INTRODUCTION
Plasmodium falciparum (Pf) malaria is one of the most important paediatric infectious diseases estimated to kill over 600 000 people annually, most of whom are children younger than 5 years of age (WHO, 2014). Yet, newborns and young infants (less than 6 months of age) are thought to be relatively protected from symptomatic malaria (Covell, Reference Covell1950; Wagner et al. Reference Wagner, Koram, McGuinness, Bennett, Nkrumah and Riley1998; Riley et al. Reference Riley, Wagner, Akanmori and Koram2001; Apinjoh et al. Reference Apinjoh, Anchang-Kimbi, Mugri, Njua-Yafi, Tata, Chi, Tangoh, Loh and Achidi2015). This protection historically has been thought to be primarily mediated by maternal antimalarial IgG antibodies transferred to the fetus in the last trimester of pregnancy. Antibodies play an important role in the host defence against malaria. This was demonstrated when serum transferred from healthy malaria immune adults to hospitalized children with malaria resulted in the rapid amelioration of symptoms and parasitaemia (Cohen et al. Reference Cohen, Mc and Carrington1961; McGregor, Reference McGregor1964). Malaria targets of protective antibodies include proteins expressed on the sporozoite needed for hepatocyte invasion, proteins expressed on the merozoite surface important for invasion of erythrocytes and variant surface antigens (VSAs) trafficked to the surface of the infected erythrocyte important for sequestration and pathogenesis (Richards and Beeson, Reference Richards and Beeson2009; Dups et al. Reference Dups, Pepper and Cockburn2014; Smith, Reference Smith2014). Antimalarial IgG antibodies may function to block sporozoite invasion of hepatocytes and merozoite invasion of erythrocytes, opsonize merozoites and infected erythrocytes expressing VSA on their surface for phagocytosis, and fix and activate complement on the merozoite surface with resultant parasite lysis (Hill et al. Reference Hill, Eriksson, Li Wai Suen, Chiu, Ryg-Cornejo, Robinson, Siba, Mueller, Hansen and Schofield2013). An increasing number of Pf antigens have been identified as relevant to naturally acquired immunity and are considered potential vaccine targets (Richards et al. Reference Richards, Arumugam, Reiling, Healer, Hodder, Fowkes, Cross, Langer, Takeo, Uboldi, Thompson, Gilson, Coppel, Siba, King, Torii, Chitnis, Narum, Mueller, Crabb, Cowman, Tsuboi and Beeson2013; Osier et al. Reference Osier, Mackinnon, Crosnier, Fegan, Kamuyu, Wanaguru, Ogada, McDade, Rayner, Wright and Marsh2014; Dent et al. Reference Dent, Nakajima, Liang, Baum, Moormann, Sumba, Vulule, Babineau, Davies, Felgner and Kazura2015), but how and whether antibodies to these antigens are acquired during the first year of life is not well described. With this review, we focus on maternal antibodies directed against Pf targets and their possible role in protecting the infant from malaria, infant susceptibility to malaria as maternal antibodies wane and infant-generated antimalarial antibodies as a result of malaria infection.
PF MALARIA INFECTION IN NEONATES AND INFANTS
Infants living in malaria endemic areas are relatively protected from clinical malaria during the first 6 months of life. However, there are instances of malaria infection in neonates. For example, congenital malaria occurs after transmission from the mother through the placenta just before or during delivery (though earlier in pregnancy cannot be excluded), with detection of asexual parasites in cord blood or in neonatal peripheral blood within the first week of life (Malhotra et al. Reference Malhotra, Mungai, Muchiri, Kwiek, Meshnick and King2006; Falade et al. Reference Falade, Mokuolu, Okafor, Orogade, Falade, Adedoyin, Oguonu, Aisha, Hamer and Callahan2007). Neonatal malaria occurs during the first 28 days of life and is due to an infective mosquito bite after birth. Both congenital and neonatal malaria infections are considered to be very rare (Covell, Reference Covell1950; Bruce-Chwatt, Reference Bruce-Chwatt1952). Though existing data on prevalence and burden of disease in young infants are still limited and often contradictory (Mwaniki et al. Reference Mwaniki, Talbert, Mturi, Berkley, Kager, Marsh and Newton2010), it appears that while subclinical asymptomatic infection is not uncommon, clinical disease due to Pf infection is quite rare in infants under 5 months of age (Sehgal et al. Reference Sehgal, Siddjiqui and Alpers1989; McGuinness et al. Reference McGuinness, Koram, Bennett, Wagner, Nkrumah and Riley1998; Snow et al. Reference Snow, Nahlen, Palmer, Donnelly, Gupta and Marsh1998; Franks et al. Reference Franks, Koram, Wagner, Tetteh, McGuinness, Wheeler, Nkrumah, Ranford-Cartwright and Riley2001). Several studies in sub-Saharan Africa demonstrate an increase in malaria prevalence and risk for clinical disease after the ages of 3–6 months (Bruce-Chwatt, Reference Bruce-Chwatt1952; Gottschau and Hogh, Reference Gottschau and Hogh1995; McGuinness et al. Reference McGuinness, Koram, Bennett, Wagner, Nkrumah and Riley1998; Snow et al. Reference Snow, Nahlen, Palmer, Donnelly, Gupta and Marsh1998; Afolabi et al. Reference Afolabi, Salako, Mafe, Ovwigho, Rabiu, Sanyaolu and Ibrahim2001).
In malaria endemic regions where mothers have existing immunity, Pf infection in young infants (less than 6 months) is characterized by low parasitaemia, often <100 parasites µL−1 of blood (Sehgal et al. Reference Sehgal, Siddjiqui and Alpers1989; McGuinness et al. Reference McGuinness, Koram, Bennett, Wagner, Nkrumah and Riley1998; Afolabi et al. Reference Afolabi, Salako, Mafe, Ovwigho, Rabiu, Sanyaolu and Ibrahim2001; Falade et al. Reference Falade, Mokuolu, Okafor, Orogade, Falade, Adedoyin, Oguonu, Aisha, Hamer and Callahan2007; Mwaniki et al. Reference Mwaniki, Talbert, Mturi, Berkley, Kager, Marsh and Newton2010). Such infections are frequently transient, with spontaneous clearance of parasites occurring more quickly than in older infants and children, suggesting that young infants possess some mechanisms for controlling and clearing parasitaemia (Kitua et al. Reference Kitua, Smith, Alonso, Masanja, Urassa, Menendez, Kimario and Tanner1996; McGuinness et al. Reference McGuinness, Koram, Bennett, Wagner, Nkrumah and Riley1998; Franks et al. Reference Franks, Koram, Wagner, Tetteh, McGuinness, Wheeler, Nkrumah, Ranford-Cartwright and Riley2001; Falade et al. Reference Falade, Mokuolu, Okafor, Orogade, Falade, Adedoyin, Oguonu, Aisha, Hamer and Callahan2007). Young infants are less likely to be febrile with Pf infection, though the parasite density thresholds for fever appears to be lower in infants (estimated at 100 parasites µL−1) than in children older than 12 months (3500 parasites µL−1) (McGuinness et al. Reference McGuinness, Koram, Bennett, Wagner, Nkrumah and Riley1998). However, very high parasite densities have been detected in afebrile, asymptomatic infants, suggesting that failure to mount a febrile response to Pf infection may be due to a specific mechanism, such as lack of immunologic priming of T cells needed to produce pro-inflammatory cytokines (McGuinness et al. Reference McGuinness, Koram, Bennett, Wagner, Nkrumah and Riley1998; Riley, Reference Riley1999). Clinical manifestations of symptomatic malaria infection in neonates and young infants are non-specific and difficult to distinguish from other diseases, such as bacterial sepsis. Signs and symptoms include fever, anaemia, pallor, splenomegaly, lethargy, poor feeding, diarrhoea, respiratory distress, cyanosis, hepatomegaly, jaundice and seizures (Ibhanesebhor, Reference Ibhanesebhor1995; Afolabi et al. Reference Afolabi, Salako, Mafe, Ovwigho, Rabiu, Sanyaolu and Ibrahim2001; Mwaniki et al. Reference Mwaniki, Talbert, Mturi, Berkley, Kager, Marsh and Newton2010).
This relative protection against clinical Pf malaria and progression to severe disease in young infants may be explained by a number of potential immunologic, physiologic and environmental mechanisms. Studies of the biting habits of anopheline mosquitoes show that infants are fed on less frequently than larger adults and older children, which may offer some protection against infection (Muirhead-Thomson, Reference Muirhead-Thomson1951; Port, Reference Port1980). Diet may also play an important role in protection. Breast milk constituents such as lactoferrin and secretory IgA have been shown to inhibit parasite growth in vitro (Kassim et al. Reference Kassim, Ako-Anai, Torimiro, Hollowell, Okoye and Martin2000). Parasite replication depends on an external source of the nutrient para-aminobenzoic acid, which is low in breast milk (Kicska et al. Reference Kicska, Ting, Schramm and Kim2003). The presence of fetal haemoglobin (HbF) may provide a physiologic mechanism for protection against clinical malaria. Some studies suggest that parasite growth is restricted in erythrocytes containing higher levels of HbF (Pasvol et al. Reference Pasvol, Weatherall and Wilson1977). A transgenic mouse model of adult persistence of HbF demonstrated delayed parasite development and protection from severe disease (Shear et al. Reference Shear, Grinberg, Gilman, Fabry, Stamatoyannopoulos, Goldberg and Nagel1998). In a more recent study, Amaratunga et al. showed that Pf parasites invaded and grew normally in cord blood erythrocytes, but that parasitized cord blood erythrocytes had impaired cytoadherence properties; the presence of immune IgG further impaired the ability of these parasitized cells to adhere, suggesting that HbF and maternal IgG act cooperatively to protect young infants (Amaratunga et al. Reference Amaratunga, Lopera-Mesa, Brittain, Cholera, Arie, Fujioka, Keefer and Fairhurst2011).
MATERNAL ANTIMALARIAL ANTIBODIES IN THE YOUNG INFANT: ROLE OF PROTECTION OR MARKER OF EXPOSURE?
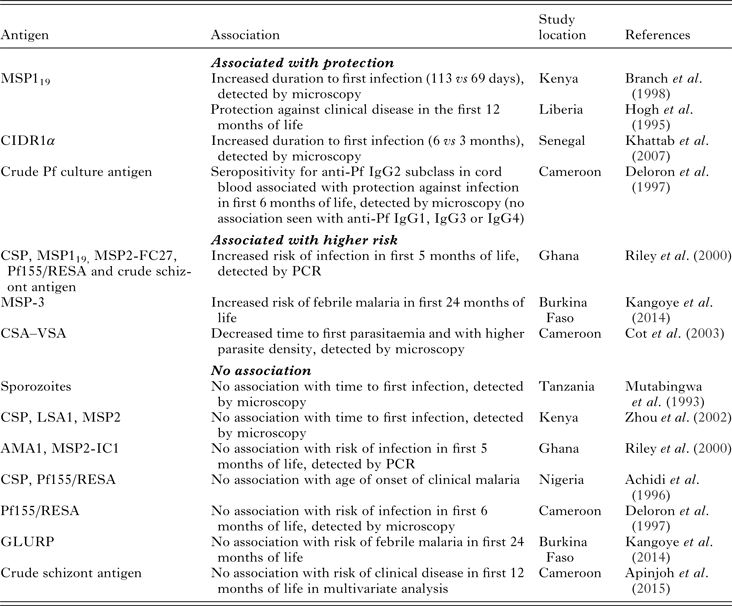
Transplacental transfer of maternal IgG antibodies to the fetus occurs primarily in the third trimester and is mediated by the neonatal Fc receptor (Simister, Reference Simister2003). After birth, maternal antibodies of all isotypes, but primarily IgA, are transferred to infants in breast milk, though these are not systemically absorbed and act primarily in the gut (Van de Perre, Reference Van de Perre2003). Studies of birth cohorts in sub-Saharan Africa have reported the waning of maternal antimalarial IgG antibodies by 6–9 months of age, which coincides with the period of time in which the risk for malaria infection and clinical disease in infants begins to increase (Achidi et al. Reference Achidi, Perlmann, Salimonu, Perlmann, Walker and Asuzu1995; Riley et al. Reference Riley, Wagner, Ofori, Wheeler, Akanmori, Tetteh, McGuinness, Bennett, Nkrumah, Anders and Koram2000; Duah et al. Reference Duah, Miles, Whittle and Conway2010; Kangoye et al. Reference Kangoye, Nebie, Yaro, Debe, Traore, Ouedraogo, Sanou, Soulama, Diarra, Tiono, Marsh, Sirima and Bejon2014; Nhabomba et al. Reference Nhabomba, Guinovart, Jimenez, Manaca, Quinto, Cistero, Aguilar, Barbosa, Rodriguez, Bassat, Aponte, Mayor, Chitnis, Alonso and Dobano2014). It has long been proposed that passive transfer of maternal antimalarial antibodies confers protection against clinical disease in young infants (McGregor et al. Reference McGregor, Rowe, Wilson and Billewicz1970; Logie et al. Reference Logie, McGregor, Rowe and Billewicz1973; McGuinness et al. Reference McGuinness, Koram, Bennett, Wagner, Nkrumah and Riley1998). However, the evidence supporting this assumption is lacking. In Table 1, we summarize the evidence in the literature regarding the question: are maternal antimalarial antibodies that are present at the time of delivery (in maternal and/or cord blood) associated with infant malaria protection? The studies referenced in the table focus on Pf infection.
Table 1. Maternal antibodies and association with protection against Pf malaria in infantsa

a Maternal antibodies are defined as antimalarial antibodies present at the time of birth in either maternal or cord blood. CSP, circumsporozoite protein; MSP119, merozoite surface protein-1 GPI-anchored 19-kD fragment; CIDR1α, cysteine-rich interdomain region of P. falciparum 732var gene; Pf155/RESA, ring-infected erythrocyte surface antigen; MSP2-FC27, merozoite surface protein-2 from FC27 P. falciparum isolate; MSP3, merozoite surface protein-3; CSA–VSA, chondroitin sulphate-A variant surface antigen; LSA1, liver stage antigen-1; AMA1, apical membrane antigen-1; MSP2-IC1, merozoite surface protein-2 from IC1 P. falciparum isolate; GLURP, glutamate-rich protein.
Longitudinal studies designed to answer this question vary in regards to antigens studied, reported outcomes (infection vs clinical disease), method for detecting parasitaemia, location and transmission intensity. A few of these studies have demonstrated an association between maternal antimalarial antibodies and protection from infection (Deloron et al. Reference Deloron, Dubois, Le Hesran, Riche, Fievet, Cornet, Ringwald and Cot1997; Khattab et al. Reference Khattab, Chia, May, Le Hesran, Deloron and Klinkert2007), clinical disease (Hogh et al. Reference Hogh, Marbiah, Burghaus and Andersen1995) or both (Branch et al. Reference Branch, Udhayakumar, Hightower, Oloo, Hawley, Nahlen, Bloland, Kaslow and Lal1998) in infants. However, several others have found an association between maternal antibodies and an increased risk for infection in infants (Riley et al. Reference Riley, Wagner, Ofori, Wheeler, Akanmori, Tetteh, McGuinness, Bennett, Nkrumah, Anders and Koram2000; Cot et al. Reference Cot, Le Hesran, Staalsoe, Fievet, Hviid and Deloron2003; Kangoye et al. Reference Kangoye, Nebie, Yaro, Debe, Traore, Ouedraogo, Sanou, Soulama, Diarra, Tiono, Marsh, Sirima and Bejon2014), or no association at all (Mutabingwa et al. Reference Mutabingwa, Malle, Verhave, Eling, Meuwissen and de Geus1993; Achidi et al. Reference Achidi, Salimonu, Perlmann, Perlmann, Berzins and Williams1996; Deloron et al. Reference Deloron, Dubois, Le Hesran, Riche, Fievet, Cornet, Ringwald and Cot1997; Riley et al. Reference Riley, Wagner, Ofori, Wheeler, Akanmori, Tetteh, McGuinness, Bennett, Nkrumah, Anders and Koram2000; Zhou et al. Reference Zhou, Xiao, Branch, Kariuki, Nahlen and Lal2002; Kangoye et al. Reference Kangoye, Nebie, Yaro, Debe, Traore, Ouedraogo, Sanou, Soulama, Diarra, Tiono, Marsh, Sirima and Bejon2014; Apinjoh et al. Reference Apinjoh, Anchang-Kimbi, Mugri, Njua-Yafi, Tata, Chi, Tangoh, Loh and Achidi2015). For example, the presence of maternal antibodies against merozoite invasion protein 1 (MSP119) were associated with delayed onset of first infection among a cohort in western Kenya and with protection of infants against clinical disease in Liberia (Gottschau and Hogh, Reference Gottschau and Hogh1995; Branch et al. Reference Branch, Udhayakumar, Hightower, Oloo, Hawley, Nahlen, Bloland, Kaslow and Lal1998). In a comprehensive study of the role of passively acquired antimalarial antibodies in infants living in Southern Ghana, Riley et al. found that antibodies against MSP119 (as well as circumsporozoite surface protein (CSP), MSP2, ring-infected erythrocyte surface antigen (Pf155/RESA) and crude schizont antigen) were positively associated with infection (Riley et al. Reference Riley, Wagner, Ofori, Wheeler, Akanmori, Tetteh, McGuinness, Bennett, Nkrumah, Anders and Koram2000). Other studies in West Africa also found a positive association between maternal antibodies against MSP3 and chondroitin sulphate A (CSA)–VSA and an increased risk for malaria (Cot et al. Reference Cot, Le Hesran, Staalsoe, Fievet, Hviid and Deloron2003; Kangoye et al. Reference Kangoye, Nebie, Yaro, Debe, Traore, Ouedraogo, Sanou, Soulama, Diarra, Tiono, Marsh, Sirima and Bejon2014). We and these authors conclude that the presence of antimalarial antibodies at birth is a biomarker for intensity of exposure to malaria in infants (Franks et al. Reference Franks, Koram, Wagner, Tetteh, McGuinness, Wheeler, Nkrumah, Ranford-Cartwright and Riley2001).
It is important to consider that different antigens have been used as markers of Pf infection so there is very little consistency between studies. In a study of malaria-exposed pregnant women in Senegal, Khattab et al. showed that maternal antibodies against the 732var Pf erythrocyte membrane protein 1 (PfEMP1) domain cysteine-rich interdomain region 1α (CIDR1α) conferred protection against malaria in infants during the first 6 months of life (Khattab et al. Reference Khattab, Chia, May, Le Hesran, Deloron and Klinkert2007). In another study of anti-VSA antibodies, Cot et al. showed that the presence of maternal antibodies against CSA–VSA was associated with a decreased time to first parasitaemia (Cot et al. Reference Cot, Le Hesran, Staalsoe, Fievet, Hviid and Deloron2003). These discrepant results are likely explained by the different function of the antigens. CIDR1α mediates parasite sequestration by binding to endothelial cell surface receptors, and this domain is expressed in parasite strains relevant to infection and disease in young children (Avril et al. Reference Avril, Brazier, Melcher, Sampath and Smith2013; Turner et al. Reference Turner, Lavstsen, Berger, Wang, Petersen, Avril, Brazier, Freeth, Jespersen, Nielsen, Magistrado, Lusingu, Smith, Higgins and Theander2013; Smith, Reference Smith2014). Antibodies directed against CSA–VSA are important in response to placental malaria, and thus likely represent recent exposure in mothers of these infants.
Several factors contribute to an infant's risk for malaria, and it is difficult to disentangle any one factor from the others. The waning of maternal antibodies occurs simultaneously with the waning of HbF, changes in infants’ diets, and decreased breastfeeding, all of which might alter risk for malaria (Colombo et al. Reference Colombo, Kim, Atencio, Molina and Terrenato1976). Transmission intensity and season of birth have been shown to be very important factors in risk for clinical disease (Riley et al. Reference Riley, Wagner, Ofori, Wheeler, Akanmori, Tetteh, McGuinness, Bennett, Nkrumah, Anders and Koram2000; Apinjoh et al. Reference Apinjoh, Anchang-Kimbi, Mugri, Njua-Yafi, Tata, Chi, Tangoh, Loh and Achidi2015). Maternal HIV and placental malaria have also been implicated (Briand et al. Reference Briand, Badaut and Cot2009).
PLASMODIUM VIVAX (PV) INFECTION IN THE NEONATE AND INFANT
Cases of congenital Pv malaria have been described in infants born to mothers without pre-existing antimalarial immunity, mostly in case reports of infants born in non-endemic countries to mothers with a travel history to endemic countries (Del Punta et al. Reference Del Punta, Gulletta, Matteelli, Spinoni, Regazzoli and Castelli2010). These infants often present several days to weeks after birth with fever, irritability and hepatosplenomegaly, and if untreated, the infection may be rapidly fatal (Menendez and Mayor, Reference Menendez and Mayor2007). Very little is known about the development of humoral immunity to Pv in the first year of life. The majority of Pv infections occur in Southeast Asia and the Western Pacific, with a significant number of cases also occurring in the Eastern Mediterranean, South and Central America, and Africa (Price et al. Reference Price, Tjitra, Guerra, Yeung, White and Anstey2007). Studies performed in Papua New Guinea, Indonesia and Vanuatu demonstrate that in areas endemic for both Pv and Pf, Pv serves as the predominant cause of malaria infection during the first 2 years of life (Maitland et al. Reference Maitland, Williams, Bennett, Newbold, Peto, Viji, Timothy, Clegg, Weatherall and Bowden1996; Karyana et al. Reference Karyana, Burdarm, Yeung, Kenangalem, Wariker, Maristela, Umana, Vemuri, Okoseray, Penttinen, Ebsworth, Sugiarto, Anstey, Tjitra and Price2008; Poespoprodjo et al. Reference Poespoprodjo, Fobia, Kenangalem, Lampah, Hasanuddin, Warikar, Sugiarto, Tjitra, Anstey and Price2009; Senn et al. Reference Senn, Rarau, Stanisic, Robinson, Barnadas, Manong, Salib, Iga, Tarongka, Ley, Rosanas-Urgell, Aponte, Zimmerman, Beeson, Schofield, Siba, Rogerson, Reeder and Mueller2012). In contrast to Pf infection in young infants, which has a low risk for progression to symptomatic or severe disease, Pv infection in infants younger than 3 months of age is a significant cause of morbidity in endemic areas, characterized by higher parasite densities and substantial risk for development of severe disease (Poespoprodjo et al. Reference Poespoprodjo, Fobia, Kenangalem, Lampah, Hasanuddin, Warikar, Sugiarto, Tjitra, Anstey and Price2009). Limited data exist regarding risk factors for Pv malaria in infants, and as with Pf, it is not known if transfer of maternal antimalarial antibodies confers any protection against Pv infection in infants (Campbell et al. Reference Campbell, Martinez and Collins1980). Clinical immunity to Pv appears to develop more rapidly than to Pf, with most children in endemic areas acquiring immunity to Pv by 5 years of age while remaining at risk for Pf illness (Michon et al. Reference Michon, Cole-Tobian, Dabod, Schoepflin, Igu, Susapu, Tarongka, Zimmerman, Reeder, Beeson, Schofield, King and Mueller2007). Further studies are needed to evaluate how protective immunity develops in infants and to determine which factors might be unique to Pv compared with Pf infant infections.
PREGNANCY-ASSOCIATED PF MALARIA AND INFANT IMMUNITY
Pregnancy-associated malaria (either peripheral or placental detection of parasites) can have devastating effects on both mother and fetus, including anaemia, stillbirth, low birthweight, intrauterine growth retardation and pre-term delivery (Brabin, Reference Brabin1991; Menendez, Reference Menendez1995; Brabin et al. Reference Brabin, Romagosa, Abdelgalil, Menendez, Verhoeff, McGready, Fletcher, Owens, D'Alessandro, Nosten, Fischer and Ordi2004; Nosten et al. Reference Nosten, Rogerson, Beeson, McGready, Mutabingwa and Brabin2004; Desai et al. Reference Desai, ter Kuile, Nosten, McGready, Asamoa, Brabin and Newman2007; Rogerson et al. Reference Rogerson, Hviid, Duffy, Leke and Taylor2007). Some studies have found that infants born to mothers with Pf placental malaria have increased susceptibility to malaria with earlier infections compared with those born to mothers with no evidence of pregnancy-associated malaria (Le Hesran et al. Reference Le Hesran, Cot, Personne, Fievet, Dubois, Beyeme, Boudin and Deloron1997; Mutabingwa et al. Reference Mutabingwa, Bolla, Li, Domingo, Li, Fried and Duffy2005; Schwarz et al. Reference Schwarz, Adegnika, Breitling, Gabor, Agnandji, Newman, Lell, Issifou, Yazdanbakhsh, Luty, Kremsner and Grobusch2008; Malhotra et al. Reference Malhotra, Dent, Mungai, Wamachi, Ouma, Narum, Muchiri, Tisch and King2009; Bardaji et al. Reference Bardaji, Sigauque, Sanz, Maixenchs, Ordi, Aponte, Mabunda, Alonso and Menendez2011; Le Port et al. Reference Le Port, Watier, Cottrell, Ouedraogo, Dechavanne, Pierrat, Rachas, Bouscaillou, Bouraima, Massougbodji, Fayomi, Thiebaut, Chandre, Migot-Nabias, Martin-Prevel, Garcia and Cot2011). The reasons for this are likely multifactorial with maternal parity, receipt of intermittent preventive treatment during pregnancy, use of insecticide treated bed nets and malaria transmission intensity playing important roles (Apinjoh et al. Reference Apinjoh, Anchang-Kimbi, Mugri, Njua-Yafi, Tata, Chi, Tangoh, Loh and Achidi2015).
In utero exposure to parasite antigens may induce fetal immune tolerance (Le Hesran et al. Reference Le Hesran, Cot, Personne, Fievet, Dubois, Beyeme, Boudin and Deloron1997). In placental malaria, infected erythrocytes sequester in the intervillous blood of the placenta such that the fetus may be exposed to infected erythrocytes or soluble malaria antigens that cross the placenta. May et al. (Reference May, Grube, Malhotra, Long, Singh, Mandaliya, Siegmund, Fusch, Schneider and King2009) showed that immune-complexed MSP1 transferred from maternal to fetal circulation using an ex vivo human placental cotyledon model. Numerous studies have shown that fetal cord blood lymphocytes can have recall responses to specific malaria antigens (Fievet et al. Reference Fievet, Ringwald, Bickii, Dubois, Maubert, Le Hesran, Cot and Deloron1996; King et al. Reference King, Malhotra, Wamachi, Kioko, Mungai, Wahab, Koech, Zimmerman, Ouma and Kazura2002; Malhotra et al. Reference Malhotra, Mungai, Muchiri, Ouma, Sharma, Kazura and King2005, Reference Malhotra, Dent, Mungai, Wamachi, Ouma, Narum, Muchiri, Tisch and King2009, Reference Malhotra, McKibben, Mungai, McKibben, Wang, Sutherland, Muchiri, King, King and LaBeaud2015; Dent et al. Reference Dent, Malhotra, Mungai, Muchiri, Crabb, Kazura and King2006; Metenou et al. Reference Metenou, Suguitan, Long, Leke and Taylor2007) and cord blood can have fetal malaria-specific IgM and IgE, immunoglobulins that do not cross the placenta (Rasheed et al. Reference Rasheed, Bulmer, De Francisco, Jawla, Jakobsen, Jepson and Greenwood1995; Xi et al. Reference Xi, Leke, Thuita, Zhou, Leke, Mbu and Taylor2003; Apinjoh et al. Reference Apinjoh, Anchang-Kimbi, Mugri, Njua-Yafi, Tata, Chi, Tangoh, Loh and Achidi2015). The consequence of fetal tolerance to malaria antigens is poorly understood, but we have shown that infants exposed to malaria in utero but lacking malaria-specific cord blood lymphocyte recall responses (putatively tolerized) make less functional MSP 119 invasion inhibitory antibodies (Dent et al. Reference Dent, Malhotra, Mungai, Muchiri, Crabb, Kazura and King2006). Bonner et al. showed that infants born to mothers with placental malaria had lower antibody responses to multiple malaria antigens, including CSP, EBA175 and MSP2 compared with infants born to placental malaria negative mothers. This difference was apparent especially after 4 months of age when maternal antibodies wane (Bonner et al. Reference Bonner, Zhou, Mirel, Ayisi, Shi, van Eijk, Otieno, Nahlen, Steketee and Udhayakumar2005). Thus, in utero exposure to malaria may have detrimental effects on infant antimalarial antibody generation through poorly understood mechanisms. It is likely, this is a generalizable immune consequence as infants born to mothers with placental malaria have increased all-cause mortality (Verhoeff et al. Reference Verhoeff, Le Cessie, Kalanda, Kazembe, Broadhead and Brabin2004; Bardaji et al. Reference Bardaji, Sigauque, Sanz, Maixenchs, Ordi, Aponte, Mabunda, Alonso and Menendez2011; Rachas et al. Reference Rachas, Le Port, Cottrell, Guerra, Choudat, Bouscaillou, Massougbodji and Garcia2012). Additionally, malaria putatively tolerized Kenyan infants, those with in utero exposure to malaria but lacking cord blood lymphocyte recall responses to malaria antigens, had lower levels of antibodies to diphtheria toxin vaccine compared with those infants who were not exposed to malaria in utero (Malhotra et al. Reference Malhotra, McKibben, Mungai, McKibben, Wang, Sutherland, Muchiri, King, King and LaBeaud2015). Thus, fetal exposure to malaria in utero may affect subsequent infant immune responses to malaria and other pathogens.
ACQUISITION OF ANTIMALARIAL ANTIBODIES IN THE INFANT AFTER 6 MONTHS OF AGE
After maternal antibodies wane, infants exposed to malaria gradually acquire antimalarial antibodies. Evaluation of infant antibody responses to Pf has relied primarily on serologic assays with Pf schizont extract, single recombinant proteins and/or protein domains. Numerous antigens on the surface and within merozoites have been identified as important targets of naturally acquired immunity. Targets include MSPs thought to be important in initial attachment of the merozoite to the erythrocyte, apical membrane antigen (AMA1) implicated in apical reorientation of merozoite, and erythrocyte-binding proteins (e.g. EBA175, EBA140 and EBA181) and reticulocyte-binding homologues (e.g. Rh4 and Rh5) thought to be important in erythrocyte invasion (Cowman and Crabb, Reference Cowman and Crabb2006). Pre-erythrocytic antigens such as CSP are also important targets as infant trials with the vaccine containing CSP named ‘RTS,S’ have indicated (Rts, Reference Rts2015). Studies of antimalarial antibodies and protection from malaria in children vary greatly in design, outcome measured (i.e. infection or symptomatic disease), age of children included, transmission intensity and duration of follow-up. Alleles and recombinant protein preparation used also vary widely. A challenge to this review is the paucity of studies conducted in infants. Thus, we extended our review of the literature to include studies that included 12-month-old infants and young children. In multiple studies including infants and young children, serologically measured antibodies against targets such as MSP1, MSP2, MSP3, AMA1, glutamate-rich protein (GLURP) and EBA175 have been associated with protection from clinical malaria (Roussilhon et al. Reference Roussilhon, Oeuvray, Muller-Graf, Tall, Rogier, Trape, Theisen, Balde, Perignon and Druilhe2007; Osier et al. Reference Osier, Fegan, Polley, Murungi, Verra, Tetteh, Lowe, Mwangi, Bull, Thomas, Cavanagh, McBride, Lanar, Mackinnon, Conway and Marsh2008; Fowkes et al. Reference Fowkes, Richards, Simpson and Beeson2010; Richards et al. Reference Richards, Stanisic, Fowkes, Tavul, Dabod, Thompson, Kumar, Chitnis, Narum, Michon, Siba, Cowman, Mueller and Beeson2010, Reference Richards, Arumugam, Reiling, Healer, Hodder, Fowkes, Cross, Langer, Takeo, Uboldi, Thompson, Gilson, Coppel, Siba, King, Torii, Chitnis, Narum, Mueller, Crabb, Cowman, Tsuboi and Beeson2013). With recent advances in genomics, proteomics and methods for protein expression, an increasing number of putative targets of naturally acquired immunity are being identified. These include hypothetical proteins and targets with no identified function. Osier et al. examined antibodies to 36 different proteins in a cohort of Kenyan children who were followed for malaria for 6 months. They found that the breadth and magnitude of antibody responses increased with age, and that the breadth of responses was a better correlate of protection than individual antibody responses (Osier et al. Reference Osier, Fegan, Polley, Murungi, Verra, Tetteh, Lowe, Mwangi, Bull, Thomas, Cavanagh, McBride, Lanar, Mackinnon, Conway and Marsh2008). In our microarray study examining antibody responses in a treatment-time to infection study in Kenya, we also found that 1–4-year-old children had the narrowest breadth and the lowest magnitude of antibody responses compared with older children and adults (Dent et al. Reference Dent, Nakajima, Liang, Baum, Moormann, Sumba, Vulule, Babineau, Davies, Felgner and Kazura2015). In our longitudinal infant study in Kenya, 12-month-old infants who had detectable serologically measured antimalarial antibodies had an increased risk of malaria in the subsequent year of life compared with 12-month-old infants with no detectable antimalarial antibodies (Dent submitted). Recently, Stanisic et al. (Reference Stanisic, Fowkes, Koinari, Javati, Lin, Kiniboro, Richards, Robinson, Schofield, Kazura, King, Zimmerman, Felger, Siba, Mueller and Beeson2015) examined antimalarial antibodies in cohorts of 1–4 and 5–14-year-old Papua New Guinean children. They found that young children who had higher levels of antibodies to MSP2, AMA1, EBA175, EBA140 and EBA181 had an increased risk of malaria compared with young children with low or no detectable antimalarial antibodies. Additionally, young children had antimalarial antibodies that were of significantly lower magnitude than those found in protected older (5–14-year-old) children. Results of this study indicate that one of the reasons why antibody responses in young infants represent biomarkers of malaria exposure rather than protection from malaria is related to failure of antibody responses to reach a critical ‘protective’ level, as determined by serology, until age 4 years or older. This is consistent with other studies that have found the magnitude of antibody responses influences protection from malaria (John et al. Reference John, Moormann, Pregibon, Sumba, McHugh, Narum, Lanar, Schluchter and Kazura2005; Osier et al. Reference Osier, Fegan, Polley, Murungi, Verra, Tetteh, Lowe, Mwangi, Bull, Thomas, Cavanagh, McBride, Lanar, Mackinnon, Conway and Marsh2008; Courtin et al. Reference Courtin, Oesterholt, Huismans, Kusi, Milet, Badaut, Gaye, Roeffen, Remarque, Sauerwein, Garcia and Luty2009; Murungi et al. Reference Murungi, Kamuyu, Lowe, Bejon, Theisen, Kinyanjui, Marsh and Osier2013). Serologically measured antimalarial antibodies may be a biomarker of exposure rather than protection especially in infants. Serology detects the presence of antibodies recognizing an antigen, but does not measure the function of these antibodies. Despite this, serology is useful to identify and prioritize antigen targets, especially in older protected children and adults, which may be valuable in a multicomponent vaccine, essential for eradicating malaria. Follow-up functional assays are invaluable tools in validating serologically identified targets.
VSAs are considered a major target of naturally acquired immunity (Bull et al. Reference Bull, Lowe, Kortok, Molyneux, Newbold and Marsh1998; Hviid, Reference Hviid2010; Chan et al. Reference Chan, Fowkes and Beeson2014) and include PfEMP1, repetitive interspersed family, subtelomeric variable open reading frame and surface-associated interspersed gene family proteins with PfEMP1 being the key target of humoral immunity (Chan et al. Reference Chan, Howell, Reiling, Ataide, Mackintosh, Fowkes, Petter, Chesson, Langer, Warimwe, Duffy, Rogerson, Bull, Cowman, Marsh and Beeson2012). PfEMP1, encoded by the var multigene family, is highly polymorphic and expression is clonally variant (Smith et al. Reference Smith, Chitnis, Craig, Roberts, Hudson-Taylor, Peterson, Pinches, Newbold and Miller1995). Antibodies that bind to VSA may function to prevent tissue sequestration by blocking VSA binding to endothelial receptors and facilitating opsonic phagocytosis by monocytes and macrophages. Serology has been used to measure antibody responses to various proteins and protein domains, but the VSA assay utilizes the whole Pf parasite to identify IgG antibodies that recognize the trafficked proteins on the surface of the infected erythrocyte. VSA antibodies are known to be highly parasite isolate-specific among children (Marsh and Howard, Reference Marsh and Howard1986; Reeder et al. Reference Reeder, Rogerson, al-Yaman, Anders, Coppel, Novakovic, Alpers and Brown1994), and may be short-lived (Kinyanjui et al. Reference Kinyanjui, Bull, Newbold and Marsh2003). It is thought that young children develop VSA antibodies to parasites expressing VSA associated with severe disease earlier in childhood than VSA associated with mild or moderate disease (Hviid and Staalsoe, Reference Hviid and Staalsoe2004). Studies in infants are limited but Vestergaard et al. (Reference Vestergaard, Lusingu, Nielsen, Mmbando, Dodoo, Akanmori, Alifrangis, Bygbjerg, Lemnge, Staalsoe, Hviid and Theander2008) showed that infants residing in a low malaria transmission region of Tanzania had low prevalence and magnitude of VSA antibodies compared with infants residing in regions of high transmission. In high transmission areas, VSA antibodies increased with age between 5 and 24 months. Nhabomba et al. (Reference Nhabomba, Guinovart, Jimenez, Manaca, Quinto, Cistero, Aguilar, Barbosa, Rodriguez, Bassat, Aponte, Mayor, Chitnis, Alonso and Dobano2014) found a lack of VSA antibody acquisition in infants up to 2 years of age in Mozambique, though their group previously found that infants acquire VSA antibodies in a study conducted in the same region at an earlier time when malaria transmission was presumably higher (Quelhas et al. Reference Quelhas, Jimenez, Quinto, Serra-Casas, Mayor, Cistero, Puyol, Wilson, Richards, Nhampossa, Macete, Aide, Mandomando, Sanz, Aponte, Alonso, Beeson, Menendez and Dobano2011). A caveat to these observations is that the parasite isolates used in these studies may not accurately represent the circulating parasites at the time the studies were conducted. A study in Papua New Guinea using a PfEMP1 microarray demonstrated that young children (<2 years old) had low prevalence, magnitude and breadth of antibodies compared with older children (Barry et al. Reference Barry, Trieu, Fowkes, Pablo, Kalantari-Dehaghi, Jasinskas, Tan, Kayala, Tavul, Siba, Day, Baldi, Felgner and Doolan2011). In general, VSA antibodies are low in infants and may not be readily acquired in the first year of life depending in large part on the geographical malaria transmission levels.
A better way to evaluate infant immunity may be to use assays that assess the function of the antimalarial antibodies. Antibodies may function by binding to merozoite surface proteins to inhibit erythrocyte invasion and intraerythrocytic growth. These can be measured with the Growth Inhibition Assay (GIA) which quantifies antibody-mediated activity against parasites in vitro and has been used to assess vaccine efficacy in animal models and malaria-naïve human volunteers (Singh et al. Reference Singh, Miura, Zhou, Muratova, Keegan, Miles, Martin, Saul, Miller and Long2006; Dutta et al. Reference Dutta, Sullivan, Grady, Haynes, Komisar, Batchelor, Soisson, Diggs, Heppner, Lanar, Collins and Barnwell2009; Spring et al. Reference Spring, Cummings, Ockenhouse, Dutta, Reidler, Angov, Bergmann-Leitner, Stewart, Bittner, Juompan, Kortepeter, Nielsen, Krzych, Tierney, Ware, Dowler, Hermsen, Sauerwein, de Vlas, Ofori-Anyinam, Lanar, Williams, Kester, Tucker, Shi, Malkin, Long, Diggs, Soisson, Dubois, Ballou, Cohen and Heppner2009; Remarque et al. Reference Remarque, Roestenberg, Younis, Walraven, van der Werff, Faber, Leroy, Sauerwein, Kocken and Thomas2012; Otsyula et al. Reference Otsyula, Angov, Bergmann-Leitner, Koech, Khan, Bennett, Otieno, Cummings, Andagalu, Tosh, Waitumbi, Richie, Shi, Miller, Otieno, Otieno, Ware, House, Godeaux, Dubois, Ogutu, Ballou, Soisson, Diggs, Cohen, Polhemus, Heppner, Ockenhouse and Spring2013). In malaria endemic populations, naturally acquired immunity measured by GIA has been associated with protection from infection in children in some studies, but this has not been a consistent finding (Dent et al. Reference Dent, Bergmann-Leitner, Wilson, Tisch, Kimmel, Vulule, Sumba, Beeson, Angov, Moormann and Kazura2008; McCallum et al. Reference McCallum, Persson, Mugyenyi, Fowkes, Simpson, Richards, Williams, Marsh and Beeson2008; Courtin et al. Reference Courtin, Oesterholt, Huismans, Kusi, Milet, Badaut, Gaye, Roeffen, Remarque, Sauerwein, Garcia and Luty2009; Crompton et al. Reference Crompton, Miura, Traore, Kayentao, Ongoiba, Weiss, Doumbo, Doumtabe, Kone, Huang, Doumbo, Miller, Long and Pierce2010). Growth inhibitory activity appears to decrease or remain relatively stable with age (Dent et al. Reference Dent, Bergmann-Leitner, Wilson, Tisch, Kimmel, Vulule, Sumba, Beeson, Angov, Moormann and Kazura2008; McCallum et al. Reference McCallum, Persson, Mugyenyi, Fowkes, Simpson, Richards, Williams, Marsh and Beeson2008; Courtin et al. Reference Courtin, Oesterholt, Huismans, Kusi, Milet, Badaut, Gaye, Roeffen, Remarque, Sauerwein, Garcia and Luty2009). Few studies have examined GIA in infants specifically. Wilson et al. examined GIA in infants from delivery to 12 months of age and found that GIA antibodies decline over time becoming essentially undetectable by 12 months of age (Wilson et al. Reference Wilson, Malhotra, Mungai, King and Dent2013). In contrast, Quelhas et al. found detectable GIA in 12-month-old infants that increased by 24 months of age but was not associated with protection from malaria (Quelhas et al. Reference Quelhas, Jimenez, Quinto, Serra-Casas, Mayor, Cistero, Puyol, Wilson, Richards, Nhampossa, Macete, Aide, Mandomando, Sanz, Aponte, Alonso, Beeson, Menendez and Dobano2011). In a longitudinal Kenyan infant study utilizing the MSP119 mediated invasion inhibition assay (MSP119 IIA) which uses transgenic parasites, by 12 months of age, infants had detectable MSP119 IIA that gradually increased by 24–30 months after birth (Dent et al. Reference Dent, Malhotra, Mungai, Muchiri, Crabb, Kazura and King2006). The MSP119 IIA measures one pathway of invasion inhibition which is only one component of the overall GIA. A drawback of GIA is that the targets of the antibodies that inhibit growth in vitro are not known and as with the VSA assay, the parasite isolates used in the GIA may not accurately represent the circulating parasites. Young children may have GIA antibodies, but this may be a reflection of malaria transmission (McCallum et al. Reference McCallum, Persson, Mugyenyi, Fowkes, Simpson, Richards, Williams, Marsh and Beeson2008). Further studies of the acquisition of functional antibodies in young children are needed.
In summary, infants acquire antimalarial antibodies with serologically measurable antibodies possibly acquired earlier than VSA or GIA measured antibodies which may reflect the late and complex acquisition of functional antibodies. Serologically measured antibodies may be a biomarker of exposure and may not protect the young child until a certain magnitude threshold is reached. These observations coupled with the immaturity of the infant immune system until 2 years of age (Jaspan et al. Reference Jaspan, Lawn, Safrit and Bekker2006) makes understanding how infants and young children acquire immunity a challenge.
Concluding remarks and future directions
Early infancy is a critical time in the development of immunity to malaria in children born in malaria endemic areas. Antibodies are known to play an important role in host defence against malaria, but the acquisition of antimalarial humoral immunity in infants is not yet well understood. Passively transferred maternal antibodies thought to protect infants younger than 6 months from clinical disease may actually be a biomarker of exposure and risk of infection rather than a correlate of protection. Infants with maternal antimalarial antibodies still become infected with low-grade parasite densities. As maternal antibodies wane, older infants gradually acquire antimalarial antibodies, with increasing breadth and magnitude of responses with age and exposure. Fetal exposure to malaria in utero may have an important effect on infant immune responses to malaria and other pathogens. In infants and young children, antibodies detected by serology may be a biomarker of exposure, with protection afforded only after a critical threshold is reached. As infants are a target population for future malaria vaccines, understanding the complexity of achieving protection in the context of an immature immune system must be considered. Further studies and development of novel assays are needed to analyse the functional capabilities of these antibodies and to evaluate the maternal, fetal and neonatal factors leading to the development of protective immunity in infants.
ACKNOWLEDGMENTS
We would like to acknowledge the important contributions made to this work by our colleagues at the Kenya Medical Research Institute and Case Western Reserve University.
FINANCIAL SUPPORT
This work was supported by the National Institutes of Health (grant AI098511 to A.E.D.)