Over one in three women of childbearing age has obesity in the USA, and postpartum weight retention (PPWR) is understood to be a major contributing factor(Reference Yang and Colditz1–Reference Soria-Contreras, Trejo-Valdivia and Cantoral3). Because PPWR leads to changes in fat deposition within the body, namely increased central adiposity, it can increase the risk for CVD, metabolic syndrome and type 2 diabetes(Reference Callo Quinte, Barros and Gigante4,Reference Gunderson, Sternfeld and Wellons5) .
Some studies suggest total energy intake and specific dietary components, including fried food and soda, contribute to PPWR(Reference Davis, Shearrer and Tao6,Reference Boghossian, Yeung and Lipsky7) . Understanding how modifiable dietary components, like sugar-sweetened beverage (SSB) intake, affect PPWR can inform the development of effective interventions to slow the progression of weight gain and adverse metabolic changes in childbearing women. There have been a number of pregnancy and postpartum dietary interventions aimed at reducing PPWR with varying results(Reference Berger, Peragallo-Urrutia and Nicholson8). Dietary PPWR interventions initiated in postpartum appear to be effective, but there is insufficient evidence for dietary interventions initiated during pregnancy(Reference Dalrymple, Flynn and Relph9). To understand if there is an optimal time frame for dietary PPWR interventions, intake patterns should be evaluated during pregnancy and the postpartum period.
While soda and other SSB consumption have been studied in the context of reduced fertility and poor fetal health outcomes in non-pregnant and pregnant women, respectively, there is relatively little information regarding maternal SSB consumption and PPWR(Reference Hatch, Wesselink and Hahn10). One 2017 study that evaluated associations between diet during the postpartum period and substantial PPWR (SPPWR), defined as a ≥ 5 kg increase compared with pre-pregnancy weight, in women with gestational diabetes found that soda intake was associated with increased odds of SPPWR(Reference Davis, Shearrer and Tao6). Although the current study examined associations of maternal SSB intake with PPWR, the findings were specific to soda intake in the postpartum period, and to women with gestational diabetes. Further, a recent study of ninety-nine Hispanic women found a statistically significant association between postpartum intake of soft drinks, sweet drinks and postpartum weight gain after adjusting for soluble fibre intake. While the current study suggests a link between maternal SSB intake and postpartum weight gain, findings were restricted to the postpartum period and to a small sample of Hispanic women(Reference Alderete, Wild and Mierau11). More generalisable studies are needed to better understand the relationship between maternal SSB intake and PPWR.
The objective of the current study was to examine associations of maternal SSB intake frequency in late pregnancy and early postpartum with weight retention at 6-month postpartum. We hypothesised that greater SSB intake frequency in late pregnancy and early postpartum would be associated with greater PPWR and increased odds of SPPWR at 6-month postpartum.
Methods
Subjects/study design
We included subjects enrolled in the ongoing Rise and SHINE (Sleep Health in Infancy and Early Childhood) prospective birth cohort for the current analysis. Mother–infant pairs from Boston, MA and surrounding areas enrolled in the Rise and SHINE study to examine the effect of sleep and feeding behaviours on accelerated weight gain in early childhood. The study followed mother–infant pairs from birth to 2 years. Maternal eligibility criteria included minimum age of 18 years, ability to speak English or Spanish, absence of psychosocial/mental health condition, sleep disorder or substance use problem in mother, no other child previously enrolled in the study, delivery at the Massachusetts General Hospital, singleton birth and delivery by at least 37-week gestation. Infant eligibility criteria included holding in the level one nursery without genetic disorders, congenital malformations or other conditions that affect sleep, plans to receive paediatric primary care at any Massachusetts General Hospital paediatric site and residence within 40 miles of Boston, MA without plans to move in the next year. Recruitment began in May 2016 and ended in June 2018.
At the time of the current analysis, 433 mother–infant pairs were consented and completed the intake visit within 2 d postpartum. Of these 433 mother–infant pairs, 352 completed the 1-month postpartum visit and 348 had non-missing outcome data and were thus included in the analytic sample. These data included dietary information for the third trimester and 1-month postpartum, pregravid and 6-month postpartum weight, and 6-month PPWR. Comparison of the 348 participants in the current analysis with the full 433 mother–infant pairs who were consented and completed the intake visit revealed some minor differences. The current analysis had a higher percentage of White (47 v. 43), a slightly smaller percentage of Hispanic (32 v. 34) and Black (7 v. 8) participants and a smaller percentage of missing/unknown data (0·3 v. 1·4). Other demographic information did not differ.
Main exposures
The main exposures were maternal SSB intake frequency in the third trimester of pregnancy (assessed within 2-d postpartum) and at 1-month postpartum (assessed between 2-week and 4-month postpartum). We measured SSB intake frequency as part of a comprehensive dietary survey using the Dietary Screener Questionnaire, which was developed and validated by the National Health and Nutritional Examination Survey 2009–2010(Reference Thompson, Midthune and Subar12,Reference Thompson, Midthune and Kahle13) . At both assessment periods, mothers recalled how frequently they consumed SSB over the last month. We asked mothers the following four questions: (1) in the past 4 weeks, on average, how often did you drink 100 % pure fruit juices such as orange, mango, apple, grape and pineapple juices? Do not count punch, Kool-Aid®, Tampico, sports drinks or Goya juice; (2) in the past 4 weeks, on average, how often did you drink punch, sweetened fruit drinks, sports drinks, Kool-Aid, Tampico, lemonade, Hi-C, cranberry drink, Goya or Vitamin Water? Do not include 100 % fruit juice or diet drinks; (3) in the past 4 weeks, on average, how often did you drink any regular sodas or soft drinks, including Manzanita, Penafiel, Coke, Pepsi, Dr. Pepper or Mountain Dew? Do not include diet sodas and (4) in the past 4 weeks, on average, how often did you drink energy drinks such as Red Bull, Monster or Rockstar? Possible answer choices included: never, less than once per week, once per week, 2–4 times/week, nearly daily or daily, 2–4 times/d, 5 or more times per day and refused/do not know. We standardised responses to times per day using the following rules: if participants answered ‘never’ or ‘less than once per week’, their average daily intake frequency was set to 0; ‘once per week’ was set to 1/7; ‘2–4 times/week’ was set to 3/7; ‘nearly daily or daily’ was set to 1; ‘2–4 times/d’ was set to 3; ‘5 or more times/d’ was set to 5 and finally, ‘refused/do not know’ was set to missing.
Outcome measures
The main outcome was PPWR. We used clinically measured weight in the electronic health record and research-measured weight at 6-month postpartum to calculate PPWR, defined as the difference between pregravid and 6-month postpartum weight. The allowable time frame for the 6-month postpartum visit was 6 months ± 8 weeks. We assessed this outcome both continuously and categorically as substantial PPWR. We defined SPPWR as an increase of ≥ 5 kg at 6-month postpartum compared with pre-pregnancy weight(Reference Davis, Shearrer and Tao6,Reference Oken, Kleinman and Belfort14) .
Covariates
To accurately estimate the relationship between maternal SSB intake and PPWR, we adjusted for the confounding factors of race/ethnicity, household income, maternal age, pre-pregnancy BMI and gestational weight gain. Pre-pregnancy BMI and gestational weight gain have been shown to be positively associated with PPWR, as is African American or Hispanic race/ethnicity(Reference Parker and Abrams15–Reference Van, Zhao and Pham17). Income and maternal age at delivery have been shown to be negatively associated with PPWR(Reference Gunderson, Abrams and Selvin18).
Statistical analysis
We first examined bivariate relationships between SSB intake frequency in the third trimester (split into quartiles) and demographics, maternal diet and anthropometric measures through means and standard deviations for continuous variables and percentages for discrete variables. Comparisons were made using either Kruskal–Wallis tests or χ 2 or Fisher’s exact tests as appropriate. We then assessed the association between SSB intake and PPWR using unadjusted and adjusted linear regression models, with robust se to account for potential heteroskedasticity in the residuals. We also examined the association between SSB intake and SPPWR through unadjusted and adjusted logistic regression models. Unadjusted models looked at the effect of SSB intake on PPWR and SPPWR; adjusted models evaluated the association between SSB intake and PPWR and SPPWR while controlling for various demographics and maternal anthropometric variables. All statistical inferences were made using two-sided tests and deemed statistically significant if P ≤ 0·05. For linear regression and logistic regression models, we reported 95 % CI. These convey the degree of uncertainty for our parameters, and the direction and strength of the demonstrated effect, with wider CI indicating greater uncertainty. In linear and logistic regression models, SSB intake frequency was a significant predictor of PPWR, or SPPWR, at P ≤ 0·05 when the 95 % CI did not contain 0 or 1, respectively. All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc.).
Missing data
As stated previously, 348 mother–infant pairs had non-missing outcome data. For our models, continuous covariates did not have missing data. We tried to create a ‘missing/unknown’ category for each discrete covariate with missing data, but our models failed to converge due to the small number of participants in the category. Thus, participants with missing data in the covariates were excluded from each model.
Results
Of the 433 women who completed intake visits, 348 were included in the final analysis. Mean age at delivery was 32·7 (sd 5·0) years. About 47 % of the women were White, 32 % were Hispanic, 14 % were Asian and 7 % were Black. Mean pre-pregnancy weight was 66·5 (sd 14·8) kg and pre-pregnancy BMI was 25·2 (sd 5·7) kg/m2. Mean gestational weight gain was 13·7 (sd 5·4) kg and at 6-month postpartum mean weight was 69·8 (sd 16·3) kg. Mean PPWR at 6-month postpartum was 3·4 (sd 5·7) kg and ranged from –11·4 to 27·9 kg. At 6-month postpartum, 31 % of the women had SPPWR. Women reported mean daily SSB intake frequencies of 0·9 (sd 1·2) and 0·7 (sd 1·0) times/d in the third trimester and at 1-month postpartum, respectively.
In bivariate analyses (Table 1), we observed that women in the top quartile for SSB intake frequency during the third trimester had the highest average PPWR at 6-month postpartum. Furthermore, women in the fourth quartile for SSB intake frequency had an average PPWR more than twice that of women in the second quartile. PPWR at 6-month postpartum was higher in the first quartile compared with the second; however, this was not statistically significant. Women who had higher SSB intake frequencies in the third trimester tended to be younger and less frequently non-Hispanic white. They also had higher pre-pregnancy weight and BMI, reported lower levels of education and household income, and had greater PPWR compared to those with lower SSB intake frequency in the third trimester. Table 1 presents the maternal characteristics of the overall study sample.
Table 1 Overall demographic characteristics and bivariate associations of maternal demographic characteristics with daily SSB intake frequency among 348 women participating in Rise and SHINE

SSB = sugar-sweetened beverage.
In the unadjusted linear regression model (Table 2), we found each 1-time/day increment in SSB intake frequency in the third trimester (β = 1·01 kg (95 % CI 0·57, 1·45)) and at 1-month postpartum (β = 0·93 kg (95 % CI 0·37, 1·50)) was associated with greater PPWR. Adjustment for race/ethnicity, household income, maternal age, pre-pregnancy BMI and gestational weight gain substantially attenuated the associations of greater SSB intake frequency in the third trimester (β = 0·46 kg (95 % CI 0·07, 0·86)) and at 1-month postpartum with greater PPWR (β = 0·52 kg (95 % CI 0·03, 1·00)) (Table 2).
Table 2 Associations of maternal daily SSB intake frequency in the third trimester and at 1-month postpartum with PPWR (n 348)
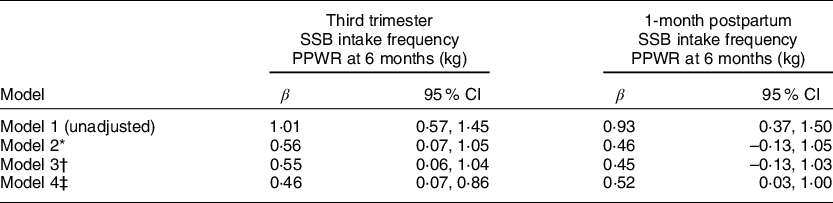
SSB = sugar-sweetened beverages; PPWR = postpartum weight retention.
* Model 2 = Model 1 + maternal age at delivery + race/ethnicity + household income.
† Model 3 = Model 2 + pre-pregnancy BMI.
‡ Model 4 = Model 3 + gestational weight gain.
In the unadjusted logistic regression models (Table 3), we found increased SSB intake frequency in the third trimester (OR: 1·57; 95 % CI 1·29, 1·95) and at 1-month postpartum (OR: 1·33; 95 % CI 1·07, 1·68) both resulted in women having higher odds of SPPWR. In the fully adjusted multivariable models adjusting for race/ethnicity, household income, maternal age, pre-pregnancy BMI and gestational weight gain, the association between greater SSB intake frequency in the third trimester (OR: 1·37; 95 % CI 1·10, 1·75) and at 1-month postpartum (OR: 1·17; 95 % CI 0·92, 1·52) with increased odds of SPPWR remained although attenuated (Table 3).
Table 3 Associations of maternal daily SSB intake frequency in the third trimester and at 1-month postpartum with SPPWR (n 348)

SSB = sugar-sweetened beverages; PPWR = postpartum weight retention.
* Model 2 = Model 1 + maternal age at delivery + race/ethnicity + household income.
† Model 3 = Model 2 + pre-pregnancy BMI.
‡ Model 4 = Model 3 + gestational weight gain.
Discussion
In the current study of 348 women in the perinatal period, linear regression models revealed that each 1-time/d increment in SSB intake frequency in the third trimester and at 1-month postpartum was associated with higher PPWR. Furthermore, logistic regression models showed increased SSB intake frequency in the third trimester and at 1-month postpartum resulted in women having higher odds of SPPWR at 6 months. Our findings emphasise the importance of diet during the perinatal period, as SSB intake during this time can have long-term adverse effects on maternal weight and overall health, as well as on fetal outcomes(Reference Beckerman, Slade and Ventura19,Reference Goran, Plows and Ventura20) .
The observed average PPWR in this sample (3·4 kg), as well as the percentage of women who have SPPWR (31 %), is consistent with findings from previous studies(Reference Gore, Brown and West21–Reference Olson, Strawderman and Hinton24). Additionally, similar to a prior study by Gamba and colleagues, our study found SSB intake during pregnancy to be higher than SSB intake during the postpartum period(Reference Gamba, Leung and Petito25). However, whether perinatal SSB intake is associated with PPWR has remained largely unexplored outside of two studies that focused on specific subsets of the general population, including women with gestational diabetes and women who identify as Hispanic(Reference Davis, Shearrer and Tao6,Reference Alderete, Wild and Mierau11) . Prior to our study, it had been unclear whether these findings extend to non-Hispanic women, women without gestational diabetes, and all SSB, considering there are many types of SSB in addition to soda and women with gestational diabetes have altered carbohydrate metabolism(Reference Butte26). By screening for over twenty different types of SSB and including a racially and ethnically diverse sample of women with and without gestational diabetes, to our knowledge, we demonstrate for the first time that perinatal SSB intake is associated with PPWR and SPPWR.
In the present study, we controlled for confounding demographic and socio-economic factors that have been demonstrated to be associated with PPWR by including them in our models as covariates(Reference Rifas-Shiman, Rich-Edwards and Kleinman27,Reference Boardley, Sargent and Coker28) . After controlling for these factors, we postulate that SSB consumption may contribute to PPWR by way of increased maternal energy intake. A recent study found each 12-ounce serving increment of SSB consumed during pregnancy was associated with an additional intake of 518·8 kJ/d (124 kcal/d), thus contributing to maternal energy intake(Reference Gamba, Leung and Petito25). This is consistent with the dose-dependent relationship of SSB intake frequency and PPWR seen in our linear regression models. Another study found increased energy intake between pregnancy and postpartum to be significantly positively associated with PPWR between 0–6 and 6–12 months(Reference Most, Altazan and St Amant29). In the same study, women who experienced PPWR had an average increase in total energy intake equal to 1046 kJ/d (250 kcal/d). Interestingly, Gamba and colleagues estimate total energy intake in pregnant women would decrease by 851·4 kJ/d (203·5 kcal/d) if SSB intake during pregnancy halted(Reference Gamba, Leung and Petito25).
We also consider inflammatory mechanisms as a plausible explanation for our observed associations between maternal SSB intake and PPWR. Food groups like fast food and SSB are considered proinflammatory and proinsulinaemic because they increase plasma levels of inflammatory biomarkers, such as C-reactive protein and C-peptide, which are proxies for inflammation and insulin, respectively(Reference Tabung, Smith-Warner and Chavarro30–Reference Tabung, Smith-Warner and Chavarro32). In fact, habitual SSB intake is positively associated with cardiometabolic inflammatory markers, such as C-reactive protein, in US women(Reference Yu, Ley and Sun33). A recent study found proinsulinaemic and proinflammatory diets are associated with substantial long-term weight gain regardless of energy intake. Although further studies are needed to support this hypothesis, the authors suggest altered metabolism in the setting of inflammation and hyperinsulinaemia as a potential mechanism(Reference Tabung, Satija and Fung34). Diets characterised as proinflammatory and/or proinsulinaemic are associated with inflammatory disease states including CVD, metabolic syndrome, overweight and obesity, yet there have been few investigations into maternal proinflammatory and proinsulinaemic diets, inflammatory biomarkers and PPWR(Reference Tabung, Smith-Warner and Chavarro32,Reference Visser, Bouter and McQuillan35–Reference Wang, Fung and Wang37) . Further studies are needed to investigate the potential inflammatory mechanisms underlying PPWR.
Our robust results adjust for confounding factors like race/ethnicity, household income, maternal age, pre-pregnancy BMI and gestational weight gain. However, our study presents with some limitations. While our study sample was racially/ethnically diverse, a large percentage of the women were college educated and had high household incomes. Thus, results may be less generalisable to socio-economically disadvantaged groups, which tend to consume low-cost beverages such as SSB at higher rates(Reference Lundeen, Park and Woo Baidal38,Reference Imoisili, Park and Lundeen39) . We used a complete case analysis, which can introduce bias if complete cases are systematically different from the sample as a whole. However, only 0·3 and 2·3 % of our analytic sample was missing race and household income, respectively; thus, major bias was not likely introduced. SSB intake frequency in the third trimester and at 1-month postpartum was collected during windows of time that allowed for maximal study participation making it possible for time periods of SSB intake frequency to overlap. Because this was a secondary analysis, we are unable to draw conclusions about the causality of the associations we observed for diet during pregnancy. We had dietary information for the third trimester of pregnancy only; thus, we could not capture and account for dietary variables earlier in pregnancy that could have impacted PPWR. Early pregnancy diets, though, tend to be higher quality than pre-pregnancy diets, so it is difficult to estimate if and how much our results would have changed had we collected dietary information from earlier in pregnancy(Reference Savard, Plante and Carbonneau40). The current study also relied on self-report for late pregnancy and early postpartum dietary information. Although subject to recall bias, administering the dietary screener questionnaires, which have been validated for use in pregnant women(Reference Cioffi, Figueroa and Welsh41,Reference Figueroa, Kalyoncu and Saltzman42) shortly after delivery and 1-month postpartum may have decreased the possibility for such bias.
Our study findings show that the frequency of maternal SSB intake in late pregnancy and early postpartum is associated with higher PPWR and higher odds of SPPWR at 6-month postpartum. Avoiding SSB during the perinatal period may help to reduce the risk for weight retention, obesity and associated complications. Understanding common, modifiable dietary patterns that contribute to PPWR allows for better development and implementation of dietary interventions. While recent data suggest an overall decline in SSB intake on a population level in US adults, whether this decline is true for pregnant and postpartum populations remains unclear(Reference Vercammen, Moran and Soto43). Pregnancy and postpartum represent times when women are particularly interested and motivated to modify their behaviours; however, simple pregnancy and postpartum dietary interventions, such as ones focused solely on SSB intake, may be more successful than cumbersome interventions.
Acknowledgements
Acknowledgements: None. Funding support: The current study was supported by grant number 1R01DK107972 (Taveras, Davison, Redline, multi-PI) from the National Institutes of Diabetes and Digestive and Kidney Diseases. Dr. Taveras’ time was supported by grant number 1K24DK105989 from the National Institute of Diabetes and Digestive and Kidney Diseases. Conflict of interest: None. Authorship: C.H. and S.G.A. performed the research. K.D., S.R. and E.M.T. designed the research study. J.M., M.S. and B.H. performed the analyses. J.M., M.S. and E.T. wrote the manuscript. All authors have read and approved the final manuscript. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Partners Healthcare Institutional Review Board. Written informed consent was obtained from all participants.





