phylogenetic reconstructions using maximum likelihood (ML) in IQ-Tree software (Minh et al. Reference Minh, Schmidt, Chernomor, Schrempf, Woodhams, von Haeseler and Lanfear2020) and Bayesian inference (BI) using MrBayes 3.2.7 (Ronquist et al. Reference Ronquist, Huelsenbeck, Teslenko, Zhang and Nylander2003).
The ML inference used support values of 1000 repetitions (bootstrap), and only nodes with bootstrap values greater than 70% were considered supported. BI was performed using the Markov chain Monte Carlo (MCMC) search, with the following parameters: lset nst = 6, rate = invgamma, ngammacat = 4. Chains with 50,000,000 generations were executed, saving only 1,000 generations. On the burn-in of the first 25% of the generations, only nodes with posterior probability greater than 90% were considered well supported. As an outgroup, we chose Serpentirhabdias viperidicus Morais, Aguiar, Muller, Narciso, Silva and Silva, 2017 (KX350054), as has been used in other studies (Müller et al. Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and da Silva2018; Willkens et al. Reference Willkens, Rebêlo, Santos, Furtado, Vilela, Tkach, Kuzmin and Melo2020; Alcantara et al. Reference Alcantara, Müller, Úngari, Ferreira-Silva, Emmerich, Giese, Morais, Santos, O’Dwyer and Silva2023). We used FigTree v1.4.4 (Rambaut Reference Rambaut2009) and Adobe Illustrator (Adobe Systems Incorporated) to visualise and edit the trees.
Results
Systematics
Family: Rhabdiasidae Railliet, 1915
Genus: Rhabdias Stiles and Hassall, 1905
Species: Rhabdias megacephala n. sp. Euclydes, Melo & Campião, 2024
Taxonomic Summary
Type host: Proceratophrys boiei (Wied-Neuwied, 1824) (Amphibia: Odontophrynidae).
Type locality: Pico Marumbi State Park (Mananciais da Serra), Piraquara, Paraná, Brazil (25°29’23” S; 48°58’37” W).
Site of infection: Lungs
Numbers of specimens/hosts, prevalence, mean infection intensity and range: 87 nematodes were found in 27 frogs, P = 66.6%; 3.2 (1–14).
Type material: Holotype (DZUP: 541909) and seven paratypes (DZUP: 541910-541913) were deposited in the invertebrate collection of the Federal University of Paraná.
GenBank, accession numbers: PP291576
ZooBank Registration: The Life Science Identifier for R. megacephala n. sp. is urn:lsid:zoobank.org:pub:C18FED99-0255-412A-AE0C-4A308282D919.
Etymology
The specific name megacephala refers to the new species’ highly distinguishing morphological feature, namely the prominent cuticular inflation around the cephalic end.
Description
See Figure 1 and Figure 2 (based on holotype and seven paratypes, all gravid hermaphrodites). Body slender, long, 9.7 (9.1; 8.1–9.9) mm in length. Body surface covered by apparent cuticular inflation in anterior and posterior regions, discrete along entire body. Very prominent cuticular inflation in cephalic region 503 (508; 466–570) in width. Cephalic inflation rounded, terminating at its connection to body wall at level of shoulder-like broadening of the body. Cuticular inflation close to shoulder-like broadening with two to three cuticular folds. Lateral pores arranged in two lines along cuticular inflation, connected by ducts with body wall. Cuticular inflation of tail prominent, with one large fold posterior to anus aperture, with second large fold reaching mid-length of tail and followed by minor folds decreasing in size. Body width at vulva 502 (513.8; 416–623), at esophagus–intestine junction 355 (359.8; 280–465). Oral opening with four enlarged and equidistant lips, situated very close to oral opening; each lip with terminal papilla on its inner edge; two amphids located laterally at some distance from oral opening. Vestibule circular in apical view, with narrow lumen. Buccal capsule cup-shaped 23 (15.2; 11–23) deep and 22 (26.3; 22–34) wide, with 1.04 (0.58; 0.46–1.04) depth/width ratio. Buccal capsule walls consisting of anterior part, with irregular internal surface and posterior one with smooth internal wall. Buccal capsule close to entrance of esophageal lumen with serrated surface. Entrance of esophagus lumen triangular, with serrated edges and esophageal gland located in dorsal region. Esophagus length 683 (722; 630–799), representing 7% (7.9%; 7–9%) of total body length; claviform, rounded apex, and dilation at anterior muscular region. Width of anterior end of esophagus 78 (73.7; 60–88), width of anterior dilatation of esophagus 75 (75.7; 60–91), width posterior dilation of the esophagus 98 (98; 83–112), width of bulb 162 (154.8; 124–176). Nerve ring around esophagus after anterior dilatation at 152 (297.7; 152–503) from anterior region. Excretory pore not observed. Intestine thick-walled. Rectum short, funnel-shaped, lined with cuticle. Contents of intestine brown throughout length. Genital system amphidelphic, transverse vagina, slightly pos-equatorial vulva located 5.1 (4.7; 4.2–5.2) mm from anterior end, representing 52.2% (51.2%; 45.2–53.6%) of total body length. Uteri with thin walls and numerous eggs (>100), with larvated eggs close to the vulva. Egg size 103 (104.2; 86–114) x 51 (52.8; 40–61) (total of 24 eggs; three eggs measured in each holotype and paratypes). Tail 293 (287.6; 244–338), representing 3% (3.1%; 2.6–3.8%) of body. Phasmid were not observed.
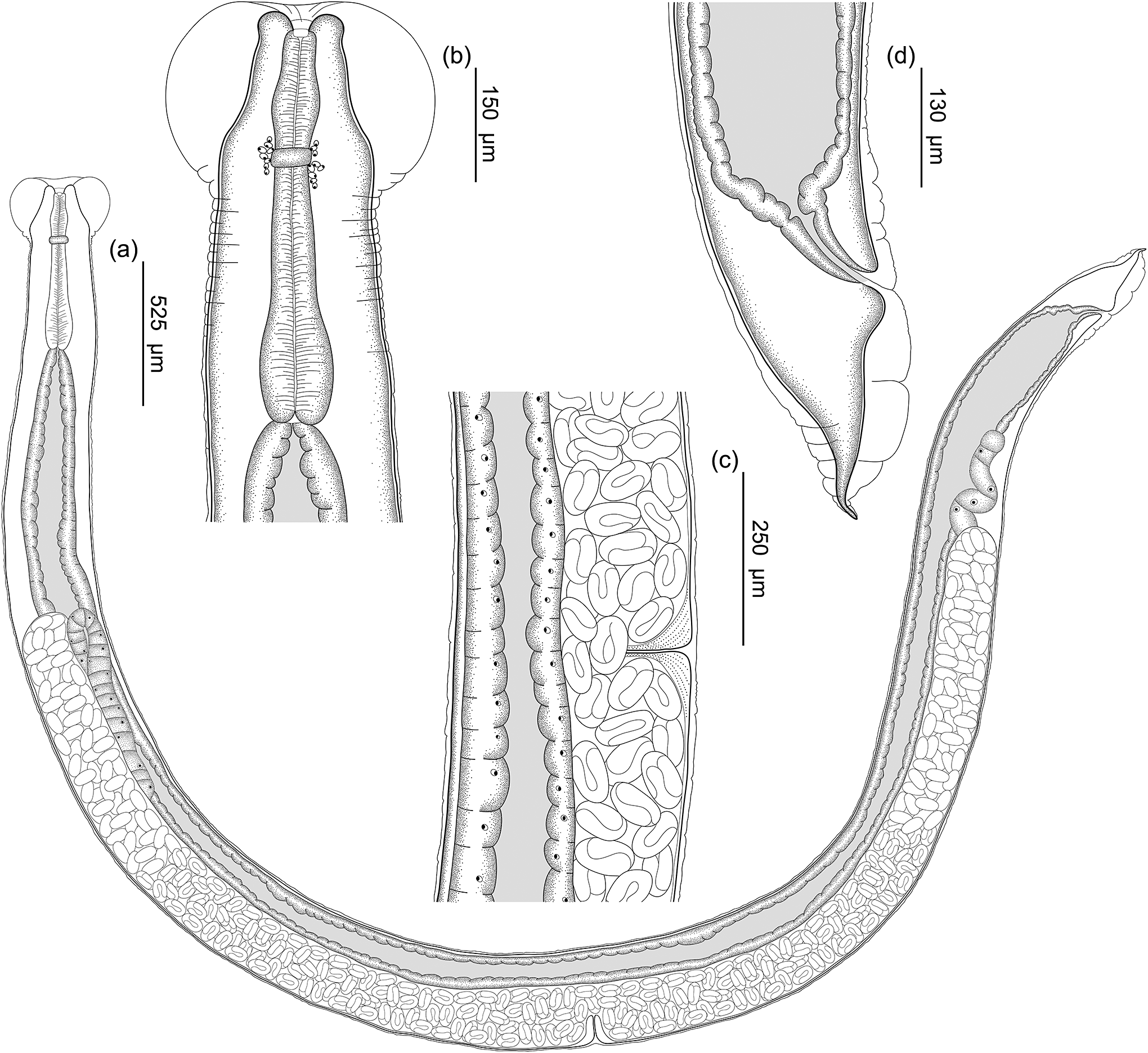
Figure 1. Line drawings of Rhabdias megacephala n. sp. from Proceratophrys boiei. A) Entire body, lateral view; B) Anterior end of the body, lateral view; C) Vulva region, lateral view; D) Caudal end, lateral view.

Figure 2. Line drawings of cross sections of anterior end and face view of Rhabdias megacephala n. sp. from Proceratophrys boiei. A) Anterior extremity end face view; B) Optical section through anterior part of buccal capsule; C) Optical section through posterior part of buccal capsule.
Remarks
Rhabdias megacephala n. sp. belongs to the genus Rhabdias because it has the following morphological characteristics: buccal capsule, inflated external cuticle, amphidelphic genital system, conical tail, and it is a parasite of the lungs of an anuran (Kuzmin et al. Reference Kuzmin, Tkach and Brooks2007; Müller et al. Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and da Silva2018; Tavares-Costa et al. Reference Tavares-Costa, Rebêlo, Müller, Jesus, Nandyara, Silva, Costa-Campos, dos Santos and Melo FT de2022). Rhabdias megacephala n. sp. has a unique set of morphological characters: a prominent cephalic cuticular dilatation, distinct from the other species, position of the nerve ring located at the anterior end of the esophagus, depth and diameter of the buccal capsule, and length of the esophagus.
The morphology of the apical region of Rhabdias spp. and the host biogeographical distribution are helpful in species differentiation. Rhabdias megacephala presents an oral opening surrounded by four lips, similar to Rhabdias leonae Martínez-Salazar, Reference Martínez-Salazar2006 and Rhabdias savagei Bursey & Goldberg, Reference Bursey and Goldberg2005 found in the Neotropical region. Rhabdias leonae is found in the lizard Anolis megapholidotus Smyth, 1933 from Mexico and differs from R. megacephala n. sp. by having a deeper buccal capsule (R. leonae 23–34 vs. R. megacephala n. sp. 11–23) and smaller width (R. leonae 11–19 vs. R. megacephala n. sp. 22–34) (Martínez-Salazar Reference Martínez-Salazar2006).
Rhabdias savagei described in Rana cf. forreri collected in Costa Rica (Boulenger, 1883), despite having four lips like R. megacephala n. sp., differs by having a much smaller body (R. savagei 4.2–5.3 mm vs. R. megacephala n. sp. 8.1–9.9 mm). Additionally, its buccal capsule is also smaller (R. savagei 18–24 x 12–18 vs. R. megacephala n. sp. 22–34 x 11–23) (Bursey & Goldberg Reference Bursey and Goldberg2005).
Rhabdias hermaphrodita Kloss, Reference Kloss1971, is a Neotropical species that has no information regarding its oral structures arrangement, with scarce morphological information, mainly concerning the presence/absence and arrangement of lips or pseudolabia. Rhabdias hermaphrodita was described in Rhinella crucifer (Wied-Neuwied, 1821). Some morphological measurements can be compared to differentiate it from R. megacephala n. sp. For example, the body can measure up to 12 mm, which is larger than that of R. megacephala n. sp. (8.1–9.9 mm). The tail of R. hermaphrodita is also larger, measuring up to 524, whereas R. megacephala n. sp. is between 244–338. Rhabdias hermaphrodita has no evident dilatation like R. megacephala n. sp. (Kloss Reference Kloss1971).
The most important characteristic of R. megacephala regarding its prominent cephalic expansion is similar to R. androgyna (R. megacephala n. sp. 466–570 vs. R. androgyna 273–706). Rhabdias androgyna Kloss, Reference Kloss1971 described in Rhinella gr. margaritifera (Laurenti, 1768) is similar to R. megacephala n. sp. in the shape of the cuticle dilation around the cephalic region. However, R. androgyna differs from R. megacephala n. sp. in having a cephalic dilation that divides into outer and inner layers, whereas R. megacephala n. sp. lacks this division and is formed by a single layer. Additionally, R. androgyna exhibits a cephalic dilation more oriented towards the apical region, while R. megacephala n. sp. presents a distinctly rounded cephalic dilation. Moreover, for species differentiation, we considered molecular data to make the distinction more integrative, given the well-conserved morphology of Rhabdias. (Kloss Reference Kloss1971).
Molecular analysis and phylogenetic study
We obtained a sequence of 630 base pairs from the COI of R. megacephala n. sp. After comparing the new sequence with that previously deposited in GenBank using BLASTn (available at NCBI), we found no other sequence with 100% similarity. Genetic distances indicated that R. megacephala n. sp. is closest to the species Rhabdias fuelleborni (OP651882, OP651884) from Rhinella diptycha and Rhinella icterica, Paraty, Brazil (Müller et al. Reference Müller, Morais, da Costa, de Vasconcelos Melo, Giese, Ávila and da Silva2023), with a genetic divergence of 10.24% (see Supplementary Table S1).
After aligning our sequence and GenBank sequences, we obtained a database with 52 sequences of 380 base pairs in length (supplementary material). The Iss index indicated no saturation in transitions or transversions and Iss.c values were greater than Iss values. The phylogenetic inferences of maximum likelihood and Bayesian inference showed similar topologies, as did the bootstrap support values (B) and posterior probability (PP). The R. megacephala n. sp. sequence is grouped with species complex Rhabdias cf. stenocephala (MH548271–MH548277) and the species complex formed by R. fuelleborni (OP651882–OP651884, OP654198) and Rhabdias sp. 4 (MH548291–MH548292), representing an outgroup of this clade formed by the two species complex (supplementary Figure S1). The phylogenetic position of R. megacephala n. sp. was not well supported by the values of B (58) and PP (56). However, we found other well-supported lineages: R. matogrossensis + R. breviensis species complex, R. fuelleborni + Rhabdias sp. 4, R. waiapi + Rhabdias sp. 5 and t R. pseudosphaerocephala species complex, which indicates that more data are needed for a robust phylogenetic hypothesis.
Discussion
Rhabdias megacephala n. sp. is the 24th species of Rhabdias from the Neotropical region. The new species is distinguished mainly by evident cephalic dilatation and size of the buccal capsule. Characteristics of apical structures arrangement of Rhabdias are important for species differentiation, such as the presence/absence of lips or pseudolabia, as well as the arrangement of these structures (Travassos Reference Travassos1930; Kuzmin et al. Reference Kuzmin, Tkach and Brooks2007; Tkach et al. Reference Tkach, Kuzmin and Snyder2014). Species from the Neotropical region can be split into three different groups based on the arrangement of apical structures: without lips or pseudolabia, with four submedial lips and two lateral pseudolabia, and with six lips (Tkach et al. Reference Tkach, Kuzmin and Snyder2014; Müller et al. Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and da Silva2018).
Bursey and Goldberg (Reference Bursey and Goldberg2005) identified a group of Rhabdias characterised by having four lips, of which only two species, Rhabdias leonae and Rhabdias savagei, have been described in the Neotropical region. Rhabdias megacephala n. sp. shares this distinctive feature, marking the third Neotropical species exhibiting the presence of these four lips. However, it differs in having a circular shaped vestibule in apical view, which does not have papillae around the oral opening. Another feature used in distinguishing species of the genus is the external cuticular inflation (Müller et al. Reference Müller, Morais, da Costa, de Vasconcelos Melo, Giese, Ávila and da Silva2023).
The sequence of R. megacephala n. sp. showed a high degree of divergence at 13.09% (11.9%) compared with the most divergent species, R. cf. stenocephala, and compared with the other 52 sequences (12 species) analysed (see Supplementary Table S1). Available phylogenies of Rhabdias have been proposed based on short sequences of the COI gene, and in this paper, we used a similar analysis. (Supplementary Figure S1) (Morais et al. Reference Morais, Müller, Melo, Aguiar, Willkens, de Sousa Silva, Giese, Ávila and da Silva2020; Tavares-Costa et al. Reference Tavares-Costa, Rebêlo, Müller, Jesus, Nandyara, Silva, Costa-Campos, dos Santos and Melo FT de2022). In fact, in phylogenetic inferences, R. megacephala n. sp. remained, with low support, as an outgroup of two clades formed by the species complex Rhabdias cf. stenocephala and the species R. fuelleborni and Rhabdias sp. 4. This low support might be related to the geographic distance between Rhabdias species and could also be a consequence of using short COI sequences.
Willkens et al. (Reference Willkens, Rebêlo, Santos, Furtado, Vilela, Tkach, Kuzmin and Melo2020) pointed out that for species from Brazil, more than geographical distance is needed to explain the high divergence in the sequences and, consequently, in the absence of phylogenetic support. However, one hypothesis for the high divergence of R. megacephala n. sp. from the other species from Brazil is the fragmentation of the Pan-Amazonian area in the early Pleistocene (Hoorn et al. Reference Hoorn, Wesselingh, Ter Steege, Bermudez, Mora, Sevink, Sanmartín, Sanchez-Meseguer, Anderson, Figueiredo, Jaramillo, Riff, Negri, Hooghiemstra, Lundberg, Stadler, Särkinen and Antonelli2010; Sobral-Souza et al. Reference Sobral-Souza, Lima-Ribeiro and Solferini2015; Tavares-Costa et al. Reference Tavares-Costa, Rebêlo, Müller, Jesus, Nandyara, Silva, Costa-Campos, dos Santos and Melo FT de2022). Pan-Amazonian fragmentation may have caused species to suffer different selective pressures according to the biome they were exposed to, with enough time to distance Rhabdias species, thereby forming a phylogenetic gap. Additional molecular sequences are necessary for more robust phylogenetic hypotheses, ideally with a larger number of base pairs. Furthermore, increased specimen collections from a broader range of locations are also essential.
The differentiation of species, as observed in Rhabdias, is a complex phenomenon that can occur through various processes. These processes involve intrinsic characteristics of the species itself or factors related to the hosts. Numerous studies have been dedicated to investigating these mechanisms, including morphological traits, genetic divergence, ecological adaptations, and functional characteristics of the parasite, providing a comprehensive understanding of parasite diversity and the elements influencing it (Poulin Reference Poulin2011; Kamiya et al. Reference Kamiya, O’Dwyer, Nakagawa and Poulin2014). This pursuit is particularly crucial in the context of species descriptions, wherein unraveling the intricacies of differentiation processes contributes to the broader scientific knowledge of parasitology.
Rhabdias species occur on most continents, except Antarctica, and the distribution of these parasites is limited by the distribution of the hosts, both anurans and reptiles (Tkach et al. Reference Tkach, Kuzmin and Snyder2014; Kuzmin et al. Reference Kuzmin, Du Preez and Junker2015). In South America, Rhabdias species are known for more than 22 host species (Campião et al. Reference Campião, Morais, Dias, Aguiar, Toledo, Tavares and Da Silva2014; Alcantara et al. Reference Alcantara, Müller, Úngari, Ferreira-Silva, Emmerich, Giese, Morais, Santos, O’Dwyer and Silva2023; Müller et al. Reference Müller, Morais, da Costa, de Vasconcelos Melo, Giese, Ávila and da Silva2023). Among the diversity of anuran parasites, 13 nominal species of the family Rhabdiasidae are known in Brazil (Alcantara et al. Reference Alcantara, Müller, Úngari, Ferreira-Silva, Emmerich, Giese, Morais, Santos, O’Dwyer and Silva2023; Müller et al. Reference Müller, Morais, da Costa, de Vasconcelos Melo, Giese, Ávila and da Silva2023). However, when we consider the diversity of Proceratophrys species (43 species) and of anurans known in Brazil (1,252 species), the diversity of Rhabdias is still poorly understood (Frost Reference Frost2024), for P. boiei, in addition to the record of R. megacephala n. sp., had been reported as host to an unidentified species of Rhabdias (Rhabdias sp.) (Aguiar et al. Reference Aguiar, Anjos, Nomura, Schwartz, Toledo, Velota and da Silva2018).
Rhabdias species are also known in other species of Proceratophrys: R. androgyna has been reported for Proceratophrys tupinamba (Boquimpani-Freitas et al. Reference Boquimpani-Freitas, Vrcibradic, Vicente, Bursey and Rocha2001; Prado & Pombal Jr. Reference Prado and Pombal2008). Other unidentified Rhabdias spp. have been reported in Proceratophrys aridus and Proceratophrys mantiqueira (Almeida-Santos Reference Almeida-Santos2017; Teles et al. Reference Teles, Brito, Filho, Dias, Ávila and Almeida2017; Müller et al. Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and da Silva2018). Given the description of R. megacephala n. sp., in comparison with other as yet undescribed species, there arises curiosity in understanding its distribution. Could R. megacephala n. sp. be endemic, or might the undescribed species actually be R. megacephala n. sp.? Aspects such as phenotypic similarities, habitat sharing, exposure to the same infective stages, and phylogenetically conserved resources among hosts may influence the exchange or sharing of different hosts (Fecchio et al. Reference Fecchio, Wells, Bell, Tkach, Lutz, Weckstein, Clegg and Clark2019; D’Bastiani et al. Reference D’Bastiani, Campião, Boeger and Araújo2020; Euclydes et al. Reference Euclydes, De La Torre, Dudczak, Melo FT de and Campião2022).
Knowledge of Rhabdias species diversity has increased, especially in recent years with species description, phylogenetic, and geographic studies (Müller et al. Reference Müller, Morais, Costa-Silva, Aguiar, Ávila and da Silva2018; Tavares-Costa et al. Reference Tavares-Costa, Rebêlo, Müller, Jesus, Nandyara, Silva, Costa-Campos, dos Santos and Melo FT de2022; Alcantara et al. Reference Alcantara, Müller, Úngari, Ferreira-Silva, Emmerich, Giese, Morais, Santos, O’Dwyer and Silva2023). Most species have been observed in the North and Midwest regions of Brazil, mainly in the Amazon region (seven species) (Willkens et al. Reference Willkens, Rebêlo, Santos, Furtado, Vilela, Tkach, Kuzmin and Melo2020; Tavares-Costa et al. Reference Tavares-Costa, Rebêlo, Müller, Jesus, Nandyara, Silva, Costa-Campos, dos Santos and Melo FT de2022; Müller et al. Reference Müller, Morais, da Costa, de Vasconcelos Melo, Giese, Ávila and da Silva2023;). Knowledge of this group of parasites in these localities may be due to a greater study effort. In the Atlantic Forest, studies show how significant the parasitic diversity of anurans is, but knowledge of the diversity of Rhabdias species is still underestimated (Martins-Sobrinho et al. Reference Martins-Sobrinho, Silva, Santos, Moura and Oliveira2017; Euclydes et al. Reference Euclydes, Dudczak and Campião2021). We describe the 24th species of Rhabdias, the first species documented in Paraná. Rhabdias megacephala n. sp. is also the first described species in P. boiei. Discovering parasites such as Rhabdias is fundamental to unveiling ecosystem diversity, species evolution, and host–parasite relationships to understand more about the evolutionary history of this cosmopolitan group prevalent among anurans.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0022149X24000385.
Acknowledgements
We thank Sanepar for the authorisation and support in carrying out the collections.
Financial support
R. Euclydes acknowledges the doctoral scholarship provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). K.M. Campião acknowledges funding from the Council for Scientific and Technological Development (CNPq - proc. 306934/2022-1). Fieldwork was partially funded by the 2018 research support call of Pró-Reitoria de Pesquisa e Pós-Graduação PRPPG/UFPR, and by Fundação Araucária and CNPq in the call to support young researchers Jovens Pesquisadores Programa Primeiros Projetos – PPP, 20/2018.
Competing interest
None.
Ethical standard
This study’s collections and observations were carried out in accordance with the license 62552-1 (Sistema de Autorização e Informação em Biodiversidade – Instituto Chico Mendes de Conservação da Biodiversidade – SISBIO). The Comitê de Ética para Uso de Animais da Seção de Ciências Biológicas da Universidade Federal do Paraná (Opinion no. 1167) certified that the procedures with the use of animals in this work were approved.
Contribution
Conceptualisation: RE and KMC; methodology: RE, HCJ, LHG, SSV; data analysis: LES, HCJ, RFJ, FTVM, and KMC. Writing: RE, KMC, and FTVM; supervision: KMC.




