INTRODUCTION
Human cytomegalovirus (HCMV) is a ubiquitous infection, which affects most people at some stage in their lives. Like other members of the herpes virus family it has a propensity to establish a latent infection within its human host and to recur throughout life. Globally HCMV prevalence is varied. In developed countries prevalence has been reported to be between 30% and 80% of the adult population, while in less developed countries this number increases to >90% [Reference Cannon, Schmid and Hyde1].
Primary HCMV infection in the immunocompetent host generally causes no serious disease, although a mononucleosis-like syndrome (tiredness, lymphadenopathy, fever) similar to primary Epstein–Barr virus (EBV) infection may occur [Reference Manfredi, Calza and Chiodo2]. However, primary or recurrent HCMV infection during pregnancy is well documented to cause serious congenital sequelae, such as sensory or neurological impairment. Worldwide, the birth prevalence of congenital HCMV is estimated at 7/1000, and each year in the UK, an estimated 900 infants develop permanent disabilities as a result of HCMV infection, two thirds of which will be asymptomatic at birth [Reference Dollard, Grosse and Ross3] (http://cmvaction.org.uk/). Counter-intuitively, maternal HCMV seropositivity is a major risk factor for congenital HCMV. Population-based predication modelling studies that take into account the likelihood of a primary infection in seronegative mothers, suggest that re-infection or reactivation in seropositive mothers accounts for the majority of congenital HCMV cases, and, for the majority of observed HCMV-related hearing loss [Reference de Vries4]. Immunocompromised individuals such as organ transplant recipients and HIV patients are also at risk of developing HCMV disease manifestations, such as pneumonia, hepatitis, retinitis and nephritis [Reference Griffiths5]. The cost to healthcare providers in treating HCMV disease can be substantial with estimates for the USA of approximately 2 billion dollars per year [Reference Modlin6]. As a result the search for a useful vaccine is a public health priority, however, before any vaccine strategy can be implemented it is critical to understand the prevalence and risk factors for HCMV infection.
The main risk factors for HCMV transmission remain unclear. Studies suggest that transmission can occur via a number of different routes including transfer of body fluids, such as saliva, urine, and placental cell transfer, or behaviours thought to be responsible for the transfer of fluids and infected cells such as breastfeeding, blood transfusion, transplantation, and sexual activity. Reports also show that those in close contact with young children, such as nursery workers/child minders, have an increased risk of HCMV seroconversion, and that infected children pose the highest risk for transmission to pregnant women [Reference Hyde, Schmid and Cannon7]. With exposure to potentially infective sources increasing with age, and sexual maturity, questions remain over the impact of transmission between young adult populations on overall HCMV prevalence. Several studies have attempted to address this question using single gender adolescent populations [Reference Stadler8, Reference Sohn9]; however, few studies have performed a longitudinal analysis [Reference Stanberry10, Reference Zanghellini11]. This study attempts to identify the risk factors for CMV transmission in a population of young adults at the start of their university career, and the potential risks factors for seroconversion during 3 years of follow-up.
METHODS
Sample population
Subjects were recruited as part of an epidemiological survey analysing the relationship between sexual behaviour and EBV infection. Details for this recruitment have been published elsewhere [Reference Crawford12]. Briefly, first-year students undertaking a 4-year degree programme who enrolled at Edinburgh University Health Centre in either October 1999 or October 2000, were asked to complete a medical/lifestyle questionnaire and to provide a blood sample for serological testing. Subjects' blood samples and questionnaires were allocated a study number and written consent was obtained from all subjects at time of recruitment. Subjects were also asked to provide a second blood sample and to complete an exit questionnaire in their final year of study. Density centrifugation was performed on all blood samples to give both plasma and peripheral blood mononuclear cell fractions and both were stored at −80°C for future use. The questionnaires collated information on medical, sexual and demographic characteristics, and social habits such as smoking, drinking and exercise.
For this study a total of 402 subjects were randomly chosen from the original cohort and investigated for the presence of IgG antibodies against the HCMV viral capsid antigen, with questionnaire data collated for analysis. Blood sample and questionnaire data were matched using the study number allocated upon recruitment. The study was approved by the Lothian Ethics Committee.
HCMV serology
Plasma samples were defrosted at room temperature and then heat-inactivated at 56°C for 20 min, followed by pulse spinning at 11 000 g for 1 min. Samples were tested for the presence of HCMV-specific viral capsid antigen IgG (VCA-IgG) detected by enzyme immunoassay (Microgen Bioproducts, UK), and according to the manufacturer's instructions. One hundred microlitres of each plasma sample was tested and absorbent values checked within 30 min of the stop solution being added. Positive samples were recorded when absorbance values at 450 nm were higher or equal to a mean value of two 0·4 IU/ml standard cut-off controls + 10%. The test was repeated for equivocal samples to confirm results.
Statistical analysis
Statistical analyses were conducted in two ways. First, an examination of whether demographic, social and sexual characteristics before attending university were associated with HCMV positivity at enrolment. Second, whether any of these pre-university characteristics, or behaviours while at university, resulted in HCMV seroconversion during the 4 years at university. Prevalence ratios (PRs) were used to compare HCMV seropositivity (and seroconversion) between groups (e.g. males vs. females). PRs, rather than odds ratios, were calculated to avoid violation of the rare disease assumption since HCMV positivity was relatively common. Wald-based 95% confidence intervals were calculated for each PR and the statistical significance of each PR was tested using Fisher's exact two-sided test. All statistical analyses were conducted using Stata software, version 8.0 (StataCorp., USA).
RESULTS
HCMV prevalence upon entry to university
A total of 402 plasma samples collected upon entry to university and GP enrolment were randomly selected and tested for HCMV seropositivity (285 females, 117 males; Table 1). One hundred and three samples tested were seropositive for HCMV VCA-IgG antibody (25·6%) with no evidence of a difference in seropositivity between males and females (25·6% male, 25·6% female; Table 1). HCMV seropositivity was higher in those born in a developing country (45% vs. 25% for developed countries, P = 0·03; Table 1), and specifically for those born in Africa (67% vs. 33%, P < 0·02; data not shown), although numbers included in this analysis were small. There was also evidence that study participants who had lived in Africa at any point in their lifetime prior to attending university had a higher HCMV prevalence than those who had never lived in Africa (53·3% vs. 24·5% HCMV seropositive, P = 0·03; Table 1). Analysis of age upon entry to university, the presence and number of siblings, the degree of crowding in the parental home prior to attending university, the number of vaccinations and EBV serostatus were all performed with no evidence of associations with HCMV (summarized in Table 1).
Table 1. Risk of human cytomegalovirus (HCMV) seropositivity and seroconversion by demographic, social and sexual characteristics
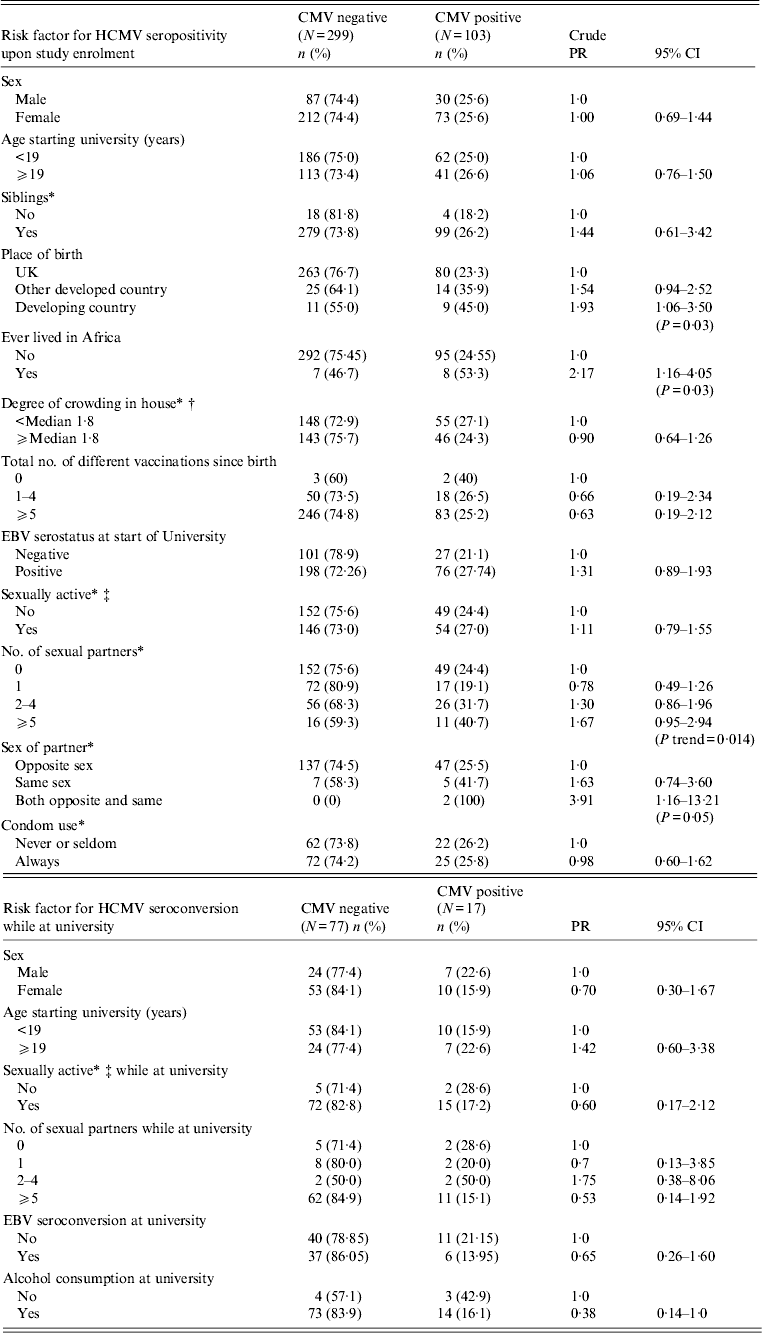
PR, Prevalence ratio; CI, confidence interval; EBV, Epstein–Barr virus.
* Missing data as follows: sibling (2), crowding (10), sexual activity prior to university (1), sexual activity (1), number of sexual partners (2), sex of partner (2), condom use (3).
† No. of rooms per person.
‡ Defined as any sexual contact or experience including genital contact not involving intercourse, oral sexual intercourse, or penetrative vaginal sex.
Being sexually active prior to the start of university was not associated with HCMV seropositivity at enrolment (27% of sexually active students were seropositive vs. 24·4% students who were not sexually active, P = 0·55, Table 1), although there was some suggestion (based on only two cases) of association in those who had ever had same-sex partners (100% HCMV seropositive, P = 0·05; Table 1). Investigation of the number of sexual partners showed that risk of HCMV seropositivity increased with the number of sexual partners prior to attending university (P trend = 0·014), although this increase was not steady across the four investigated groups (no partners, 24%; 1 partner, 19%; 2–4 partners, 32%; ⩾5 partners, 41%; Table 1). The analyses found no association between condom use and HCMV prevalence (26·2% HCMV seropositive of those that never/seldom use condoms vs. 25·8% HCMV seropositive that always use condoms, P = 0·95; Table 1).
HCMV serostatus upon exit from university
Of the 402 participants included in the enrolment analyses, 299 [87 (29%) male, 212 (71%) female] were seronegative and therefore included in the ‘seroconversion while at university’ analyses. Ninety-four [31 (33%) male, 63 (67%) female] of the 299 participants completed a follow-up questionnaire and provided a blood sample in their final year of study (∼3 years since enrolment sampling). Seventeen (18%) of the 94 patients were HCMV seropositive upon exit. Risk factors for HCMV seroconversion while at university are summarized in Table 1. We found no association between HCMV seroconversion and gender (22·6% male vs. 15·9% female, P = 0·57), age upon entry to university (15·9% aged <19 years vs. 22·6% aged >19 years, P = 0·57), sexual activity (28·6% not sexually active vs. 17·2% sexually active, P = 0·61), the number of sexual partners (no partners, 28·6%; 1 partner, 20%; 2–4 partners, 50%; ⩾5 partners, 15·1%; P = 0·18), EBV serostatus (21·1% EBV seropositive vs. 14% EBV seronegative, P = 0·42), behavioural factors (e.g. alcohol consumption, smoking) or particular allergies (e.g. eczema).
DISCUSSION
This study aimed to identify potential risk factors for HCMV prevalence in young adults attending university. By linking blood samples taken at the start of university with questionnaire data on social/behavioural factors and demographic status prior to attending university, a number of potential associations were identified, although none were strongly significant. These included being born in a developing country, ever having lived in Africa and sexual activity with a same-sex partner.
Follow-up blood samples and questionnaire data from students who were HCMV negative at the start of university provide an insight into risk factors for HCMV seroconversion in a specific young adult population over time, Sexual activity was not found to be a risk factor for HCMV seroconversion, which is in contrast to findings from previous reports [Reference Sohn9, Reference Staras13]. A possible explanation is that these studies reported on HCMV risk factors and serostatus, with no longitudinal analysis of seroconversion. In contrast, our study followed HCMV seronegative individuals for 3–4 years while at university, thus eliminating factors that influence seropositivity earlier in life (i.e. childhood HCMV seroconversion). We have no evidence at present to suggest that baseline sexual activity in this population may have changed during this time period; however, this variable should be considered in future studies to eliminate any statistical effect. Reports of HCMV in semen, saliva, and cervical secretions would suggest an increased risk of infection during sexual activity. However, it is possible that HCMV seropositive individuals in this age group are not actively shedding virus thereby preventing infection of a HCMV seronegative sexual partner. Although difficult to obtain, an analysis of body fluids would provide a more accurate picture as to whether or not most HCMV seropositive individuals are actively shedding virus in this age group.
Female gender was also not found to be a significant risk factor, again in contrast with a previous report by Hecker et al. [Reference Hecker14]. Following the increased risk of HCMV infection during childhood, females who remain HCMV seronegative may be exposed to another period of high risk around the age they begin a family. At this time they may be exposed to possible infection from nurseries, through childcare or their own children. Since the age of this study population is generally below the age of first childbirth in the UK (29·4 years), these risk factors may not yet have an impact in this age group (Office for National Statistics 2010; www.ons.gov.uk).
In summary we observed an increased prevalence upon enrolment to university associated with ever being born in a developing country and ever being resident in Africa. No particular risk factor was found for HCMV seroconversion during the university study time period (3–4 years). These findings should be verified in a larger study with improved statistical power and more specific questionnaire data, particularly questions regarding contact with potentially infective sources such as sharing drinks, degree of kissing (mouth to mouth or orogenital), types of sexual contact, etc. However, this survey is a good indicator of risk and transmission in this age group. With an annual seroconversion rate of 6%, this population of young adults represent a potential group of healthy volunteers for the evaluation of vaccines or therapies designed to protect from HCMV primary infection. Further studies, particularly those focussed on seroconversion within a study cohort, may help provide a more detailed insight into HCMV transmission.
ACKNOWLEDGEMENTS
We thank the staff of the University of Edinburgh Health Centre and all the student volunteers for their help with this study. Financial support was received from the UK Medical Research Council (grant no. G9826804 to D. H. Crawford).
DECLARATION OF INTEREST
None.



