Introduction
Besnoitia besnoiti, an obligate intracellular apicomplexan parasite, was firstly described in 1912 (Besnoit and Robin, Reference Besnoit and Robin1912). Several reports on bovine besnoitiosis (i.e. Portugal (Cortes et al., Reference Cortes, Reis, Waap, Vidal, Soares, Marques, Pereira da Fonseca, Fazendeiro, Ferreira, Caeiro, Shkap, Hemphill and Leitão2006), Spain (Fernández-García et al., Reference Fernández-García, Risco-Castillo, Pedraza-Díaz, Aguado-Martínez, Álvarez-García, Gómez-Bautista, Collantes-Fernández and Ortega-Mora2009), France (Jacquiet et al., Reference Jacquiet, Liénard and Franc2010), Germany (Schares et al., Reference Schares, Basso, Majzoub, Cortes, Rostaher, Selmair, Hermanns, Conraths and Gollnick2009), Italy (Gollnick et al., Reference Gollnick, Gentile and Schares2010; Rinaldi et al., Reference Rinaldi, Maurelli, Musella, Bosco, Cortes and Cringoli2013), Switzerland (Basso et al., Reference Basso, Lesser, Grimm, Hilbe, Sydler, Trösch, Ochs, Braun and Deplazes2013) and Hungary (Hornok et al., Reference Hornok, Fedák, Baska, Hofmann-Lehmann and Basso2014)) clearly indicate the spread of this disease within Europe (Álvarez-García et al., Reference Álvarez-García, Frey, Mora and Schares2013). Based on the increased number of cattle besnoitiosis cases and its geographic expansion into previous non-endemic countries, the European Food Safety Authority (EFSA) classified bovine besnoitiosis as an emerging disease within EU in 2010 (European Food Safety Authority, 2010). Besides Europe, bovine besnoitiosis is also a vastly endemic disease in the Middle East, Asia, South America (Trujillo and Benavides, Reference Trujillo and Benavides2011; Vogelsang and Gallo, Reference Vogelsang and Gallo1941) and Africa (Bigalke and Prozesky, Reference Bigalke, Prozesky, Coetzer and Tustin2014; Cortes et al., Reference Cortes, Leitão, Gottstein and Hemphill2014) causing significant economic losses in cattle industry due to significant reduction of productivity (Jacquiet et al., Reference Jacquiet, Liénard and Franc2010; Maqbool et al., Reference Maqbool, Bhat, Shah, Ganayi and Sheikh2012).
Typically, bovine besnoitiosis is characterized by an acute and a chronic phase with different clinical signs. In the acute phase, B. besnoiti-infected cattle present pyrexia, intensive respiratory disorders, increased heart rates, subcutaneous oedema, anasarca, swollen joints, conjunctivitis, nasal discharge, photophobia, reduced milk yield and orchitis associated with permanent infertility in bulls (Bigalke, Reference Bigalke, Ristic and McIntyre1981; Álvarez-García et al., Reference Álvarez-García, Frey, Mora and Schares2013; Cortes et al., Reference Cortes, Leitão, Gottstein and Hemphill2014). During the chronic phase of disease, B. besnoiti bradyzoites proliferate slowly within the epidermis, subcutaneous tissues, mucous membranes and/or sclera, and form characteristic cysts within mesenchymal host cells, related to dramatic thickening, hardening, folding, wrinkling of the skin (also termed ‘elephant skin’), alopecia, and gradual deterioration of body condition and weight loss (Pols, Reference Pols1960). Until now, the complete life cycle of B. besnoiti is not entirely known and final host species are unidentified carnivores. Nevertheless, direct contact between infected and non-infected animals (e.g. natural mating, naso-pharyngeal route) and insect-mediated transmission through biting flies (i. e. tabanids (Tabanus spp.), stable flies (Stomoxys calcitrans)) have been suggested as suitable transmission routes (Gollnick et al., Reference Gollnick, Scharr, Schares and Langenmayer2015; Gutiérrez-Expósito et al., Reference Gutiérrez-Expósito, Ferre, Ortega-Mora and Álvarez-García2017; Tainchum et al., Reference Tainchum, Shukri, Duvallet, Etienne and Jacquiet2018) and of epidemiological relevance (Sharif et al., Reference Sharif, Jacquiet, Prevot, Grisez, Raymond-Letron, Semin, Geffré, Trumel, Franc, Bouhsira and Liénard2019).
So far, very limited knowledge exists on early interactions between circulating polymorphonuclear neutrophils (PMN) of host innate immune system with B. besnoiti, although these cells are the first ones to be recruited to infection sites. As such, PMN are the most abundant granulocytes in the blood and being the first line of defence against invading pathogens including parasites (Weissmann et al., Reference Weissmann, Smolen and Korchak1980; Behrendt et al., Reference Behrendt, Ruiz, Zahner, Taubert and Hermosilla2010; Villagra-Blanco et al., Reference Villagra-Blanco, Silva, Muñoz-Caro, Yang, Li, Gärtner, Taubert, Zhang and Hermosilla2017a). Upon activation, and in addition to phagocytosis (Behrendt et al., Reference Behrendt, Taubert, Zahner and Hermosilla2008) and degranulation (Lacy, Reference Lacy2006), PMN also combat efficiently invading pathogens by releasing neutrophil extracellular traps (NETs) (Fuchs et al., Reference Fuchs, Abed, Goosmann, Hurwitz, Schulze, Wahn, Weinrauch, Brinkmann and Zychlinsky2007; Brinkmann and Zychlinsky, Reference Brinkmann and Zychlinsky2012; Brinkmann, Reference Brinkmann2018). These NETs are composed of nuclear DNA decorated with different histones (H1, H2A/H2B, H3, H4) and various antimicrobial granular effector molecules and commonly released via a novel cell death process known as suicidal NETosis. Suicidal NETosis is characterized by nuclear and cell membrane rupture and the loss of main PMN functions such as chemotaxis, degranulation and phagocytosis (Fuchs et al., Reference Fuchs, Abed, Goosmann, Hurwitz, Schulze, Wahn, Weinrauch, Brinkmann and Zychlinsky2007; Remijsen et al., Reference Remijsen, Kuijpers, Wirawan, Lippens, Vandenabeele and Vanden Berghe2011b; Yipp and Kubes, Reference Yipp and Kubes2013). In contrast, vital NETosis can also occur by not affecting the continuation of mentioned PMN functions (Yipp and Kubes, Reference Yipp and Kubes2013). Vital NETosis have been demonstrated in response to bacteria (Pilsczek et al., Reference Pilsczek, Salina, Poon, Fahey, Yipp, Sibley, Robbins, Green, Surette and Sugai2010), fungi (Byrd et al., Reference Byrd, O'Brien, Johnson, Lavigne and Reichner2013), LPS-activated platelets (Clark et al., Reference Clark, Ma, Tavener, McDonald, Goodarzi, Kelly, Patel, Chakrabarti, McAvoy and Sinclair2007) and even the protozoan parasite Leishmania amazonensis (Rochael et al., Reference Rochael, Guimaraes-Costa, Nascimento, DeSouza-Vieira, Oliveira, Garcia e Souza, Oliveira and Saraiva2015). A landmark of vital NETosis is its rapid induction, normally within 30 min after PMN stimulation (Yipp and Kubes, Reference Yipp and Kubes2013) or as early as 10 min after neutrophil-L. amazonensis interaction (Rochael et al., Reference Rochael, Guimaraes-Costa, Nascimento, DeSouza-Vieira, Oliveira, Garcia e Souza, Oliveira and Saraiva2015). In previous studies, it was shown that suicidal NETosis was able to efficiently trap B. besnoiti tachyzoites in vitro and that released suicidal NETosis was capable of hampering tachyzoites from active host cell invasion (Muñoz Caro et al., Reference Muñoz Caro, Hermosilla, Silva, Cortes and Taubert2014a). Furthermore, also bovine monocyte-derived extraellular traps (METosis) occurred when these phagocytes have been exposed to vital and motile B. besnoiti tachyzoites (Muñoz Caro et al., Reference Muñoz Caro, Hermosilla, Silva, Cortes and Taubert2014a), thereby expanding the spectrum of leukocytes undergoing ETosis (Villagra-Blanco et al., Reference Villagra-Blanco, Silva, Conejeros, Taubert and Hermosilla2019).
Conversely, no data are available so far neither on interactions of bovine PMN with B. besnoiti bradyzoites nor the role of autophagy in parasite-induced NETosis. Autophagy has recently been indicated to play a crucial role not only influencing classical PMN-mediated effector mechanisms (e.g. phagocytosis) (Mitroulis et al., Reference Mitroulis, Kourtzelis, Kambas, Rafail, Chrysanthopoulou, Speletas and Ritis2010; Skendros et al., Reference Skendros, Mitroulis and Ritis2018) but also actively regulating NETosis (Skendros et al., Reference Skendros, Mitroulis and Ritis2018). Thus, in the present study, we intended firstly to investigate rapid vital- as well as suicidal-NETosis in bovine PMN exposed to freshly isolated bradyzoites of B. besnoiti from subdermal tissue cysts and further to analyse the possible correlation of autophagy in B. besnoiti bradyzoite-mediated NETosis.
Materials and methods
Animal data
In early 2018, a 4-year-old Limousine heifer (502 kg BW) from South France presented inappetence, limb oedema with desquamation and emaciation. Natural B. besnoiti infection was confirmed by polymerase chain reaction investigation. For animal treatment, flunixine meglumine (2.2 mg/kg; Finadyne®) and sulfamethoxine (40 mg/kg; Sulfaron®) were given. Three months later, the same animal was admitted to the Ecole Nationale Veterinaire Toulouse (ENVT) because of weakness, hyperkeratosis, and multifocal alopecia, and presence of multiple visible cysts within the sclera. One week later, the animal was euthanized due to severe emaciation. At necropsy, bovine besnoitiosis in the scleroderma phase was confirmed: multiple whitish punctuated cysts were observed in sclera and in mucocutaneous junctions of mouth and anus. Moreover, the skin of neck, shoulders, base of tail, hocks, and pasterns presented marked hyperkeratosis with crusty appearance. No other relevant clinical alterations were further noticed. Skin samples of affected areas have been collected, stored in sterile saline solution (4°C) and later on processed for histopathological evaluation as well as for parasite isolation.
Isolation of vital Besnoitia besnoiti bradyzoites
Skin biopsies were placed in a sterile Petri dish (Nunc) containing a small volume of sterile RPMI 1640 cell culture medium without phenol red (Sigma-Aldrich) supplemented with 1% penicillin-streptomycin (Sigma-Aldrich). A sterile tweezer was used to hold the skin and with a sterile scalpel, the skin surface was carefully scraped in order to release vital bradyzoites from these cysts. As soon as the RPMI 1640 cell culture medium became turbid, it was collected and filtered through a sterile gauze swab in a sieve into a 50-mL Falcon tube followed by centrifugation at 200 × g for 1 min at room temperature (RT). Then the supernatant was collected and transferred into a new 50-mL Falcon tube, centrifuged (400 × g, 12 min), and the pellet was washed again with RPMI 1640 medium to collect released bradyzoites. All supernatants were collected and centrifuged at 1500 × g for 10 min, and then the supernatant was discarded and the pellet containing bradyzoites was resuspended in sterile RPMI 1640 cell medium. Vital and extremely motile B. besnoiti bradyzoites were isolated and afterwards counted in a Neubauer haemocytometer chamber (Supplementary data video 1). Isolated B. besnoiti bradyzoites were firstly stored for 30 min at 4°C and afterwards at −80°C in RPMI 1640 cell medium supplemented with 10% DMSO (Merck).
Histopathological examination
After successful bradyzoites isolation, parts of skin samples (5 × 5 mm2) were stored in 10% phosphate-buffered formalin for histopathological examinations. Shortly, formalin-fixed samples were dehydrated using an ascending ethanol series, embedded in paraffin wax at 56°C and finally sectioned at 3 μm tissue samples at the Institute of Veterinary Pathology, Faculty of Veterinary Medicine, Justus Liebig University Giessen, Germany. Histological tissue samples have been stained using haematoxylin and eosin (HE), periodic acid-Schiff (PAS) and Giemsa staining according to routine protocols and pathological findings/changes of B. besnoiti-infected skin samples were then evaluated under a light microscope (Nikon Eclipse 80i) equipped with a DS-Fi1 digital camera (Nikon).
Isolation of bovine PMN
Healthy adult dairy cows (n = 3) were served as blood donors. Animals were bled by puncture of the jugular vein and 30 ml peripheral blood was collected in 12 ml heparinized sterile plastic tubes (Kabe Labortechnik). Approximately 20 ml of heparinized blood was re-suspended in 20 ml sterile PBS with 0.02% EDTA (Sigma-Aldrich), slowly layered on top of 12 ml Biocoll® separating solution (density = 1.077 g/L; Biochrom AG), and centrifuged (800 × g, 45 min). After extraction of plasma and peripheral mononuclear blood cells (PBMC), the pellet was washed in 25 ml distilled water and gently shaken during 40 s in order to lyse erythrocytes. Osmolarity was rapidly restored by Hank's balanced salt solution (4 ml, HBSS 10×; Biochrom AG). To complete erythrocyte lysis, this step was repeated twice and PMN were later re-suspended in sterile RPMI 1640 medium (Gibco). Finally, freshly isolated bovine PMN were allowed to rest at 37°C and 5% CO2 atmosphere for 30 min until further use (Behrendt et al., Reference Behrendt, Ruiz, Zahner, Taubert and Hermosilla2010).
For each experiment, purity and viability of neutrophils were determined. Only samples with a purity of neutrophils higher than 93% and viability greater than 96% (tested by trypan blue exclusion assay (Sigma-Aldrich)) were used.
Scanning electron microscopy (s.e.m.) analysis
Bovine PMN were co-cultured with vital B. besnoiti bradyzoites (ratio 1:4) for 3 h on coverslips (10 mm diameter; Nunc) pre-coated with 0.01% poly-L-lysine (Sigma-Aldrich) in an incubator at 37°C and 5% CO2 atmosphere. After incubation, cells were fixed in 2.5% glutaraldehyde (Merck), post-fixed in 1% osmium tetroxide (Merck), washed in distilled water, dehydrated, critical point dried by CO2-treatment and sputtered with gold. Finally, all samples were visualized via a Philips® XL30 scanning electron microscope at the Institute of Anatomy and Cell Biology, Justus Liebig University Giessen, Germany.
Immunofluorescence microscopy analysis for visualization of B. besnoiti bradyzoite-triggered NETosis
Freshly isolated bovine PMN were co-cultured on 0.01% poly-L-lysine pre-treated coverslips (15 mm diameter) with B. besnoiti bradyzoites (ratio 1:4) for 3 h (37°C and 5% CO2 atmosphere), then fixed by adding 4% paraformaldehyde (Merck) for 15 min and stored at 4°C until further epifluorescence microscopy experiments.
For visualization of suicidal NETosis-related structures, Sytox Orange® (Life Technologies) was used to stain extracellular DNA, anti-histone 3 (H3; clone H11-4, 1:1000; Merck Millipore) and anti-neutrophil elastase (NE) antibodies (AB68672, 1:1000, Abcam) were used to label H3 and NE on NETosis structures. In brief, fixed samples were washed thrice with sterile PBS, then blocked with 1% bovine serum albumin (BSA; Sigma-Aldrich) at RT for 15 min, incubated with corresponding primary antibodies (1 h; RT), and then incubated with secondary antibodies (Alexa Fluor 488 goat anti-mouse IgG or Alexa Fluor 405 goat anti-rabbit IgG, both Life Technologies, 60 min, 1:1000, RT), and finally incubated for 15 min with Sytox Orange® (Life Technologies). After incubation, samples were carefully mounted with the anti-fading solution (ProLong Gold® anti-fading buffer; Thermo Fisher Scientific) and thereafter visualized using an inverted IX81® epifluorescence microscope (Olympus) equipped with a digital camera XM10® (Olympus).
Live cell interactions between bovine PMN and B. besnoiti bradyzoites investigated by live cell 3d holotomographic microscopy
Isolated PMN (1 × 106) were centrifuged at 300 × g for 10 min at RT, the supernatant was carefully discarded and cells were suspended in 2 ml of pre-warmed RPMI 1640 cell medium. One ml of this PMN solution was placed in an Ibidi® cell plate 35 mm low profile, and the plate was incubated in an Ibidi® chamber at 5% CO2 and 37°C. PMN were allowed to settle down (30 min) to bottom of the plate and then 2 × 106 B. besnoiti bradyzoites were added to the center of the plate. The acquisition was set for refractive index (RI; 3D tomography) for a time-lapse of 155 min every 30 s in a Nanolive Fluo-3D Cell Explorer® (Nanolive) microscope. At the end of the experiment, images were exported using Steve software v.2.6® (Nanolive). Using Image J software (Fiji version 1.7, NIH), every frame was exported using z-projection, maximum intensity algorithm and the video was constructed using 1 frame per 5 s of speed. For zoomed video, the region of interest was cropped and the same procedure described above was applied. Digital staining and 3D rendering of activated PMN was performed by using Steve software v.2.6® (Nanolive).
Autophagosome detection by immunofluorescence analysis
LC3 has been used as a classical marker for autophagosomes (Karim et al., Reference Karim, Kanazawa, Daigaku, Fujimura, Miotto and Kadowaki2007), being LC3-I cytosolic and LC3-II membrane bound and enriched in the autophagic vacuole. Therefore, we tested whether LC3 expression might be present during B. besnoiti bradyzoite-induced NETosis as described elsewhere (Zhou et al., Reference Zhou, Conejeros, Velásquez, Muñoz-Caro, Gärtner, Hermosilla and Taubert2019). Briefly, bovine PMN (n = 3) were added on 0.01% poly-L-lysine pre-coated coverslips (15 mm diameter; Nunc), then stimulated by B. besnoiti bradyzoites for 1 h at RT. After incubation, cells were fixed with 4% paraformaldehyde for 10 min, permeabilized with cold methanol (Merck) for 3 min and blocked by using the following blocking buffer (5% BSA (Sigma-Aldtich), 0.1% Triton X-100 (Sigma-Aldrich) in sterile PBS) for 60 min at RT. After removing blocking buffer, cells were incubated overnight at 4°C with rabbit anti-LC3B antibodies (Cat#2775, 1:200, Cell Signaling Technology) diluted in blocking buffer, washed three times with PBS, incubated with goat anti-rabbit IgG conjugated with Alexa Fluor 488 (Invitrogen) for 1 h in the dark at RT. After being washed three times with PBS, coverslips were mounted by prolonged anti-fading reagent with DAPI (Invitrogen) on glass slides (Nunc), and five randomly images were taken per condition using an inverted epifluorescence microscope IX 81® (Olympus) and/or by using confocal microscopy analysis (LSM 710®; Zeiss).
Statistical analysis
Results are illustrated as means ± s.e.m. of at least three independent experimental settings. One-way analysis of variance and Dunnett's multiple comparison test and Spearman correlation test were performed here by using GraphPad Prism 7®. Differences were considered significant at a level of P ⩽ 0.05.
Results
Histopathological examination of B. besnoiti-infected skin
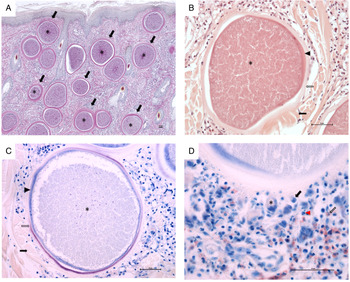
Histopathological examination revealed multifocal large-sized round to ovoid B. besnoiti-cysts present in the dermis, panniculus and underlying muscle layer (Fig. 1A, highlighted exemplary by black arrows). Rare early tissue cysts were small, approximately 10–20 μm and contained a parasitophorous vacuole (PV) with few banana-shaped 3–5 μm structures (bradyzoites). Mature B. besnoti-cysts (up to 400 μm) were filled with thousands of bradyzoite stages (Fig. 1A-C, asterisks). Cysts containing numerous typical banana-shaped B. besnoiti bradyzoites had a three-layered wall (Fig. 1B and C, thickness of 10–30 μm thick): the outer wall composed of compressed collagen type I fibres (Fig. 1B and C, black arrows), the middle layer representing a thick hyaline capsule composed of extracellular matrix (Fig. 1B and C, clear arrows) and the inner layer composed of a small rim of host cell cytoplasm with often multiple flattened nuclei containing the PV (Fig. 1B and C, arrowheads). The outer wall of compressed collagen, as well as the bradyzoites, stained mildly and the middle hyaline layer were brightly stained with PAS (Fig. 1A). Using Giemsa stain, the inner rim of the middle hyaline layer of mature cysts stained purple, while the outer rim of the hyaline layer was translucent (Fig. 1C, clear arrow) and the inner layer containing the host cell cytoplasm as well as the bradyzoites themselves stained blue (Fig. 1C, arrowhead and asterisk). Surrounding the tissue cysts there was a mild to moderate multifocal to coalescing infiltrate (Fig. 1D) composed of macrophages (Fig. 1D, black arrow), fewer lymphocytes, plasma cells (Fig. 1D, black arrowhead), neutrophils (Fig. 1D, red arrowhead) and eosinophils (Fig. 1D, clear arrow) and rare multinucleated giant cells (Fig. 1D, asterisk). Few tissue cysts were found ruptured and surrounded by abundant macrophages including multinucleated giant cells, numerous neutrophils and eosinophils, which were sometimes arranged in clusters as well as fewer lymphocytes and plasma cells. Also, throughout the affected skin, there was mild to moderate diffuse epithelial hyperplasia and moderate orthokeratotic hyperkeratosis.

Fig. 1. Histopathological examination of skin biopsy (scale bars = 50 μm). (A) Characteristic mature cysts of Besnoitia besnoiti within the dermis, Periodic acid–Schiff (PAS) staining, 40 × total magnification; (B) A mature cyst of Besnoitia besnoiti with a three-layered wall composed of an outer (black arrow), middle (clear arrow) and inner wall (arrowhead), haematoxylin and eosin (H&E) staining, 200 × total magnification; (C) A mature cyst of Besnoitia besnoiti with a three-layered wall composed of an outer (black arrow), middle (clear arrow) and inner wall (arrowhead), Giemsa staining, 200 × total magnification; (D) Vicinity of a mature Besnoitia besnoiti cyst with an inflammatory infiltrate composed of macrophages (black arrow), fewer lymphocytes and plasma cells (black arrowhead), neutrophils (red arrowhead) and eosinophils (clear arrow) as well as rare multinucleated giant cells (asterisk), Giemsa staining, 400 × total magnification. Scale bar = 50 μm.
Besnoitia besnoiti bradyzoite-triggered NETosis was unveiled via s.e.m.- and immunofluorescence microscopy-analysis
To investigate whether B. besnoiti bradyzoites were capable to induce NETosis, bovine PMN exposed to bradyzoites were analysed by s.e.m. Fine network structures were observed in B. besnoiti bradyzoites-stimulated bovine PMN (Fig. 2), and many bradyzoites were trapped by those structures (Fig. 2) visualized in s.e.m. analysis. Alongside, different morphologies of bovine PMN were observed around these fine networks. Whilst typical smooth rounded PMN have been found in close proximity to bradyzoites indicating a rather inactivation status, other exposed PMN showed disrupted cell membrane surfaces and thereby releasing extracellular filaments entrapping firmly bradyzoites by cell death. Former PMN status corresponded well to previously described suicidal (lytic) ETosis against this apicomplexan protozoa where extracellular fibres mainly derived from dead PMN and monocytes (Muñoz-Caro et al., Reference Muñoz Caro, Hermosilla, Silva, Cortes and Taubert2014a,Reference Muñoz-Caro, Silva, Ritter, Taubert and Hermosillab).

Fig. 2. NETosis of bovine PMN after a confrontation with Besnoitia besnoiti bradyzoites. Scanning electron microscopy (s.e.m.) analysis revealed NETosis being formed by bovine PMN co-cultured with B. besnoiti bradyzoites, and these extracellular structures resulted in a fine meshwork containing bardyzoites as indicated by white arrows. Scale bar = 5 μm.
In order to confirm whether bovine PMN were undergoing suicidal NETosis, main components of NET formation (i.e. DNA, histones (H3) and NE) were visualized via immunostaining. In the control group (non-exposed PMN), no NETosis-like structures were observed by co-localization of H3 and NE (Fig. 3A). In contrast, the classical characteristics of suicidal NETosis were demonstrated in bovine PMN exposed to B. besnoiti bradyzoites by co-localization of extracellular DNA adorned with H3 and NE (Fig. 3B), and several bradyzoites being firmly trapped by NETosis as indicated by white arrows in Fig. 3B.

Fig. 3. Suicidal NETosis was visualized by co-localization of DNA with histones (H3) and neutrophil elastase (NE) in B. besnoiti bradyzoite-exposed bovine PMN. After 3 h of incubation, co-cultures of bovine PMN and B. besnoiti bradyzoites in a 1:4 ratio were fixed, permeabilized, and then suicidal NETosis was visualized via immunostaining. Panel A: PMN alone group; Panel B: PMN + bradyzoites group. Bradyzoites were indicated by arrows. Scale bar = 20 μm.
3D-holotomographic microscopy live cell imaging of B. besnoiti bradyzoite-triggered vital NETosis
Activation of bovine PMN and NETosis were additionally analysed by live cell 3D-holotomographic microscopy technology (Nanolive®). Activation of PMN occurred within the first 5 to 30 min of interaction with motile bradyzoites thereby showing pseudopod formation and rapid migration and crawling activities of PMN into the vision field showing bradyzoites. Noteworthy to mention was the observation of an elongated structure being rapidly tossed out from PMN after 30 min of parasite interaction. Due to the time point of occurrence and the non-lytic PMN phenotype of this ‘chameleon tongue-like’ reactions we interpreted this response as vital NETosis (Fig. 4A-B; please refer to Video S2). The digital staining and 3D reconstruction of vital NETosis showed clearly that neither the overall cell phenotype nor crawling activities were altered by the protrusion of this elongated structure (Video S3; Fig. 4C).

Fig. 4. Besnoitia besnoiti bradyzoites induced vital NETosis. Live cell 3D holotomographic microscopy (Nanolive®) analysis under controlled temperature and atmosphere conditions was performed for 1 h of interactions registering images every 30 s (A). At 31 min of incubation a tossing vital NETosis is observed without compromising the overall structure of PMN (B). Digital staining and 3D holotomographic reconstruction of tossed vital NETosis (C). (A) Scale bar = 20 μm, (B) Scale bar = 10 μm.
Autophagy occurred during B. besnoiti-triggered suicidal NETosis
Autophagy is a highly conserved intracellular degradation process not only to keep homeostasis or energy source of mammalian cells but also pivotal in several host innate immune functions (Germic et al., Reference Germic, Frangez, Yousefi and Simon2019). During autophagy, LC3 (microtubule-associated protein 1A/1B-light chain 3) is an important protein being involved in autophagosome formation, and it has been used as a classical marker of autophagosomes. As shown in Fig. 5, the majority of bovine PMN (see Fig. 5A) were still round and inactive without stimulation of B. besnoitia bradyzoites. In contrast, most of bovine PMN exposed to B. besnoitia bradyzoites (see Fig. 5B) were undergoing autophagy alongside with NETosis resulting in bradyzoite entrapment (Fig. 5B), indicating an association of these two cellular processes.

Fig. 5. Autophagy occurs in Besnoitia besnoiti-triggered suicidal NETosis. Bovine PMN (n = 3) were exposed to B. besnoiti bradyzoites on coverslips for 1 h at 37°C, 5% CO2. Samples were fixed and thereafter permeabilized for LC3B-based immunostaining in order to determine autophagosome formation by confocal microscopy analysis. Bradyzoites were indicated by arrows in merged images. Scale bar = 10 μm.
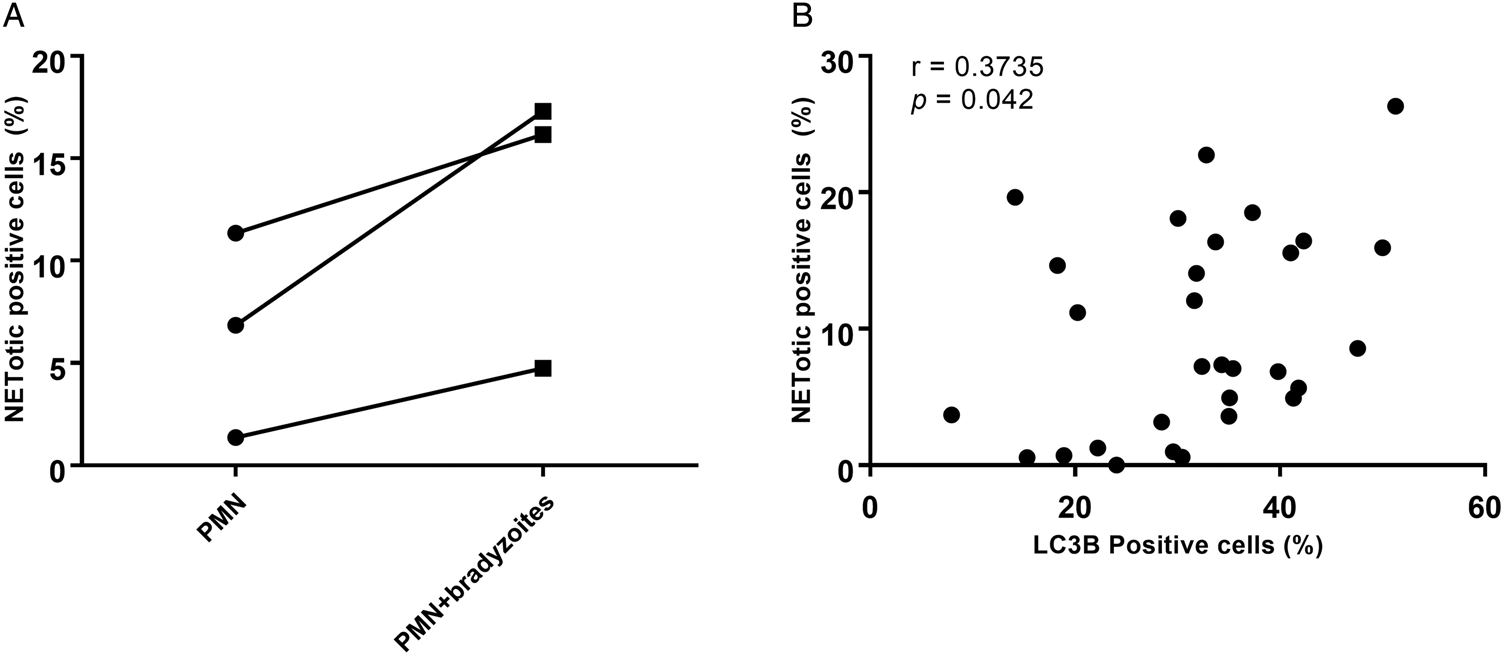
To investigate in more detail concomitant autophagy while B. besnoiti-induced NETosis, the percentages of ‘NETotic cells’ and LC3B-positive cells were calculated, and thereafter a Spearman correlation test was performed. As seen in Fig. 6A, more cells were undergoing suicidal NETosis in B. besnoiti bradyzoite-stimulated bovine PMN when compared to non-stimulated PMN (not statistically significant). Moreover, a significant low positive correlation (r = 0.3735, P = 0.042) was found between suicidal NETosis and autophagy in B. besnoiti bradyzoite-stimulated bovine PMN (Fig. 6B) compared to negative controls.

Fig. 6. Autophagy has a significant correlation with Besnoitia besnoiti bradyzoite-triggered suicidal NETosis. Five images were randomly taken from each sample, the number of NETotic (A) and LC3B-positive PMN were counted using ImageJ and the percentages over total cells was calculated. Positive correlation between B. besnoiti-induced LC3B expression and NETotic cells was analysed by Spearman test (B). Results are represented as a before-after graph with data derived from three different animals (n = 3). P values of <0.05 were considered as statistically significant.
Discussion
Bovine besnoitiosis is caused by the parasite B. besnoiti which is a cyst forming apicomplexan parasite closely related to Toxoplasma gondii and Neospora caninum (Ellis et al., Reference Ellis, Holmdahl, Ryce, Njenga, Harper and Morrison2000). Chronic bovine besnoitiosis is characterized by tissue cyst formation, especially in the skin and in the mucosa of diverse organs (e.g. eyes, genitals) with associated progressive thickening, folding, hardening, wrinkling lesions of affected skin or mucosa. Characteristic large-sized cysts of B. besnoiti containing thousands of bradyzoites were identified in the skin samples of this naturally infected heifer by histopathological examinations in accordance to previous reports (Cortes et al., Reference Cortes, Leitao, Vidal, Vila-Vicosa, Ferreira, Caeiro and Hjerpe2005; Jacquiet et al., Reference Jacquiet, Liénard and Franc2010; Rostaher Ana et al., Reference Rostaher, Mueller Ralf, Majzoub, Schares and Gollnick2010; Gentile et al., Reference Gentile, Militerno, Schares, Nanni, Testoni, Bassi and Gollnick2012; Frey et al., Reference Frey, Gutiérrez-Expósito, Ortega-Mora, Benavides, Marcén, Castillo, Casasús, Sanz, García-Lunar, Esteban-Gil and Álvarez-García2013). The affected animal did not benefit from implemented treatments and its deteriorated clinical status determined euthanasia. However, even if the clinical status of this animal had remained unaltered, culling would have been the better control measurement for cattle besnoitiosis in the farm.
NETosis is an effective defence process of activated PMN to ensnare and eliminate invading pathogens by releasing web-like extracellular traps which consist of DNA as a backbone, histones (H1, H2A/H2B, H3, H4), and diverse anti-microbial peptides/proteases such as cathepsin G, α-defensin, pentraxin, cathelicidin (LL37), lactoferrin, calprotectin and others (Amulic and Hayes, Reference Amulic and Hayes2011; Hermosilla et al., Reference Hermosilla, Caro, Silva, Ruiz and Taubert2014). Recently, more attention has been paid on pivotal role of NETosis against protozoan- and metazoan-parasites in various terrestrial and marine mammalian species (Behrendt et al., Reference Behrendt, Ruiz, Zahner, Taubert and Hermosilla2010; Silva et al., Reference Silva, Muñoz Caro, Gerstberger, Vila-Viçosa, Cortes, Hermosilla and Taubert2014; Muñoz Caro et al., Reference Muñoz Caro, Hermosilla, Silva, Cortes and Taubert2014a; Reichel et al., Reference Reichel, Muñoz-Caro, Sanchez Contreras, Rubio García, Magdowski, Gärtner, Taubert and Hermosilla2015; Rochael et al., Reference Rochael, Guimaraes-Costa, Nascimento, DeSouza-Vieira, Oliveira, Garcia e Souza, Oliveira and Saraiva2015; Villagra-Blanco et al., Reference Villagra-Blanco, Silva, Aguilella-Segura, Arcenillas-Hernández, Martínez-Carrasco, Seipp, Gärtner, Ruiz de Ybañez, Taubert and Hermosilla2017b) as well as gastropods (Lange et al., Reference Lange, Penagos-Tabares, Muñoz-Caro, Gärtner, Mejer, Schaper, Hermosilla and Taubert2017). Consequently, PMN-derived NETosis and monocyte-derived METosis exerted potent entrapment capacities against B. besnoiti tachyzoites indicating that these two leukocyte populations might reduce parasite replication during the acute phase of infection as previously postulated. Nevertheless, no data in the literature are still available on early NETosis against B. besnoiti bradyzoites although bradyzoites are released from tissue cysts in vivo (Langenmayer et al., Reference Langenmayer, Gollnick, Majzoub-Altweck, Scharr, Schares and Hermanns2015).
Here, for the first time, we demonstrated that bradyzoites of B. besnoiti were also able to induce NETosis in a similar manner as tachyzoites, and thus proving that B. besnoiti-triggered NETosis is a rather parasite stage-independent effector mechanism. Bradyzoites and tachyzoites of B. besnoiti are known to exhibit different antigens (Fernandez-Garcia et al., Reference Fernandez-Garcia, Alvarez-Garcia, Risco-Castillo, Aguado-Martinez, Marugan-Hernandez and Ortega-Mora2009; Schares et al., Reference Schares, Basso, Majzoub, Rostaher, Scharr, Langenmayer, Selmair, Dubey, Cortes and Conraths2010), and whether bradyzoite-specific antigens induced bovine NETosis could not be answered here (Silva et al., Reference Silva, Muñoz Caro, Gerstberger, Vila-Viçosa, Cortes, Hermosilla and Taubert2014; Muñoz-Caro et al., Reference Muñoz-Caro, Mena Huertas, Conejeros, Alarcón, Hidalgo, Burgos, Hermosilla and Taubert2015, Reference Muñoz-Caro, Conejeros, Zhou, Pikhovych, Gärtner, Hermosilla, Kulke and Taubert2018). In line with these findings, other reports have also shown that different apicomplexan parasitic stages of the same species are able to induce NETosis (Silva et al., Reference Silva, Muñoz Caro, Gerstberger, Vila-Viçosa, Cortes, Hermosilla and Taubert2014; Muñoz-Caro et al., Reference Muñoz-Caro, Mena Huertas, Conejeros, Alarcón, Hidalgo, Burgos, Hermosilla and Taubert2015; Villagra-Blanco et al., Reference Villagra-Blanco, Silva, Aguilella-Segura, Arcenillas-Hernández, Martínez-Carrasco, Seipp, Gärtner, Ruiz de Ybañez, Taubert and Hermosilla2017b). s.e.m. analysis unveiled the presence of classical web-like structures released by bovine PMN exposed to B. besnoiti bradyzoites, as previously observed for tachyzoites (Muñoz Caro et al., Reference Muñoz Caro, Hermosilla, Silva, Cortes and Taubert2014a; Maksimov et al., Reference Maksimov, Hermosilla, Kleinertz, Hirzmann and Taubert2016). Additionally, the main components of NETosis (i.e. DNA, histones (H3), NE) were identified here and visualized via immunostaining and proving that these web-like structures were mainly suicidal NETosis. In addition, live cell imaging by 3D holotomographic microscopy showed rapid vital NETosis within the first 30 min without compromising the general structure of PMN cell membrane as well as crawling activity. In the past, it has been proposed that PMN subpopulations are able to elicit different types of NETosis and that only 20–30% undergo suicidal NETosis (Fuchs et al., Reference Fuchs, Abed, Goosmann, Hurwitz, Schulze, Wahn, Weinrauch, Brinkmann and Zychlinsky2007; Yipp and Kubes, Reference Yipp and Kubes2013). Interestingly, suicidal NETosis seems to be more related to chemical stimuli as PMA, requiring hours to occur, meanwhile, vital NETosis might be more related to biological triggering agents of NETosis such as bacteria, fungi (Yipp and Kubes, Reference Yipp and Kubes2013) or parasites (Rochael et al., Reference Rochael, Guimaraes-Costa, Nascimento, DeSouza-Vieira, Oliveira, Garcia e Souza, Oliveira and Saraiva2015). Currently, it is not clear how vital NETosis could contribute to the control of infection, but it has been suggested that this process is the first effector mechanism against invasive pathogens to occur (Yipp and Kubes, Reference Yipp and Kubes2013) and afterwards followed by suicidal NETosis, which results in stronger DNA release. Additionally, it has been speculated that this phenomenon could be initialized by TRL mediated responses (de Buhr and von Köckritz-Blickwede, Reference de Buhr and von Köckritz-Blickwede2016), but further investigations are necessary to better understand such an intriguing feature in bovine besnoitiosis. To our knowledge, this is the first time that vital NETosis is evidenced by 3D live cell imaging as a PMN response to motile parasite stages.
As stated above, bradyzoites can be released from tissue cysts either after host induced- or after mechanical rupture (Schulz, Reference Schulz1960; Langenmayer et al., Reference Langenmayer, Gollnick, Majzoub-Altweck, Scharr, Schares and Hermanns2015). Further, Langenmayer et al. (Reference Langenmayer, Gollnick, Majzoub-Altweck, Scharr, Schares and Hermanns2015) suggested that during chronic bovine besnoitiosis intravascular circulation of ‘zoites’ might be possible after mechanical rupture of cysts located directly underneath vascular endothelium or after reactivation of tissue cysts and stage conversion into tachyzoite stages. Irrespective of these in vivo scenarios, released bradyzoites would be immediately in close contact to PMN and extruded NETosis might ultimately hamper bradyzoite dissemination. Released bradyzoites might not be immediately identified in the bloodstream of infected animals due to pro-inflammatory host innate immune reactions as proposed elsewhere (Langenmayer et al., Reference Langenmayer, Gollnick, Majzoub-Altweck, Scharr, Schares and Hermanns2015) and in vivo PMN are among the first ones to be recruited to inflammation/infection sites (Fuchs et al., Reference Fuchs, Abed, Goosmann, Hurwitz, Schulze, Wahn, Weinrauch, Brinkmann and Zychlinsky2007; Villagra-Blanco et al., Reference Villagra-Blanco, Silva, Conejeros, Taubert and Hermosilla2019; Zhou et al., Reference Zhou, Conejeros, Velásquez, Muñoz-Caro, Gärtner, Hermosilla and Taubert2019).
Our present results also demonstrated that autophagy was associated with bradyzoite-triggered NETosis. These findings corresponded well to recent data on B. besnoiti tachyzoite-mediated suicidal NETosis with concomitant autophagy (Zhou et al., Reference Zhou, Conejeros, Velásquez, Muñoz-Caro, Gärtner, Hermosilla and Taubert2019). While autophagy process has recently been tightly associated with major neutrophil functions, including degranulation, reactive oxygen species production, and release of neutrophil extracellular traps, the exact molecular mechanisms and autophagy pathways are still not completely clear (Remijsen et al., Reference Remijsen, Berghe, Wirawan, Asselbergh, Parthoens, De Rycke, Noppen, Delforge, Willems and Vandenabeele2011a; Ullah et al., Reference Ullah, Ritchie and Evans2017; Skendros et al., Reference Skendros, Mitroulis and Ritis2018). Also, autophagy is an essential intracellular degradation mechanism to regulate protein and organelle turnover in many living cells thereby maintaining homeostasis and intracellular energy balance (Levine and Kroemer, Reference Levine and Kroemer2008). During the process of autophagy, intracellular autophagosomes ultimately fuse with lysosomes to degrade and recycle the inside cargo (Bernard and Klionsky, Reference Bernard and Klionsky2013). LC3 is a small soluble protein, which is distributed ubiquitously in mammalian tissues and known to form stable associations with the membrane of autophagosomes (Tanida et al., Reference Tanida, Ueno, Kominami and Deretic2008). Thus, LC3 is widely used as a classical marker for microscopical detection of autophagosomes (Koukourakis et al., Reference Koukourakis, Kalamida, Giatromanolaki, Zois, Sivridis, Pouliliou, Mitrakas, Gatter and Harris2015; Park et al., Reference Park, Shrestha, Youn, Kim, Kim, Kim, Park, Ahn, Kim, Lee, Jung, Park, Mo, Ko, Lee, Koh, Park, Song and Hong2017). Previous studies have revealed that autophagy is required for NETosis (Remijsen et al., Reference Remijsen, Berghe, Wirawan, Asselbergh, Parthoens, De Rycke, Noppen, Delforge, Willems and Vandenabeele2011a; Ullah et al., Reference Ullah, Ritchie and Evans2017), and that autophagy induction significantly increased NETosis (Park et al., Reference Park, Shrestha, Youn, Kim, Kim, Kim, Park, Ahn, Kim, Lee, Jung, Park, Mo, Ko, Lee, Koh, Park, Song and Hong2017). Accordingly, LC3B-stained autophagosomes were detected concomitantly in PMN extruding suicidal NETosis towards B. besnoiti bradyzoites. These findings confirm that autophagy is required in bovine NETosis not only against B. besnoiti tachyzoite- (Zhou et al., Reference Zhou, Conejeros, Velásquez, Muñoz-Caro, Gärtner, Hermosilla and Taubert2019) but also against bradyzoite-stages as Spearman test revealed a significant low positive correlation between these two processes. In agreement to our findings, autophagy has also been reported to prime PMN not only for increased NETosis but also for increased phagocytosis during sepsis (Park et al., Reference Park, Shrestha, Youn, Kim, Kim, Kim, Park, Ahn, Kim, Lee, Jung, Park, Mo, Ko, Lee, Koh, Park, Song and Hong2017). PMN-derived phagocytosis through autophagy is expected to occur in the chronic phase of cattle besnoitosis in vivo, however this process needs further investigations.
In summary, we describe for the first time the ability of bovine PMN to cast NETosis against motile B. besnoiti bradyzoites evidencing the importance of this ancient and well-conserved effector mechanism of early host innate immune system in cattle. Furthermore, LC3B-stained autophagosomes were detected in B. besnoiti bradyzoite-exposed PMN casting NETs resulting in a significant low positive correlation of autophagy and parasite-induced suicidal NETosis. Nevertheless, further autophagy-related investigations should elucidate other molecular mechanisms in this cell pathway, such as potential membrane changes of mitochondria and oxygen consumption rates, as well as the role of AMPK in autophagy (Zhou et al., Reference Zhou, Conejeros, Velásquez, Muñoz-Caro, Gärtner, Hermosilla and Taubert2019). Finally, B. besnoiti-mediated vital NETosis resulted in a rapid extrusion and retraction of a ‘chameleon tongue-like’ structure, which is the first hint for this type of NETosis against these apicomplexan parasites. Exact machinery, B. besnoiti-specific antigens and PMN receptors leading to fast parasite-triggered vital NETosis need further investigations.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182019001707
Acknowledgements
The authors would like to acknowledge Anika Seipp (Institute of Anatomy and Cell Biology, Justus Liebig University Giessen, Germany) for her excellent assistance in processing samples for scanning electron microscopy analysis. We would also like to thank doctoral student Elfi Schlohsarczyk (Institute for Veterinary Pathology, Justus Liebig University Giessen, Germany) for macroscopic descriptions and embedding the formalin fixed skin tissue for histological examination.
Financial support
The present work was partially financed by the DFG project: 216337519 (TA291/4-1) granted to Prof. Dr Anja Taubert. Mr. Ershun Zhou PhD was funded by China Scholarship Council (file number: 201506170042). The publication fees were partially funded by the Open Access Publication Fund from Justus Liebig University of Giessen (JLU).
Conflict of interest
The authors declare no conflict of interest.
Ethical standards
This work was performed in accordance with the Justus Liebig University Giessen Animal Care Committee Guidelines. Protocols were approved by the Ethic Commission for Experimental Animal Studies of the Federal State of Hesse (Regierungspräsidium Giessen; A9/2012; JLU-No.521_AZ), and in accordance to prevalent European Animal Welfare Legislation (ART13TFEU) and current applicable German Animal Protection Laws.