While chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are confirmed major determinants for liver cirrhosis (LC) and hepatocellular carcinoma (HCC), a proportion of patients diagnosed with either cirrhosis or cancer of the liver are free of HBV or HCV infection(Reference Bartsch, Rojas, Nair, Nair and Alexandrov1), suggesting that other environmental or genetic factors contribute to the development of these disorders. Taiwan, with high prevalence rates of both cirrhosis and liver cancer, has an increasing rate of betel (Areca catechu) chewing; the nuts are chopped and, with Piper betle vine flower heads, used as betel-chews by a tenth of the 23 million inhabitants currently(2). This habit is recognized to be carcinogenic in man(2) and has been reported to be associated with LC and with HCC in Taiwan(Reference Tsai, Jeng and Chuang3–Reference Sun, Wu, Lin, Lu, You, Wang, Wu and Chen6).
Experimental studies demonstrate persistent hepatocyte necroinflammation after chronic betel feeding(Reference Prokopczyk, Rivenson, Bertinato, Brunnemann and Hoffmann7). Betel chewing is hepatotoxic and hepatocarcinogenic, owing to the high concentration of aflatoxin B1 in A. catechu nuts(Reference Raisuddin and Misra8), the high concentration of safrole in P. betle plants(Reference Tsai, Chuang, Jeng, Ho, Hsieh, Lin and Wang4) and the specific carcinogenic nitrosamines formed from arecal alkaloids and bacterial nitrite products during betel chewing and after acidification of swallowed chew materials in the stomach(2). Previous case–control and cohort studies have demonstrated positive associations between betel chewing, LC(Reference Tsai, Jeng and Chuang3) and HCC(Reference Tsai, Chuang, Jeng, Ho, Hsieh, Lin and Wang4–Reference Sun, Wu, Lin, Lu, You, Wang, Wu and Chen6). However, hospital-based, age- and gender-matched case–control studies are liable to recall bias with possible overestimation of associations. Moreover, they may not allow for the fact that exposure to betel quid chewing and HCV infection rates vary with age, or the possibility that these may be interactive risk factors, or that the impact of betel chewing on cirrhosis and cancer of the liver may vary with age and over time. These aspects of risk could be important in the search for new aetiologies for LC and HCC, but have not previously been considered and cannot be evaluated in matched case–control studies. The aims of the present study were therefore: (i) to confirm associations between betel chewing and the risk of LC and HCC; (ii) to determine, in particular, whether there are dose–response relationships for quantity or duration of chewing or age at first chewing; and (iii) to assess interactions between betel chewing and infection with HBV, HCV and age, using a large population-based study.
Subjects and methods
Study subjects
The study group was derived from the Keelung Community-based Integrated Screening (KCIS) programme, which has been in operation since 1999. It is a population-based recruitment programme with community-based screening for multiple diseases in Keelung, the northernmost county of Taiwan. The programme was set up to offer periodic screening for a variety of cancers and chronic diseases to a target population of 212 954 registry-listed residents aged over 30 years. Details of the study design, identification and surveillance of high-risk groups and of confirmatory tests for diagnosis of relevant cancers or chronic diseases have been described elsewhere(Reference Chen, Chiu and Luh9). In brief, the KCIS programme for liver disease used two-stage screening for liver cancer and non-neoplastic liver diseases (cirrhosis, haemangioma, pseudotumour and chronic hepatitis). Stage 1 used five serum markers – hepatitis B surface antigen (HBsAg), hepatitis C antibody (anti-HCV), raised serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) and high levels of α-fetoprotein (AFP ≥ 20 ng/ml) – to identify ‘high-risk’ subjects(Reference Chen, Chen, Yen, Lu, Sun, Huang, Yang, Lee and Duffy10). In Stage 2, subjects with one or more positive results at Stage 1 underwent ultrasound examination of the liver. Ultrasound examination of the liver was then offered to all recruits: 3-monthly for those found to have LC, haemangioma or pseudotumour; 6-monthly for those with early LC, manifested as patchy fibrosis; and annually for those in whom no abnormalities had been found at ultrasound. Subjects suspected of having HCC at ultrasonography (i.e. with hypoechoic, isoechoic or hyperechoic areas compared with surrounding liver parenchyma) were confirmed by AFP concentration >400 mg/ml, enhanced computed tomography or angiography or by histological findings at biopsy, where clinically indicated.
A total of 61 652 habitants aged 30–79 years were enrolled in the KCIS programme by the end of 2003. Of these, 1326 participants were excluded (no information on betel chewing, n 1274; pre-existing HCC, n 50; death before initial assessment completed, n 2) leaving 60 326 participants forming the present study population dataset. A further 481 subjects were excluded as having previously diagnosed LC for the analyses of associations for LC (n 59 845). Because the screening procedures were community-based, liver ultrasonography was used as the sole imaging method for screening and routine follow-up for parenchymal disorders; the characteristics assessed were ‘brightness’ and ‘coarseness’. Cirrhosis was diagnosed by coarseness of liver parenchyma, an uneven or nodular surface and widening of the angle of the left lateral lobe of the liver, and by signs of portal hypertension (enlarged splenic hilar vessels or patent ductus umbilicus) on ultrasound. Five hundred and eighty-eight subjects were found to have LC at enrolment or at subsequent follow-up. Since the KCIS programme is an ongoing community-based screening project for multiple diseases, participants have been followed over different times. Information on diseases of interest ascertained from referral systems to hospitals or clinics up to the end of 2003 were, as planned, included for analyses, allowing ascertainment of 131 of the 588 diagnoses of LC whose Stage 1 markers had been negative but who had presented to health-care institutions during the study period. Similarly, the 131 confirmed cases of HCC also included those identified through the screening programme at enrolment, at subsequent follow-up or identified through linkage of the study population with the Taiwan cancer and national mortality registers by the end of 2003. Both the Keelung study and the provisions for data linkage and maintaining patient confidentiality were approved by the local ethics committee (Health Bureau of Keelung city). Written informed consent was obtained from all live participants. Subjects in the present study had all been followed up for 1 year or more after recruitment (mean follow-up 2·45 (sd 1·20) years) and the data included for analyses, in each case, were those from the latest assessment made before the end of 2003.
Data collection
Information on betel-chewing habits and other lifestyle and demographic factors was collected using structured questionnaires at initial screening. The most popular type of betel-chew used in Keelung city is called ‘Lao-Hwa’ locally; it is prepared by combining pieces of unripe A. catechu palm nuts with a piece of the inflorescence (flower head) of the P. betle vine and red lime paste, and is swallowed after chewing(2). Subjects were classified as current chewers, ex-chewers or never-chewers at initial interview; the duration of betel chewing, age at starting chewing and number of betel portions chewed daily were recorded for current chewers and ex-chewers. The duration of chewing (in years) and the years since chewing cessation were also requested from ex-chewers. Smoking and alcohol consumption status were defined as current, ex or never. Information on duration (in years) and quantity of these habits was recorded. Cumulative exposure to these habits was expressed by a combination of duration and quantity: pack-years for cigarette consumption, glass-years for alcohol consumption and portion-days for consumption of betel chews, as follows.
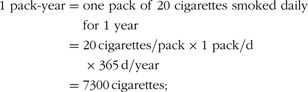

Various cut-off points were defined for use in analyses of the effects of cumulative exposure to each of these habits.
HBsAg status in KCIS programme attendees was assessed using radioimmunoassay kits (Abbott Laboratories, Chicago, IL, USA); anti-HCV was detected using a third-generation enzyme immunoassay (Abbott Laboratories).
Statistical analyses
Age-specific prevalence rates for LC and HCC were calculated by betel-chewing status using the year 2000 of the ‘world standard population’ as the standard population(Reference Ahmad, Boschi-Pinto, Lopez, Murray, Lozano and Inoue11). The relationships of betel chewing to LC or HCC at different ages were assessed using Cox proportional hazards regression models; hazard ratios and their 95 % confidence intervals were calculated with ‘survival’ time defined by the age at diagnosis for LC or HCC, whichever had come first. Censored cases included those who died from other causes and those alive but without any evidence of LC or HCC by the end of 2003, and hazard ratios for these disorders were calculated in relation to betel-chewing status before and after adjustment for other lifestyle and demographic risk factors. Interactions between HBsAg, anti-HCV and betel chewing were examined using synergy indices(Reference Hosmer and Lemeshow12). Trend tests were carried out on the associations with betel chewing for dose–response effects in relation to LC and HCC after adjustment for viral infection and other risk factors, using likelihood ratio tests.
Results
Morbidity rates for liver cirrhosis and hepatocellular carcinoma in current and ex-betel chewers v. never-chewers
Table 1 shows age-stratified and age-standardized rates of liver cirrhosis and liver cancer by betel-chewing status. Ever-chewers had a higher risk of developing LC than never-chewers in those under but not those over 50 years old. Relative age-standardized rates were higher in younger chewers (2·73-fold) than in older chewers (1·45-fold) with the interaction between age and betel chewing for LC (P < 0·01) suggesting that the effect of betel chewing on the risk of LC does vary with age. No such variation of betel-chewing effect with age was found for HCC although ever-betel chewers had a twofold increase in risk for liver cancer compared with never-chewers using age-standardized HCC rates. Sixty-seven per cent of LC cases were under 50 years of age whereas 73 % of HCC cases were aged 50 or older, suggesting that betel chewing impacts initially on the liver as cirrhosis, followed by increases in primary liver cancer risk over time.
Table 1 Age-specific and age-standardized morbidity rates of liver cirrhosis (n 59 845)Footnote * and hepatocellular carcinoma (n 60 326) by betel-chewing status: Keelung Community-based Integrated Screening programme, Taiwan, 1999–2003
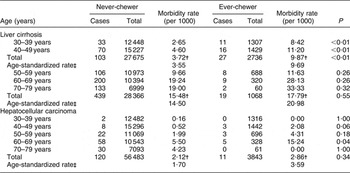
* Excluding 481 previously diagnosed cases of liver cirrhosis.
† Crude morbidity rate.
‡ Standardized population is by the year 2000 of ‘world standard population’(Reference Ahmad, Boschi-Pinto, Lopez, Murray, Lozano and Inoue11).
Association of betel chewing with liver cirrhosis and hepatocellular carcinoma
As suggested by the data in Table 1, the effects of betel chewing on LC and HCC appear to be a continuum over time. These conditions were, therefore, combined in a survival model. Table 2 shows that there was an increased risk of either LC or HCC, whichever was first diagnosed, in both betel chewers and ex-chewers compared with never-chewers, as well as in relation to the well-recognized risk factors, HBsAg or anti-HCV positivity and current alcohol use. Table 3 shows dose–response effects for the relationships between duration, quantity and cumulative exposure to betel chewing and also for the starting age of the betel-chewing habit and the risk of LC or HCC; this table also shows that these relationships were linear after controlling for gender, HBV and HCV infections, alcohol use and cigarette smoking.
Table 2 AdjustedFootnote * hazard ratios (HR) for the associations between relevant risk factors and exposure to betel chewing and liver cirrhosis (LC) or hepatocellular carcinoma (HCC): Keelung Community-based Integrated Screening programme, Taiwan, 1999–2003
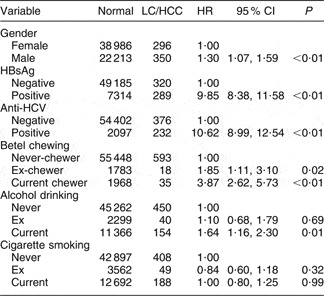
HBsAg, hepatitis B surface antigen; anti-HCV, hepatitis C antibody.
* Adjusted for gender, HBsAg, anti-HCV, exposure to alcohol drinking and exposure to cigarette smoking.
Table 3 AdjustedFootnote * hazard ratios (HR) for the associations between duration, quantity and cumulative exposure to betel chewing and liver cirrhosis (LC) or hepatocellular carcinoma (HCC): Keelung Community-based Integrated Screening programme, Taiwan, 1999–2003
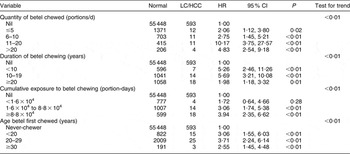
* Adjusted for gender, hepatitis B surface antigen, hepatitis C antibody, cumulative exposure to alcohol drinking and cumulative exposure to cigarette smoking.
Interaction between hepatitis virus infection and betel chewing
Since HBsAg and anti-HCV were the dominant factors accounting for LC and HCC in our data, the interactions between betel chewing and these factors are shown in Table 4, using data stratified by these infections. The risk for LC in chewers was increased in the absence of either HBV or HCV infection. Additive synergetic effects of betel chewing were found for LC or HCC with either HBV or HVC infection.
Table 4 AdjustedFootnote * hazard ratios (HR) of interactions between hepatitis B virus infection, hepatitis C virus infection and betel chewing and the risk of liver cirrhosis (LC) or hepatocellular carcinoma (HCC): Keelung Community-based Integrated Screening programme, Taiwan, 1999–2003
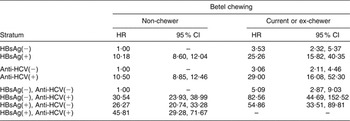
HBsAg, hepatitis B surface antigen; anti-HCV, hepatitis C antibody.
* Adjusted for gender, HBsAg, anti-HCV, cumulative exposure to alcohol and cumulative exposure to cigarettes. The synergy indices for the presence of HCC or LC, in combination with HBsAg and anti-HCV, are 2·07 (95 % CI 1·28, 3·37) and 2·42 (95 % CI 1·31, 4·47), respectively; with HBsAg but not anti-HCV it is 1·83 (95 % CI 1·14, 2·95), and with anti-HCV but not HBsAg it is 2·43 (95 % CI 1·32, 4·42).
Discussion
The present findings show marked associations of betel-nut (A. catechu) chewing with LC and HCC, after controlling for other recognized risk factors including HBV and HCV infections, in a population-based study of approximately 60 000 community dwellers in Taiwan, confirming previous smaller case–control and cohort study findings(Reference Wang, You, Lu, Ho, Wu, Sun, Yang and Chien-Jen5, Reference Srivatanakul, Parkin, Khlat, Chenvidhya, Chotiwan, Insiripong, L'Abbé and Wild13). These findings are supported by the dose–response effects found for quantity, duration, age at first chewing and cumulative exposure to betel chewing, independent of other recognized risk factors. HBV and HCV infections are the predominant risk factors for liver disease in Taiwanese subjects over 30 years old, infected before HBV vaccination programmes began in Taiwan in 1985, but independent associations of LC and HCC with betel chewing were also present in subjects free of HBV and/or HCV infection. Additive synergistic effects were found for betel chewing in those with HBV or HCV infection, as suspected in earlier case–control studies(Reference Tsai, Jeng and Chuang3, Reference Tsai, Chuang, Jeng, Ho, Hsieh, Lin and Wang4) and cohort studies(Reference Wang, You, Lu, Ho, Wu, Sun, Yang and Chien-Jen5, Reference Sun, Wu, Lin, Lu, You, Wang, Wu and Chen6) for LC and HCC risks.
Betel chewing could have a causal role in LC and HCC since increased risks for both were found in the absence of HBV or HCV infection. The habit is recognized to be carcinogenic in man(2) because safrole (from P. betel leaves)–DNA adducts are found in hepatocellular cancer(Reference Liu, Chen, Chang, Chu and Liu14).
Our findings for cirrhosis risk are biologically plausible because fibrosis results from exposure to betel-chew components, including Cu(Reference Trivedy, Meghji, Warnakulasuriya, Johnson and Harris15). Undue inhibition of matrix metalloproteinase (MMP) enzymes by their inhibitors also leads to excessive collagen deposition, as seen in betel-chew-related submucous fibrosis(Reference Tilakaratne, Klinikowski, Saku, Peters and Warnakulasuriya16, Reference Chang, Yang, Tai, Chou and Hsieh17) and HCV- and HBV-related LC(Reference Lichtinghagen, Michels, Haberkorn, Arndt, Bahr, Flemming, Manns and Boeker18, Reference Flisiak, Maxwell, Prokopowicz, Timms and Panasiuk19); and both arecoline and safrole increase expression of the tissue inhibitor of MMP-9 (tissue inhibitor of metalloproteinase-1; TIMP-1)(Reference Shieh, Chiang and Shieh20). Independent and dose-related increases in circulating TIMP-1 are found in betel chewers, suggesting that betel-related fibrosis need not be limited to the oral cavity(Reference Timms, Mannan, Hitman, Noonan, Mills, Syndercombe-Court, Aganna, Price and Boucher21). Hepatic stellate cell angiotensin-2 production induces excess collagen production through NADPH oxidase activation(Reference Bataller, Sancho-Bru, Gines and Brenner22) and blockade of the renin–angiotensin system (RAS) reduces fibrosis experimentally and in human liver(Reference Yokohama, Tokusashi and Nakamura23, Reference Yoshiji, Yoshii, Ikenaka, Noguchi, Tsujinoue, Nakatani, Imazu, Yanase, Kuriyama and Fukui24). Since the RAS is inhibited by vitamin D(Reference Basak, Bhattacharya and Chatterjee25), hypovitaminosis D may increase the risk for liver fibrosis, while activated vitamin D is also known to inhibit nitroso-related hepatocarcinogenesis(Reference Yokohama, Tokusashi and Nakamura23). There is an ‘urgent need to identify RAS inhibitors for trials in treatment for inhibition of liver fibrosis’(Reference Bataller, Sancho-Bru, Gines and Brenner22). Hypovitaminosis D is common worldwide, being especially severe in south Asians and more common in south Asians than in other populations in the UK, as are both LC and HCC(Reference Douds, Cox, Iqbal and Cooper26–Reference Boucher28); furthermore, increased activity of the enzyme catabolic for hormonal vitamin D, a 24-hydroxylase, has been reported in south Asians(Reference Awumey, Mitra, Hollis, Kumar and Bell29). Dose-related increases in expression of this 24-hydroxylase have been found in circulating blood mononuclear cells in man in relation to betel chewing(Reference Chiang, Huang, Wang, Liu, Kuo, Hahn and Kuo30, 31). It is, therefore, possible that avoidance of hypovitaminosis D could reduce risk for LC and HCC in betel chewers and others, and this postulate requires further investigation.
The dose-related effects we report for hepatocellular cancer are also biologically plausible since the triggers for hepatocyte necroinflammation and HCC include carcinogenic betel-chew adducts such as the arecal nitrosamines which, like betel leaf safrole, induce precancerous changes such as p53 mutations(Reference Chiang, Huang, Wang, Liu, Kuo, Hahn and Kuo30–Reference Wong, Liu, Chang, Lin, Chao, Li and Chang32), over-expression of c-myc protein, activation of the ras oncogene(Reference Baral, Patnaik and Das33) and subsequent over-expression of the cell cycle regulatory protein, cyclin D1(2, Reference Kuo, Lin, Hahn, Cheng and Chiang34).
Nitroso adducts of areca alkaloids are formed in the nut and in the mouth (in the presence of nitrite-generating bacteria) and formation increases with acidification of swallowed material(2). In addition, these carcinogens target foregut-derived tissues, including liver, whether administered orally or by intravenous injection(2).
The increased association of LC with betel chewing in younger onset chewers provides some indication of potential years of life lost from the habit – information needed in planning betel cessation programmes. In Taiwan, reduction in LC following HBV immunization programmes has been matched by increased betel chewing, mainly in young adolescents(Reference Wen, Tsai, Cheng, Chen, Levy, Yang and Eriksen35); thus, this habit could become a major risk factor for LC in younger subjects. In 1999, 21 % of Taiwanese subjects under 50 years old had chewed betel(Reference Wen, Tsai, Cheng, Chen, Levy, Yang and Eriksen35). The potential for reduction in LC risk in ex-chewers, calculated from data on LC risk in betel chewers (Table 2), suggests a 29 % reduction in combined prevalence of LC and HCC for cessation at a 21 % usage rate(Reference Wen, Tsai, Cheng, Chen, Levy, Yang and Eriksen35), a comparable reduction to that for elimination of HCV infection (27 %), although only half of the figure anticipated with elimination of HBV infection (54 %). In London, where death rates for HCC are doubled in south Asians(Reference Leon and McCambridge36) and betel chewing is much more prevalent(Reference Boucher and Mannan37), reductions could be much higher.
In conclusion, we have confirmed independent dose–response relationships of betel chewing with increased risk for either LC or HCC in a large population-based study, after adjustment for the high prevalence of viral hepatitis. The betel-related risk of cirrhosis is larger in the under-50s than in over-50s, while the risk of betel-related primary hepatocellular cancer is larger in over-50s than in the under-50s. Increased risks were found in those free of HBV or HCV infections and, in addition, the risks of betel chewing were synergistically additive to those of HBV and HCV infections. Thus, risk reductions with effective anti-betel chewing programmes could be sizeable.
Acknowledgements
Funding source:This study was supported financially by the Taiwan National Science Council (NSC 91-2320-B002-171; NSC 91-2320-B002-172).
Acknowledgements:We are indebted to those who were involved in this work, including Po-En Wang (Director of Keelung Health Bureau), Ting-Ting Wang (Chief of Health Prevention Division) and other health staff in Keelung Health Bureau.
Author contributions and conflict of interest:G.H.-M.W. contributed to data retrieval, data management, statistical analysis, interpretation of results and writing the manuscript. B.J.B. contributed intellectually to the concepts studied, to discussion of the findings and to writing the manuscript. Y.-H.C. contributed to data retrieval, data management, statistical analysis, interpretation of results and writing the manuscript. C.-S.L. contributed to data retrieval, data management, statistical analysis, interpretation of results and writing the manuscript. T.H.-H.C. directed the research and contributed to the proposal of study hypothesis, study conception, study design, data retrieval, interpretation of the findings and writing the manuscript. The authors declare that they have no competing interests.






