Introduction
Increasing interest surrounds the possibility that n-3 long-chain PUFA (n-3PUFA) may be implicated in the regulation of mood and behaviour. This interest stems from evidence of effects of n-3PUFA on cell membrane structure and function, and early evidence suggesting a role for n-3PUFA in the development and/or treatment of various disorders of mood and behaviour. More recent evidence, however, questions the robustness of this early evidence. The present paper aims to provide a comprehensive review of the evidence to date investigating a role for n-3PUFA in the regulation of mood and behaviour.
n-3 Long-chain polyunsaturated fatty acids
n-3PUFA (also named omega-3 fatty acids) are a family of PUFA, named as such due to the positioning of the first double carbon bond on the third atom from the methyl end of the acyl chain. All members of the family are derived from the parent fatty acid 18: 3n-3 (α-linolenic acid (ALA)), via desaturation and elongation(Reference Haag1, Reference Ruxton, Calder, Reed and Simpson2), as demonstrated in Fig. 1. Closely related to the n-3PUFA are the n-6 long-chain PUFA (n-6PUFA), named from the positioning of the first double bond on the sixth carbon atom from the methyl end of the acyl chain. n-6PUFA are derived from the parent fatty acid 18 : 2n-6 (linoleic acid), and for synthesis share the same desaturases and elongases as n-3PUFA. The n-3 and n-6PUFA thus compete for synthesis from their parent fatty acids. The parent fatty acids, however, cannot be synthesised by man(Reference Haag1, Reference Ruxton, Calder, Reed and Simpson2).
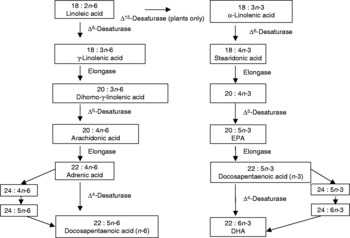
Fig. 1 Desaturation and elongation of n-3 long-chain PUFA.
As essential fatty acids, ALA and linoleic acid must be obtained from the diet. Longer-chain n-3 and n-6PUFA can be formed in man, but biological conversion is slow and inefficient, making diet an important source for these fatty acids as well(Reference Ma, Folsom, Eckfeldt, Lewis and Chambless3). Dietary sources of ALA include certain nuts and seeds, such as walnuts, flaxseed and rapeseed oil, and dietary sources of the longer n-3PUFA EPA and DHA include fatty fish, some white fish, shellfish and other seafoods such as seaweed, and certain eggs and animal products dependent on the animals' diet(Reference Ruxton, Calder, Reed and Simpson2, 4–Reference Simopoulos6). Dietary sources of linoleic acid and all n-6PUFA include plant and vegetable seeds and oils, as found in margarines and the majority of processed foods(Reference James, Gibson and Cleland5, Reference Simopoulos6). Dietary intakes of n-3 and n-6PUFA, however, have changed dramatically over recent decades. Our traditional diet is thought to have contained approximately equal amounts of energy from n-3PUFA and n-6PUFA(Reference Simopoulos6). By comparison, a current Western diet is estimated to contain approximately five to twenty times more energy from n-6PUFA than from n-3PUFA(Reference Simopoulos6, Reference Gregory, Foster, Tyler and Wiseman7). The increasing imbalance between n-3 and n-6PUFA is currently thought to impact on cell membrane structure and function.
n-3 Long-chain polyunsaturated fatty acids in cell membrane structure and function
Both n-3 and n-6PUFA are integral components of all cell membranes as part of the phospholipid bilayer. Within the phospholipid bilayer, n-3PUFA and n-6PUFA can be interchanged, where incorporation into the membrane depends largely on PUFA availability, both from the diet and from chemical synthesis(Reference Haag1, Reference Ruxton, Calder, Reed and Simpson2, Reference James, Gibson and Cleland5). Incorporation into cell membranes, dependent on availability, has been clearly demonstrated(Reference Bourre, Faivre, Dumont, Nouvelot, L'Oudes, Puymirat and Tixier-Vidal8, Reference Bourre, Pascal, Durand, Masson, Dumont and Piciotti9). Membrane composition, however, affects membrane function. First, n-3PUFA molecules, due to their size and shape, influence the physical state of the membrane, resulting in increased fluidity and permeability. Ehringer et al. (Reference Ehringer, Belcher, Wassall and Stillwell10) found DHA administration to result in increased permeability of the phospholipid bilayer in vitro, Tappia et al. (Reference Tappia, Ladha, Clark and Grimble11) found supplementation with fish oil to result in increases in membrane fluidity in rats and Hirashima et al. (Reference Hirashima, Parow, Stoll, Demopulos, Damico, Rohan, Eskesen, Zuo, Cohen and Renshaw12) found supplementation with n-3PUFA to result in a decrease in T2 values (brain water proton transverse relaxation times) indicative of increased membrane fluidity in humans. This increased fluidity may aid cross-cell membrane transport, aiding cell communication and functionality(Reference Haag1).
Second, n-3 and n-6PUFA are also thought to have different effects on surrounding molecules and cell functions. Specifically, n-3 and n-6PUFA are considered important in cell signalling and signal transduction(Reference Haag1, Reference Ruxton, Calder, Reed and Simpson2, Reference James, Gibson and Cleland5). n-3 and n-6PUFA are thought to impact directly on the activity of a number of enzymes in various neurotransmitter pathways, resulting in changes in the activities of these systems(Reference Haag1). n-3PUFA-deficient diets have been associated with lower levels of serotonin and dopamine in piglets(Reference De la Presa Owens and Innis13). In rats, n-3PUFA-deficient diets have been found to result in increased serotonin 5-HT2 receptor density in the frontal cortex(Reference Delion, Chalon, Herault, Guilloteau, Besnard and Durand14, Reference Delion, Chalon, Guilloteau, Besnard and Durand15), elevated 5-HT2A receptor binding density in the prefrontral cortex(Reference McNamara, Richtand and Levant16), lower levels of dopamine in the cortex, hippocampus and striatum(Reference Delion, Chalon, Herault, Guilloteau, Besnard and Durand14, Reference Delion, Chalon, Guilloteau, Besnard and Durand15, Reference Takeuchi, Fukumoto and Harada17), a decreased density of D2 receptors in the frontal cortex(Reference Delion, Chalon, Herault, Guilloteau, Besnard and Durand14, Reference Delion, Chalon, Guilloteau, Besnard and Durand15), reduced D2 receptor binding density in the prefrontal cortex(Reference McNamara, Richtand and Levant16), decreased activity in the mesocortical dopamine pathway and increased activity in the mesolimbic dopamine pathway(Reference Zimmer, Vancassel, Cantagrel, Breton, Delamanche, Guilloteua, Durand and Chalon18), and higher levels of noradrenalin in the cortex, hippocampus and striatum(Reference Takeuchi, Fukumoto and Harada17), compared with control diets. Supplementation with n-3PUFA has also been found to result in enhanced 5-HT responsivity(Reference Yao, Magan, Sonel, Gurklis, Sanders and Reddy19) and reductions in noradrenalin(Reference Hamazaki, Itomura, Huan, Nishizawa, Sawazaki, Tanouchi, Watanabe, Hamazaki, Terasawa and Yazawa20, Reference Sawazaki, Hamazaki, Yazawa and Kobayashi21) in humans.
n-3 and n-6PUFA are also thought to affect enzymes which result in the release of fatty acids from the phospholipid bilayer to form a number of eicosanoids, prostaglandins and leucotrienes. These compounds can have effects on signal transduction, resulting again in increased activity and increased cell signalling(Reference Haag1, Reference Ruxton, Calder, Reed and Simpson2, Reference James, Gibson and Cleland5). These compounds can also have either pro- or anti-inflammatory properties depending on their derivation – prostaglandins formed from the n-6PUFA arachidonic acid are typically pro-inflammatory, prostaglandins formed from EPA are typically anti-inflammatory(Reference Ruxton, Calder, Reed and Simpson2, Reference James, Gibson and Cleland5, Reference Calder22). Supplementation with EPA has been found to result in reduced production of inflammatory cytokines in animals and in humans(Reference James, Gibson and Cleland5, Reference Calder22). Low levels of docosapentaenoic acid n-3 in human erythrocyte membranes have also been associated with high levels of the pro-inflammatory cytokine IL-6(Reference Yao, Sistilli and van Kammen23), and supplementation with ALA has been found to result in reductions in inflammatory cytokines – TNFα and IL- 1β(Reference Caughey, Mantzioris, Gibson, Cleland and James24), and reductions in IL-6, C-reactive protein and serum amyloid A(Reference Rallidis, Paschos, Liakos, Velissaridou, Anastasiadis and Zampelas25).
n-3 and n-6PUFA have also been found to modulate ion channels important in cell signalling and transmission. Ion transfer is vital for neurotransmission, and n-3PUFA have been associated with an inhibition of enzymes that maintain ion gradients(Reference Kearns and Haag26). Deficiencies in dietary ALA have also been found to result in a reduction of neural enzyme activity in the rat brain(Reference Bourre, Francois, Youyou, Dumont, Piciotti, Pascal and Durand27), and deficiencies in dietary DHA have been found to result in decreased neuron size in several areas of the rat brain(Reference Ahmad, Moriguchi and Salem28), diminished nerve growth factor levels in the hippocampus and increased nerve growth factor in the piriform cortex(Reference Ikemoto, Nitta, Furukawa, Ohishi, Nakamura, Fujii and Okuyama29).
The serotonergic, dopaminergic and adrenergic neurotransmitter systems are known to be important in the regulation of mood and behaviour(Reference Haag1). The anti-inflammatory effect of n-3PUFA are also thought to be important in a number of behavioural conditions(Reference James, Gibson and Cleland5). The biochemical evidence thus suggests a potential role for n-3PUFA in the regulation of mood and behaviour, and has resulted in the development of a number of hypotheses centring around a role for n-3PUFA in a number of mood and behavioural conditions. These hypotheses include the biogenic amine hypothesis of depression(Reference Hibbeln and Salem30), the immune/inflammatory hypothesis of psychiatric disease(Reference Smith31) and the membrane hypothesis of schizophrenia(Reference Horrobin32).
While biochemical work continues to examine these various hypotheses, epidemiological, clinical and trial evidence investigating a role for n-3PUFA in the regulation of mood and behaviour is also available.
n-3 Long-chain polyunsaturated fatty acids in mood and behaviour
To date, studies have investigated the effects of n-3PUFA in relation to various aspects of mood and behaviour. The majority of this work has focused on a role for n-3PUFA in the development and treatment of depression and a variety of depressive illnesses, but work is also available on anxiety and anxiety-related disorders, aggression, hostility and anti-social disorders, inattention, impulsivity and attention deficit hyperactivity disorder (ADHD) and other psychiatric symptoms and disorders, such as schizophrenia. The present review will consider each of these in turn. Where studies measure more than one aspect of mood, these studies are included separately in the discussion of each condition. Aspects of cognitive function, such as vigilance, concentration and disorders of cognitive function, such as Alzheimer's' disease, while closely associated with mood and behaviour, are not covered.
Depression, depressive illness and suicidal behaviour
Depression is characterised by high levels of depressed or low mood, a loss of interest or pleasure in nearly all activities, changes in appetite, weight, sleep or activity, decreased energy, difficulties thinking, concentrating or making decisions, feelings of worthlessness or guilt, and recurrent thoughts of death or suicidal ideation, plans or attempts. Depressive disorders are defined by two consecutive weeks of depressed mood or loss of interest in nearly all activities, plus verification of four additional symptoms(33). Epidemiological, clinical and trial evidence investigating a role for n-3PUFA in depression and depressive illness is available.
Epidemiological evidence
Studies investigating the association between the dietary intake of n-3PUFA and depression are given in Table 1(Reference Hibbeln34–Reference Lovibond and Lovibond59). Four ecological studies have found negative linear and non-linear associations between national fish consumption and national prevalence of major depression(Reference Hibbeln34, Reference Peet39), postpartum depression(Reference Hibbeln36) and bipolar disorders(Reference Noaghiul and Hibbeln37). These studies, however, use crude population (rather than individual) measures of n-3PUFA intake and depressive illness, and few potential confounders of n-3PUFA intake and depression are considered.
Table 1 Epidemiological evidence investigating a role for n-3 long-chain PUFA (n-3PUFA) in depression and depressed mood
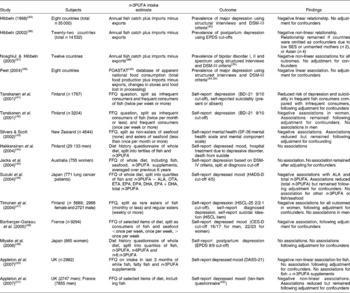
DSM, Diagnostic and Statistical Manual of Mental Disorders; EPDS, Edinburgh Postnatal Depression Scale(Reference Cox, Holden and Sagovsky58); SES, socio-economic status; FOASTAT, Food and Agriculture Organization of the United Nations Statistics; BDI-21, Beck Depression Inventory (twenty-one-item version)(Reference Beck and Steer53); SF-36, MOS Short Form Health Survey(Reference Ware and Sherbourne54); ALA, α-linolenic acid; OTA, octadecatetraenoic acid; ETA, eicosatetraenoic acid; DPA, docosapentaenoic acid; HADS-D, Hospital Anxiety and Depression Scales (depression scale)(Reference Zigmond and Snaith55); HSCL-25, Hopkins Symptom Checklist (twenty-five-item version)(Reference Winokur, Winokur, Rickels and Cox56); CES-D, Center for Epidemiologic Studies (depression scale)(Reference Radloff57); n-6PUFA, n-6 long-chain PUFA; DASS-21, Depression, Anxiety and Stress Scales (twenty-one-item version)(Reference Lovibond and Lovibond59).
The majority of epidemiological studies investigating n-3PUFA intakes and depression have been conducted within individuals. Studies have used FFQ and diet recalls to record fish consumption, seafood consumption and the whole diet, and all studies measured self-reported depression, although different questionnaires have been used. Of the eleven (within-individual) studies reported in Table 1, eight studies found a negative association between fish or n-3PUFA intake and depression(Reference Tanskanen, Hibblen, Hintikka, Haatainen, Honkalampi and Viinamaki41–Reference Silvers and Scott43, Reference Suzuki, Akechi, Kobayashi, Taniguchi, Goto, Sasaki, Tsugane, Nishiwaki, Miyaoka and Uchitomi46–Reference Barberger-Gateau, Jutand, Letenneur, Larrieu and Tavernier48, Reference Appleton, Peters, Hayward, Heatherley, McNaughton, Rogers, Gunnell, Ness and Kessler50, Reference Appleton, Woodside and Yarnell51), although in two studies, associations were only found in female participants(Reference Tanskanen, Hibbeln and Tuomilehto42, Reference Timonen, Horrobin, Jokelainen, Laitinen, Herva and Rasanen47), and in one study associations were found for fish intake but not for fish +n-3PUFA supplement intake(Reference Appleton, Peters, Hayward, Heatherley, McNaughton, Rogers, Gunnell, Ness and Kessler50), whereas in another study associations were found for calculated total n-3PUFA intake but not for fish intake(Reference Suzuki, Akechi, Kobayashi, Taniguchi, Goto, Sasaki, Tsugane, Nishiwaki, Miyaoka and Uchitomi46). Three studies found no associations(Reference Hakkarainen, Partonen, Haukka, Virtamo, Albanes and Lonnqvist44, Reference Jacka, Pasco, Henry, Kotowicz, Nicholson and Berk45, Reference Miyake, Sasaki and Yokoyama49). In seven of the studies that found associations, the relationship between n-3PUFA intake and depression was found following adjustment for confounders(Reference Tanskanen, Hibblen, Hintikka, Haatainen, Honkalampi and Viinamaki41–Reference Silvers and Scott43, Reference Suzuki, Akechi, Kobayashi, Taniguchi, Goto, Sasaki, Tsugane, Nishiwaki, Miyaoka and Uchitomi46–Reference Barberger-Gateau, Jutand, Letenneur, Larrieu and Tavernier48, Reference Appleton, Woodside and Yarnell51). In three of these studies, however, associations were reduced following adjustment for confounders(Reference Silvers and Scott43, Reference Suzuki, Akechi, Kobayashi, Taniguchi, Goto, Sasaki, Tsugane, Nishiwaki, Miyaoka and Uchitomi46, Reference Appleton, Woodside and Yarnell51), and in one study no association between n-3PUFA intake and depressed mood remained following adjustment for the confounders, age and deprivation(Reference Appleton, Peters, Hayward, Heatherley, McNaughton, Rogers, Gunnell, Ness and Kessler50).
Clinical evidence
Clinical studies typically investigate associations between n-3PUFA status and depressive illness, and either compare individuals suffering from depressive symptoms with controls, or investigate the continuous relationship between n-3PUFA status and depressive symptom severity. Details of studies investigating differences between those with depressive symptoms and those without are given in Table 2(Reference Ellis and Sanders60–Reference Kaiya, Horrobin, Manku and Morse-Fisher83). The majority of studies have compared individuals with diagnosed clinical depression and controls with no depression, although some studies have also included individuals with undiagnosed, sub-clinical depression(Reference Maes, Smith, Christophe, Cosyns, Desnyder and Meltzer62, Reference Tiemeier, van Tuijl, Hofman, Kiliaan and Breteler66), and some studies have used self-report measure cut-offs to define high levels of depression as opposed to clinical diagnosis(Reference Mamalakis, Tornaritis and Kafatos73–Reference Mamalakis, Jansen, Cremers, Kiriakakis, Tsibinos and Kafatos76). Studies on postpartum depression(Reference Otto, de Groot and Hornstra77, Reference Browne, Scott and Silvers78), suicide attempt(Reference Huan, Hamazaki, Sun, Itomura, Liu, Kang, Watanabe, Terasawa and Hamazaki79), bipolar disorder(Reference Chiu, Huang, Su, Lu, Huang, Chen and Shen80, Reference Ranjekar, Hinge, Hegde, Ghate, Kale, Sitasawad, Wagh, Debsikdar and Mahadik81), self-harm(Reference Garland, Hallahan, McNamara, Carney, Grimes, Hibbeln, Harkin and Conroy82) and affective and paranoid disorders(Reference Kaiya, Horrobin, Manku and Morse-Fisher83) are also available. Studies used a variety of different biological samples for assessment of n-3PUFA status. The majority of studies involving individuals diagnosed with clinical depression show low levels of a number of n-3PUFA and high ratios of n-6PUFA:n-3PUFA in depressed individuals compared with controls. All studies, however, involve assays of a number of fatty acids, where associations are found for some fatty acids and not others, with no consistent patterns emerging for those comparisons that yield associations or those that do not. Low levels of n-6PUFA are also often reported, again in inconsistent patterns, and few studies use adjusted P values to take account of multiple testing. Two studies also demonstrate higher levels of n-3PUFA in depressed individuals compared with controls(Reference Ellis and Sanders60, Reference Fehily, Bowey, Ellis and Meade61). Comparisons of individuals with other depressive illnesses reveal some differences between cases and controls, where cases have lower levels of some n-3PUFA, but again patterns of associations are inconsistent(Reference Huan, Hamazaki, Sun, Itomura, Liu, Kang, Watanabe, Terasawa and Hamazaki79–Reference Kaiya, Horrobin, Manku and Morse-Fisher83). Comparisons between individuals with high and low levels of undiagnosed depression reveal few differences between groups(Reference Mamalakis, Tornaritis and Kafatos73–Reference Browne, Scott and Silvers78).
Table 2 Clinical evidence investigating a role for n-3 long-chain PUFA (n-3PUFA) in depression and depressed mood: comparisons between depressed cases and non-depressed controls
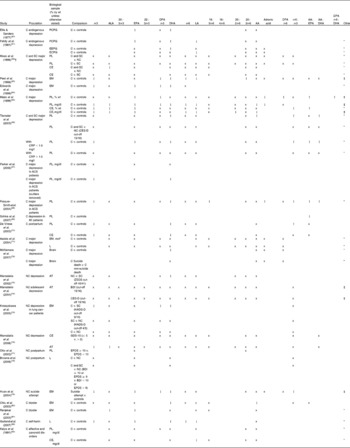
ALA, α-linolenic acid; DPA, docosapentaenoic acid; LA, linoleic acid; AA, arachidonic acid; C, clinical; PCPG, plasma choline phosphoacylglycerols; ↑ , higher PUFA in depressed cases compared with comparison; x, no association; ↓ , lower PUFA in depressed cases compared with comparison; EEPG, erythrocyte ethanolamine phosphoacylglycerols; ECPG, erythrocyte choline phosphoacylglycerols; SC, sub-clinical; PL, plasma phospholipids; NC, non-clinical; CE, plasma cholesteryl esters; EM, erythrocyte membranes; CES-D, Center for Epidemiologic Studies (depression scale)(Reference Radloff57); CRP, C-reactive protein; ACS, acute coronary syndromes; MI, myocardial infarction; mol*, concentration calculated with references to the internal standard 18-methylnonadecanoic acid; L, plasma lipids; AT, adipose tissue; ZSDS, Zung Self-Rating Depression Scale(Reference Biggs, Wylie and Ziegler84); BDI, Beck Depression Inventory(Reference Beck and Steer53); HADS-D, Hospital Anxiety and Depression Scales (depression scale)(Reference Zigmond and Snaith55); GDS-15, Geriatric Depression Scale (fifteen-item version)(Reference Sheikh and Yesavage85); EPDS, Edinburgh Postnatal Depression Scale(Reference Cox, Holden and Sagovsky58).
* Other n-3PUFA tested but no associations found.
† Results following adjustment for multiple testing.
‡ Other n-3PUFA tested and associations found.
Studies investigating relationships between n-3PUFA status and depressive symptomatology are given in Table 3(Reference Beck and Steer53, Reference Zigmond and Snaith55, Reference Radloff57, Reference Cox, Holden and Sagovsky58, Reference Fehily, Bowey, Ellis and Meade61, Reference Maes, Smith, Christophe, Cosyns, Desnyder and Meltzer62, Reference Edwards, Peet, Shay and Horrobin64, Reference Maes, Christophe, Delanghe, Altamura, Neels and Meltzer65, Reference Parker, Heruc, Hilton, Olley, Brotchie, Hadzi-Pavlovic, Friend, Walsh and Stocker67, Reference Mamalakis, Tornaritis and Kafatos73, Reference Mamalakis, Kiriakakis, Tsibinos and Kafatos74, Reference Mamalakis, Jansen, Cremers, Kiriakakis, Tsibinos and Kafatos76, Reference Otto, de Groot and Hornstra77, Reference Huan, Hamazaki, Sun, Itomura, Liu, Kang, Watanabe, Terasawa and Hamazaki79, Reference Garland, Hallahan, McNamara, Carney, Grimes, Hibbeln, Harkin and Conroy82, Reference Biggs, Wylie and Ziegler84–Reference Beck, Schuyler, Herman, Beck, Resnick and Lettieri97). These studies show similar patterns to those above. Negative associations between depressive symptoms and n-3PUFA status, and positive associations between depressive symptoms and n-6PUFA:n-3PUFA balance have been found, but again patterns with individual n-3PUFA are inconsistent and the majority of assays do not find associations.
Table 3 Clinical evidence investigating a role for n-3 long-chain PUFA (n-3PUFA) in depression and depressed mood: associations between n-3PUFA status and depressive symptom severity
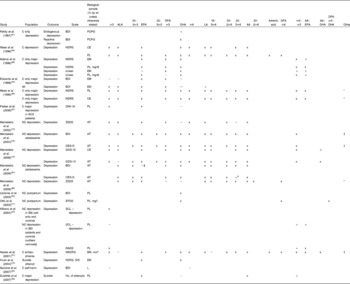
ALA, α-linolenic acid; DPA, docosapentaenoic acid; LA, linoleic acid; AA, arachidonic acid; C, clinical; BDI, Beck Depression Inventory(Reference Beck and Steer53); PCPG, plasma choline phosphoacylglycerols; +, positive correlation; x, no correlation; HDRS, Hamilton Depression Rating Scale(Reference Hamilton93); CE, plasma cholesteryl esters; PL, plasma phospholipids; EM, erythrocyte membranes; − , negative correlation; DMI-18, Depression in the Medically Ill (eighteen-item version)(Reference Parker, Hilton, Bains and Hadzi-Pavlovic94); NC, non-clinical; ZSDS, Zung Self-Rating Depression Scale(Reference Biggs, Wylie and Ziegler84); AT, adipose tissue; CES-D, Center for Epidemiologic Studies (depression scale)(Reference Radloff57); GDS-15, Geriatric Depression Scale (fifteen-item version)(Reference Sheikh and Yesavage85); EPDS, Edinburgh Postnatal Depression Scale(Reference Cox, Holden and Sagovsky58); IBS, irritable bowel syndrome; SCL, Symptom Checklist (ninety-item version)(Reference Arrindell and Ettema95); HADS-D, Hospital Anxiety and Depression Scales (depression scale)(Reference Zigmond and Snaith55); MADRS, Montgomery–Asberg Depression Rating Scale(Reference Montgomery and Asberg96); mol*, concentration calculated with references to the internal standard 18-methylnonadecanoic acid; SIS, Suicide Intent Scale(Reference Beck, Schuyler, Herman, Beck, Resnick and Lettieri97); L, plasma lipids.
* Other n-3PUFA tested but no associations found.
† Other n-3PUFA tested and associations found.
‡ No correlation, but significant negative predictor in regression model.
$ Significant correlation and significant positive predictor in regression model.
A few clinical studies have also investigated the association between n-3PUFA intake and depressive illness in groups of patients. One study again found negative associations between n-3PUFA intake and depression(Reference Edwards, Peet, Shay and Horrobin64), although no association between n-3PUFA intake and a number of depressive illnesses and behaviours has also been reported(Reference Fehily, Bowey, Ellis and Meade61, Reference Browne, Scott and Silvers78, Reference Huan, Hamazaki, Sun, Itomura, Liu, Kang, Watanabe, Terasawa and Hamazaki79). These studies, however, are typically small, often fail to account adequately for confounding factors as above, and due to the difficulty of accurately measuring usual diet, are far from conclusive.
Trial evidence
Trials measure the effects of n-3PUFA supplementation either compared with placebo (placebo-controlled trials) or with no comparison (open-label trials). A number of open-label trials have investigated the impact of supplementation with n-3PUFA on depression. Studies are typically small (between six and thirty-seven participants), and the majority of studies have found decreases in depression or depressive symptoms. Freeman et al. found decreases in diagnosed major depression(Reference Freeman, Hibbeln, Wisner, Watchman and Gelenberg98) and diagnosed postpartum depression(Reference Freeman, Hibbeln, Wisner, Brumbach, Watchman and Gelenberg99) following different doses of EPA+DHA, Osher et al. (Reference Osher, Bersudsky and Belmaker100) found decreases in bipolar depression following supplementation with EPA, and Wozniak et al. (Reference Wozniak, Biederman, Mick, Waxmonsky, Hantsoo, Best, Cluette-Brown and Laposata101) found decreases in diagnosed childhood bipolar depression following supplementation with EPA+DHA. Wozniak et al. (Reference Wozniak, Biederman, Mick, Waxmonsky, Hantsoo, Best, Cluette-Brown and Laposata101) also found decreases in mania and psychotic symptoms following supplementation and Sagduyu et al. (Reference Sagduyu, Dokucu, Eddy, Craigen, Baldassano and Yildiz102) found decreases in mania and bipolar symptoms following supplementation with EPA+DHA. Case reports of treatment with n-3PUFA for depressive disorders have also yielded benefits(Reference Chiu, Huang, Shen and Su103, Reference Puri, Richardson and Horrobin104). Marangell et al. (Reference Marangell, Martinez, Zboyan, Chong and Puryear105), however, found no benefits of EPA+DHA on depression in a non-clinical sample of women with a history of postpartum depression, and Kaplan et al. (Reference Kaplan, Matar, Kamin and Cohen106) found no benefits of EPA on depression in a sample diagnosed with post-traumatic stress disorder.
Placebo-controlled trials investigating the effects of n-3PUFA on depression and depressive symptoms are shown in Table 4(Reference Hirashima, Parow, Stoll, Demopulos, Damico, Rohan, Eskesen, Zuo, Cohen and Renshaw12, 33, Reference Beck and Steer53, Reference Lovibond and Lovibond59, Reference Llorente, Jensen, Voigt, Fraley, Berretta and Heird89, Reference Hamilton93, Reference Montgomery and Asberg96, Reference Nemets, Stahl and Belmaker107–Reference Coccaro, Harvey, Kupsaw-Lawrence, Herbert and Bernstein137). Most studies have involved individuals diagnosed with major depression, but some studies have involved individuals diagnosed with bipolar disorders(Reference Hirashima, Parow, Stoll, Demopulos, Damico, Rohan, Eskesen, Zuo, Cohen and Renshaw12, Reference Stoll, Severus, Freeman, Rueter, Zboyan, Diamond, Cress and Marangell114–Reference Keck, Mintz and McElroy116) and some studies have involved volunteers without diagnosis of depression(Reference Llorente, Jensen, Voigt, Fraley, Berretta and Heird89, Reference Ness, Gallacher, Bennett, Gunnell, Rogers, Kessler and Burr117–Reference Rogers, Appleton, Kessler, Peters, Gunnell, Hayward, Heatherley, Christian, McNaughton and Ness119) or individuals with other psychiatric conditions(Reference Behan, Behan and Horrobin120–Reference Hallahan, Hibbeln, Davis and Garland126). Of the studies conducted in individuals with diagnosed major depression, two studies found decreases in depression following supplementation with EPA+DHA compared with placebo(Reference Su, Huang, Chiu and Shen110, Reference Nemets, Nemets, Apter, Bracha and Belmaker112), two studies found decreases in depression following supplementation with EPA ethyl ester (E-EPA) compared with placebo(Reference Nemets, Stahl and Belmaker107, Reference Peet and Horrobin108), although Peet & Horrobin(Reference Peet and Horrobin108) only found benefits for 1 g E-EPA, and not for 2 g E-EPA or 4 g E-EPA, and three studies found no differences between treatment and placebo groups following supplementation with DHA(Reference Marangell, Martinez, Zboyan, Kertz, Seung Kim and Puryear109) or EPA+DHA(Reference Silvers, Woolley, Hamilton, Watts and Watson111, Reference Grenyer, Crowe, Meyer, Owen, Grigonis, Caputi and Howe113). Of the studies involving individuals with bipolar disorder, two studies found decreases in depression and in bipolar symptoms following supplementation with EPA+DHA compared with placebo(Reference Stoll, Severus, Freeman, Rueter, Zboyan, Diamond, Cress and Marangell114) or E-EPA compared with placebo(Reference Frangou, Lewis and McCrone115), although neither study found similar improvements in mania. Two studies, however, found no benefit of supplementation with EPA+DHA(Reference Hirashima, Parow, Stoll, Demopulos, Damico, Rohan, Eskesen, Zuo, Cohen and Renshaw12) or E-EPA(Reference Keck, Mintz and McElroy116) for depression, mania or bipolar symptoms. All four studies involving volunteers with no diagnosis of depression also found no differences between treatment and placebo groups following supplementation with DHA(Reference Llorente, Jensen, Voigt, Fraley, Berretta and Heird89), EPA+DHA(Reference Rogers, Appleton, Kessler, Peters, Gunnell, Hayward, Heatherley, Christian, McNaughton and Ness119), EPA+DHA+other n-3PUFA(Reference Fontani, Corradeschi, Felici, Alfatti, Bugarini, Fiaschi, Cerretani, Montorfani, Rizzo and Berra118) or advice to eat fish or supplementation with EPA(Reference Ness, Gallacher, Bennett, Gunnell, Rogers, Kessler and Burr117). Of the studies involving individuals with other psychiatric conditions, one study found a beneficial effect of EPA+DHA compared with placebo on depression in chronic fatigue patients(Reference Behan, Behan and Horrobin120), one study found a beneficial effect of E-EPA compared with placebo on depression in patients with borderline personality disorder(Reference Zanarini and Frankenburg124) and one study found a benefit of EPA+DHA compared with placebo on depression in individuals who self-harm(Reference Hallahan, Hibbeln, Davis and Garland126). Hallahan et al. (Reference Hallahan, Hibbeln, Davis and Garland126) also found a beneficial effect of EPA+DHA supplementation on the presence or absence of suicidal ideation. However, one study also found no improvement in depression following EPA+DHA supplementation in chronic fatigue patients(Reference Warren, McKendrick and Peet121), one study found no improvement in depression following supplementation with E-EPA in patients with obsessive–compulsive disorder(Reference Fux, Benjamin and Nemets125) and two studies found no improvements in depression following E-EPA supplementation in schizophrenic patients(Reference Fenton, Dickerson, Boronow, Hibbeln and Knable122, Reference Peet and Horrobin123).
Table 4 Trial evidence investigating a role for n-3 long-chain PUFA (n-3PUFA) in depression and depressed mood: placebo-controlled trials

C, clinical; E-EPA, ethyl ester EPA; HDRS, Hamilton Depression Rating Scale(Reference Hamilton93); MADRS, Montgomery–Asberg Depression Rating Scale(Reference Montgomery and Asberg96); BDI, Beck Depression Inventory(Reference Beck and Steer53); GAF, Global Assessment of Functioning(33); HDRS-SF, Hamilton Depression Rating Scale, short form(Reference Reynolds and Kobak127); CDRS, Children's Depression Rating Scale(Reference Poznanski, Cook and Carroll128); CDI, Children's Depression Inventory(Reference Kovacs, Beck, Schulterbrandt and Raskin129); CGI, Clinical Global Impression(Reference Guy130); YMRS, Young Mania Rating Scale(Reference Young, Biggs, Ziegler and Meyer131); NOS, not otherwise specified; IDS-C, Inventory of Depressive Symptomology(Reference Rush, Gullion, Basco, Jarrett and Trivedi132); CGI-BP, Clinical Global Impression – bipolar disorder(Reference Spearing, Post, Leverich, Brandt and Nolen133); NC, non-clinical; EPDS, Edinburgh Postnatal Depression Scale(Reference Cox, Holden and Sagovsky58); SCID-CV, Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV axis I disorders – clinical version(Reference First, Spitzer, Gibbon and Williams134); DSP, Derogatis Stress Profile(Reference Derogatis135); w-s, within-subjects; POMS, Profile of Mood States (depression question)(Reference McNair, Lorr and Droppleman136); DASS, Depression, Anxiety and Stress Scales (depression scale)(Reference Lovibond and Lovibond59); MOAS, Modified Overt Aggression Scale(Reference Coccaro, Harvey, Kupsaw-Lawrence, Herbert and Bernstein137).
*Advised to eat more fatty fish (mackerel, herring, kipper, pilchard, sardine, salmon, trout) or given EPA capsules; recommended dose is not reported.
Evaluation
Evidence of a role for n-3PUFA in depressive disorders is inconclusive. Epidemiological studies provide some evidence that n-3PUFA intake is associated with depressed mood, but not all studies have found associations, and some studies have found effects that have subsequently disappeared on consideration of confounders. Clinical studies also provide some evidence that depression may be associated with reduced n-3PUFA status, although the results are inconclusive. With some exceptions, the majority of studies are small, conducted on highly selected samples, and studies that find no associations between n-3PUFA or reverse associations are also available.
Evidence on the effects of n-3PUFA supplementation on depression is also inconclusive. Greatest evidence for a beneficial effect of supplementation with n-3PUFA can be found in the studies involving individuals diagnosed with major depression, but even here studies finding no benefit are also available. Evidence from individuals with bipolar disorders and other psychiatric conditions is equivocal. Evidence from the three trials involving individuals with non-diagnosed depression suggests no benefit of n-3PUFA supplementation on depression in these individuals.
Four recent meta-analyses have attempted to evaluate the evidence from placebo-controlled trials investigating a role for n-3PUFA in depression(Reference Appleton, Hayward, Gunnell, Peters, Rogers, Kessler and Ness138–Reference Appleton, Rogers, Ness and Heikkinen141). Differences between analyses exist dependent on inclusion criteria, but all four suggest a beneficial effect of n-3PUFA for depressive illness – combined effect sizes range from 0·13 (95 % CI 0·01, 0·25) to 0·61 (95 % CI 0·21, 1·01). All four meta-analyses also report clear heterogeneity between study findings, and the three conducted following a systematic review of the published literature(Reference Appleton, Hayward, Gunnell, Peters, Rogers, Kessler and Ness138, Reference Lin and Su140, Reference Appleton, Rogers, Ness and Heikkinen141) also suggest considerable publication bias, where small studies reporting positive findings are more likely to be published than small studies showing negative findings(Reference Appleton, Hayward, Gunnell, Peters, Rogers, Kessler and Ness138). The heterogeneity and publication bias in these analyses argue for caution when interpreting the overall effect sizes. The combined effect size and heterogeneity from the most recent meta-analysis conducted(Reference Appleton, Rogers, Ness and Heikkinen141) are clearly demonstrated in the relevant Forest plot (see Fig. 2). A beneficial effect of n-3PUFA compared with placebo was also found by Lin & Su(Reference Lin and Su140) when combining trials investigating a role for n-3PUFA in bipolar disorder (combined effect size 0·69 (95 % CI 0·28, 1·10), although heterogeneity and publication bias were also found. However, no differences between treatment and placebo were found by Appleton et al. (Reference Appleton, Hayward, Gunnell, Peters, Rogers, Kessler and Ness138) when combining trials investigating a role for n-3PUFA in individuals with no diagnosis of depressive disorder (combined standardised mean difference − 0·13 (95 % CI − 0·29, 0·02) sd).
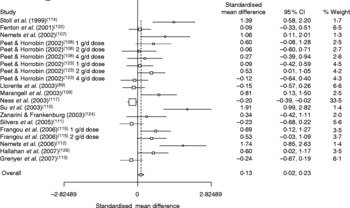
Fig. 2 Forest plot for the meta-analysis of all randomised controlled trials investigating the effects of n-3 long-chain PUFA on depressed mood up to September 2007 (taken from Appleton et al. (Reference Appleton, Rogers, Ness and Heikkinen141)).
Anxiety
Anxiety is defined as a state of uneasiness or tension caused by apprehension of possible misfortune or danger(142). Anxiety disorders include panic attacks, phobias, specific anxiety disorders, obsessive–compulsive disorder and generalised anxiety disorder. All are characterised by an intense or persistent apprehension, worry, fearfulness or terror(33).
Epidemiological evidence
No studies of which we are aware have investigated the association between n-3PUFA intake and anxiety or anxiety-related conditions.
Clinical evidence
One study has investigated associations between anxiety and n-3PUFA status(Reference Green, Hermesh, Monselise, Marom, Presburger and Weizman143). Individuals clinically diagnosed with social anxiety disorder were found to have lower levels of n-3PUFA, higher levels of n-6PUFA and higher ratios of n-6PUFA:n-3PUFA than controls. Severity of anxiety symptoms was also negatively correlated with n-3PUFA levels and positively correlated with n-6PUFA levels.
Trial evidence
Five studies have investigated the effects of n-3PUFA supplementation on anxiety and anxiety-related conditions. Ness et al. (Reference Ness, Gallacher, Bennett, Gunnell, Rogers, Kessler and Burr117) measured anxiety following advice to eat fish or supplementation with EPA in a large sample (n 452) of angina sufferers, Fontani et al. (Reference Fontani, Corradeschi, Felici, Alfatti, Bugarini, Fiaschi, Cerretani, Montorfani, Rizzo and Berra118) measured anxiety following supplementation with EPA+DHA+other n-3PUFA in thirty-three healthy volunteers, Rogers et al. (Reference Rogers, Appleton, Kessler, Peters, Gunnell, Hayward, Heatherley, Christian, McNaughton and Ness119) measured anxiety following supplementation with EPA+DHA in 218 individuals with mild–moderate depressed mood, Fux et al. (Reference Fux, Benjamin and Nemets125) studied anxiety and obsessive–compulsive behaviour following E-EPA in eleven individuals diagnosed with obsessive–compulsive disorder, and Yehuda et al. (Reference Yehuda, Rabinovitz and Mostofsky144) studied mood and organisational abilities following supplementation with ALA in a group of 126 test anxiety sufferers. Improvements following treatment compared with placebo were found in two studies(Reference Fontani, Corradeschi, Felici, Alfatti, Bugarini, Fiaschi, Cerretani, Montorfani, Rizzo and Berra118, Reference Yehuda, Rabinovitz and Mostofsky144), but no benefits of treatment compared with placebo were found in the other three studies(Reference Ness, Gallacher, Bennett, Gunnell, Rogers, Kessler and Burr117, Reference Rogers, Appleton, Kessler, Peters, Gunnell, Hayward, Heatherley, Christian, McNaughton and Ness119, Reference Fux, Benjamin and Nemets125).
Evaluation
Evidence investigating a role for n-3PUFA in anxiety and anxiety related conditions is very limited and, at present, equivocal. One clinical and two supplementation studies suggest that n-3PUFA may be implicated in anxiety, three supplementation studies suggest no role for n-3PUFA in anxiety. Further research is clearly required, however, before clear judgements can be made.
Fatigue
Fatigue is defined as a physical or mental exhaustion due to exertion(142) and is a key component of several behavioural conditions such as chronic fatigue syndrome and post-viral fatigue syndrome(Reference Holmes, Kaplan and Gantz145).
Epidemiological evidence
No studies of which we are aware have investigated the association between n-3PUFA intake and fatigue or fatigue-related conditions.
Clinical evidence
Three studies of which we are aware have investigated associations between n-3PUFA status and fatigue. Two of these studies found no differences between individuals diagnosed with chronic fatigue and controls in n-3PUFA status(Reference Behan, Behan and Horrobin120, Reference Warren, McKendrick and Peet121), although differences in n-6PUFA and saturated fats were found in one study(Reference Behan, Behan and Horrobin120). The third study(Reference Maes, Mihaylova and Leunis146) found elevated DHA levels and higher ratios of n-6PUFA:n-3PUFA in chronic fatigue patients, and found positive associations between n-6PUFA:n-3PUFA balance and fatigue symptoms. This study, however, also found associations between fatigue symptoms and levels of n-6PUFA.
Trial evidence
Five studies have included measurement of fatigue following n-3PUFA supplementation compared with placebo(Reference Grenyer, Crowe, Meyer, Owen, Grigonis, Caputi and Howe113, Reference Fontani, Corradeschi, Felici, Alfatti, Bugarini, Fiaschi, Cerretani, Montorfani, Rizzo and Berra118, Reference Behan, Behan and Horrobin120, Reference Warren, McKendrick and Peet121, Reference Yehuda, Rabinovitz and Mostofsky144). Of these studies, Behan et al. (Reference Behan, Behan and Horrobin120) found decreases in fatigue following supplementation with EPA+DHA compared with placebo in sixty-three individuals diagnosed with chronic fatigue syndrome, Fontani et al. (Reference Fontani, Corradeschi, Felici, Alfatti, Bugarini, Fiaschi, Cerretani, Montorfani, Rizzo and Berra118) found decreases following supplementation with EPA+DHA compared with placebo in thirty-three healthy volunteers, and Yehuda et al. (Reference Yehuda, Rabinovitz and Mostofsky144) found decreases in fatigue following supplementation with ALA +n-6PUFA compared with placebo in 126 individuals suffering from test anxiety. Benefits of n-3PUFA supplementation for individuals with chronic fatigue syndrome have also been reported in several individual cases(Reference Puri147). However, no differences between treatment and placebo groups were found by Warren et al. (Reference Warren, McKendrick and Peet121) using a supplement of EPA+DHA +n-6PUFA in fifty individuals, or by Grenyer et al. (Reference Grenyer, Crowe, Meyer, Owen, Grigonis, Caputi and Howe113) using a supplement of EPA+DHA in eighty-three individuals.
Evaluation
Evidence investigating a role for n-3PUFA in fatigue and related conditions is very limited and, at present, equivocal. One clinical and three supplementation studies suggest that n-3PUFA may be implicated in fatigue; however, two clinical studies and two supplementation studies also suggest no role for n-3PUFA in the development or treatment of fatigue. Further research is clearly required, however, before clear judgements can be made.
Aggression, hostility and anti-social behaviour
Aggression is defined as ‘a hostile or destructive mental attitude or behaviour’(142), hostility is defined as ‘enmity or antagonism’(142), and at extremes, both aggressive and hostile behaviours can result in a diagnosis of one of a number of impulsive control disorders, such as intermittent explosive disorder. Impulse control disorders are characterised by ‘the failure to resist an impulse drive or temptation to perform an act that is harmful to the individual or to others’(33). For the majority of disorders, the individual feels an increasing sense of tension or arousal before committing an aggressive act, experiences pleasure, gratification or relief at the time of committing the act, and then may or may not feel regret, self-reproach or guilt(33).
Epidemiological evidence
Epidemiological investigation of associations between n-3PUFA intake and hostility have been undertaken in one study, using diet histories and self-report hostility measured using the Cook–Medley Hostility Scale(Reference Cook and Medley148) in 3581 young adults(Reference Iribarren, Markovitz, Jacobs, Schreiner, Daviglus and Hibbeln149). This study found negative associations between hostility and DHA content of the diet and consumption of fish rich in n-3PUFA. No associations, however, were found between hostility and other n-3PUFA, n-6PUFA:n-3PUFA balance or consumption of all fish.
Clinical studies
Three studies of which we are aware have investigated n-3PUFA status in relation to aggressive or violent behaviour. One of these studies found lower levels of n-3PUFA and DHA, and almost higher n-6PUFA:n-3PUFA balance in aggressive compared with non-aggressive cocaine dependants(Reference Buydens-Branchey, Branchey, McMakin and Hibbeln150). The other two studies, however, found no differences between violent and non-violent controls or between individuals diagnosed with intermittent explosive disorder and controls in n-3PUFA status(Reference Umhau, Dauphinais, Patel, Nahrwold, Hibbeln and Rawlings151, Reference Virkkunen, Horrobin, Jenkins and Manku152). Virkkunen et al. (Reference Virkkunen, Horrobin, Jenkins and Manku152) did find lower levels of DHA in individuals diagnosed with personality disorder compared with controls, but greater differences were found in levels of n-6PUFA, which were markedly higher in patients than controls.
Trial evidence
One open-label study investigated the effects of n-3PUFA supplementation on irritability in thirty-four patients suffering from bipolar disorder, and found benefits(Reference Sagduyu, Dokucu, Eddy, Craigen, Baldassano and Yildiz102). A further open-label study, however, investigated the effects of n-3PUFA supplementation on anger and hostility in individuals suffering from post-traumatic stress disorder and found no effects(Reference Kaplan, Matar, Kamin and Cohen106).
Placebo-controlled trials investigating the effects of n-3PUFA on aggression, anger, hostility, tension, irritability and anti-social behaviour are given in Table 5(Reference Fontani, Corradeschi, Felici, Alfatti, Bugarini, Fiaschi, Cerretani, Montorfani, Rizzo and Berra118, Reference Rogers, Appleton, Kessler, Peters, Gunnell, Hayward, Heatherley, Christian, McNaughton and Ness119, Reference Zanarini and Frankenburg124, Reference Hallahan, Hibbeln, Davis and Garland126, Reference McNair, Lorr and Droppleman136, Reference Coccaro, Harvey, Kupsaw-Lawrence, Herbert and Bernstein137, Reference Cook and Medley148, Reference Hamazaki, Sawazaki, Itomura, Asaoka, Nagao, Nishimura, Yazawa, Kuwamori and Kobayashi153–Reference Aman, Singh, Stewart and Field166). Of the twelve studies reported, two studies found decreases in aggression following supplementation with E-EPA(Reference Zanarini and Frankenburg124), or EPA+DHA compared with placebo(Reference Hamazaki, Thienprasert, Kheovichai, Samuhaseneetoo, Nagasawa and Watanabe155), one study found decreases in anger following supplementation with EPA+DHA+other n-3PUFA compared with placebo(Reference Fontani, Corradeschi, Felici, Alfatti, Bugarini, Fiaschi, Cerretani, Montorfani, Rizzo and Berra118), one study found decreases in tension following supplementation with EPA+DHA+other n-3PUFA compared with placebo(Reference Buydens-Branchey and Branchey159), and one study found decreases in anti-social behaviour following supplementation with EPA+DHA +n-6PUFA+vitamins+minerals compared with placebo(Reference Gesch, Hammond, Hampson, Eves and Crowder160). Two further studies also found improvements in aggression following supplementation compared with placebo, where aggression increased in the placebo group but remained stable in the group treated with EPA+DHA(Reference Hamazaki, Sawazaki, Itomura, Asaoka, Nagao, Nishimura, Yazawa, Kuwamori and Kobayashi153, Reference Itomura, Hamazaki, Sawazaki, Kobayashi, Terasawa, Watanabe and Hamazaki156). Four of the studies report no differences in aggression/irritability between treatment and placebo groups following supplementation(Reference Hallahan, Hibbeln, Davis and Garland126, Reference Hamazaki, Thienprasert, Kheovichai, Samuhaseneetoo, Nagasawa and Watanabe155, Reference Hirayama, Hamazaki and Terasawa157, Reference Amminger, Berger, Schafer, Klier, Friedrich and Feucht158), one study reports no differences in hostility between treatment and placebo groups(Reference Hamazaki, Sawazaki, Nagao, Kuwamori, Yazawa, Mizushima and Kobayashi154) and one study reports no differences in anger between treatment and placebo groups(Reference Rogers, Appleton, Kessler, Peters, Gunnell, Hayward, Heatherley, Christian, McNaughton and Ness119). One study also reports increases in aggression following supplementation with EPA+DHA compared with placebo(Reference Itomura, Hamazaki, Sawazaki, Kobayashi, Terasawa, Watanabe and Hamazaki156), and one study reports decreases in aggression following placebo compared with treatment following supplementation with EPA+DHA(Reference Hamazaki, Sawazaki, Nagao, Kuwamori, Yazawa, Mizushima and Kobayashi154).
Table 5 Trial evidence investigating a role for n-3 long-chain PUFA (n-3PUFA) in aggression, hostility and anti-social behaviour: placebo-controlled trials
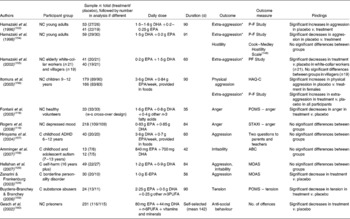
NC, non-clinical; P-F Study, Picture Frustration Study(Reference Rosenzweig161, Reference Hayashi, Sumita, Ichitani, Nakata, Hata, Tsuda, Nishio and Nishikawa162); HAQ-C, Hostility–Aggression Questionnaire for Children(Reference Yamasaki, Sakai, Soga, Ohdo, Shimai and Ohkate163, Reference Buss and Perry164); w-s, within-subjects; POMS, Profile of Mood States (anger question, tension question)(Reference McNair, Lorr and Droppleman136); STAXI, State-Trait Anger Expression Inventory(Reference Spielberger165); C, clinical; ADHD, attention deficit hyperactivity disorder; ABC, Aberrant Behaviour Checklist(Reference Aman, Singh, Stewart and Field166); MOAS, Modified Overt Aggression Scale(Reference Coccaro, Harvey, Kupsaw-Lawrence, Herbert and Bernstein137); E-EPA, ethyl ester EPA; n-6PUFA, n-6 long-chain PUFA.
* Extra-aggression is outward aggression towards other people or objects.
Evaluation
Evidence of the effects of n-3PUFA on aggression and hostility is again equivocal. Epidemiological evidence suggests relationships between some aspects of n-3PUFA intake and aggression or hostility, but not others. Some clinical studies have found associations whereas others have not. Trial studies also provide equivocal evidence of a benefit from and an absence of effects of n-3PUFA supplementation on aggression or hostility. Authors in this area have suggested that the absence of clear effects may be due to an effect of n-3PUFA which is only demonstrated in stressful situations or individuals under stress(Reference Hamazaki, Thienprasert, Kheovichai, Samuhaseneetoo, Nagasawa and Watanabe155), and that n-3PUFA may be beneficial in protecting against an increase in aggression in vulnerable situations or in individuals predisposed to aggressive or violent behaviour.
Inattention, hyperactivity, impulsivity and attention deficit hyperactivity disorder
Inattention is defined as not paying attention, hyperactivity is defined as abnormal activity and impulsivity is defined by actions based on sudden desires, whims or inclinations, rather than careful thought(142). ADHD is characterised by a persistent pattern of inattention and hyperactivity–impulsivity that is more frequent and severe than is typically observed in individuals at a comparable level of development. For formal diagnosis, some hyperactivity–impulsivity symptoms that cause impairments must have been present before the age of 7 years, some impairment from symptoms must be present in at least two settings, and clear evidence of developmentally inappropriate social, academic and occupational functioning must exist(33). Symptoms of inattention and/or hyperactivity–impulsivity of insufficient severity to warrant formal diagnosis also occur(33).
Epidemiological evidence
Only one study of which we are aware has used epidemiological evidence to investigate the association between n-3PUFA intake and inattention, impulsivity or ADHD, although this study used maternal n-3PUFA intake during pregnancy, and measures of inattention, hyperactivity and behavioural disorders in offspring(Reference Hibbeln, Davis, Steer, Emmett, Rogers, Williams and Golding167). The study found increased seafood consumption during pregnancy was associated with decreased behavioural problems; however, only associations with prosocial behaviour and social development remained after adjustment for confounders.
Clinical evidence
Several studies have investigated the associations between n-3PUFA status and inattention, hyperactivity and impulsivity(Reference Mitchell, Aman, Turbott and Manku168–Reference Antalis, Stevens, Campbell, Pazdro, Ericson and Burgess172). Four of these studies found low levels of n-3PUFA in individuals with ADHD symptoms compared with controls(Reference Stevens, Zentall, Deck, Abate, Watkins, Lipp and Burgess169–Reference Antalis, Stevens, Campbell, Pazdro, Ericson and Burgess172), and all studies found low levels of DHA in these individuals. Two studies also found high ratios of n-6PUFA:n-3PUFA in those with ADHD symptoms compared with controls(Reference Stevens, Zentall, Deck, Abate, Watkins, Lipp and Burgess169, Reference Antalis, Stevens, Campbell, Pazdro, Ericson and Burgess172). Four studies found low levels of n-6PUFA in those with symptoms(Reference Mitchell, Aman, Turbott and Manku168–Reference Stevens, Zhang, Peck, Kuczek, Grevstad, Mahon, Zentall, Arnold and Burgess170, Reference Antalis, Stevens, Campbell, Pazdro, Ericson and Burgess172). Stevens et al. (Reference Stevens, Zentall, Deck, Abate, Watkins, Lipp and Burgess169) also found a continuous association between DHA levels and ADHD symptom severity as measured by parents, but no associations were found based on teacher ratings, and similar associations were not found by Young et al. (Reference Young, Maharaj and Conquer171) using self-report questionnaires. Investigating behavioural symptoms in children with high and low n-3PUFA status, Stevens et al. (Reference Stevens, Zentall, Abate, Kuczek and Burgess173) also found associations between low n-3PUFA status and high parental ratings of hyperactivity, impulsivity and conduct disorders, although no associations were found in teachers' ratings. Conversely, Stevens et al. (Reference Stevens, Zhang, Peck, Kuczek, Grevstad, Mahon, Zentall, Arnold and Burgess170) also found higher levels of n-3PUFA and lower n-6PUFA: n-3PUFA balance in individuals with ADHD symptoms compared with controls, using measurements from erythrocytes as opposed to plasma lipids.
Antalis et al. (Reference Antalis, Stevens, Campbell, Pazdro, Ericson and Burgess172) and Stevens et al. (Reference Stevens, Zentall, Deck, Abate, Watkins, Lipp and Burgess169) also studied n-3PUFA intake in young adults diagnosed with ADHD compared with controls. Antalis et al. (Reference Antalis, Stevens, Campbell, Pazdro, Ericson and Burgess172) found no differences in intakes of n-3PUFA or n-6PUFA in cases and controls, although Stevens et al. (Reference Stevens, Zentall, Deck, Abate, Watkins, Lipp and Burgess169) found higher intakes of PUFA in cases than in controls.
Trial evidence
One open-label study investigated the effects of supplementation with ALA and vitamins on inattention, hyperactivity and impulsivity in thirty children diagnosed with ADHD, and found reductions in all three measures(Reference Joshi, Lad, Kale, Patwardhan, Mahadik, Patni, Chaudhary, Bhave and Pandit174). One further open-label study investigated the effects of n-3PUFA supplementation on impulsivity in individuals suffering from post-traumatic stress disorder, but this study found no effects(Reference Kaplan, Matar, Kamin and Cohen106).
Nine studies of which we are aware have investigated the effects of n-3PUFA on inattention, impulsivity and related conditions, as given in Table 6(Reference Hallahan, Hibbeln, Davis and Garland126, Reference Itomura, Hamazaki, Sawazaki, Kobayashi, Terasawa, Watanabe and Hamazaki156–Reference Amminger, Berger, Schafer, Klier, Friedrich and Feucht158, Reference Aman, Singh, Stewart and Field166, Reference Stevens, Zhang, Peck, Kuczek, Grevstad, Mahon, Zentall, Arnold and Burgess170, Reference Voigt, Llorente, Jensen, Fraley, Berretta and Heird175–Reference Dougherty, Marsh and Mathias186). The majority of studies have been conducted on children diagnosed with ADHD(Reference Hirayama, Hamazaki and Terasawa157, Reference Stevens, Zhang, Peck, Kuczek, Grevstad, Mahon, Zentall, Arnold and Burgess170, Reference Voigt, Llorente, Jensen, Fraley, Berretta and Heird175, Reference Harding, Judah and Gant176), but studies are also available involving children with no clinical mood diagnoses(Reference Itomura, Hamazaki, Sawazaki, Kobayashi, Terasawa, Watanabe and Hamazaki156, Reference Richardson and Puri177), children diagnosed with developmental co-ordination disorder(Reference Richardson and Montgomery178), children and adolescents diagnosed with autism(Reference Amminger, Berger, Schafer, Klier, Friedrich and Feucht158) and adults with diagnosed self-harm(Reference Hallahan, Hibbeln, Davis and Garland126). Three studies found improvements in inattention following supplementation with EPA+DHA +n-6PUFA(Reference Stevens, Zhang, Peck, Kuczek, Grevstad, Mahon, Zentall, Arnold and Burgess170, Reference Richardson and Puri177, Reference Richardson and Montgomery178) compared with placebo, two studies found improvements in hyperactivity following supplementation with EPA+DHA(Reference Amminger, Berger, Schafer, Klier, Friedrich and Feucht158) and EPA+DHA+n-6PUFA(Reference Richardson and Montgomery178) compared with placebo, one study found improvements in impulsivity following supplementation with EPA+DHA(Reference Itomura, Hamazaki, Sawazaki, Kobayashi, Terasawa, Watanabe and Hamazaki156), two studies found improvements in general ADHD symptoms following supplementation with EPA+DHA +n-6PUFA(Reference Richardson and Puri177, Reference Richardson and Montgomery178) compared with placebo, and one study found improvements in disruptive behaviour following supplementation with EPA+DHA + n-6PUFA(Reference Stevens, Zhang, Peck, Kuczek, Grevstad, Mahon, Zentall, Arnold and Burgess170). The majority of these studies, however, measured inattention, hyperactivity, impulsivity and conduct as described by parents and teachers, but found effects only in selected measures. Three studies also found no benefits of n-3PUFA compared with placebo for inattention, hyperactivity or impulsivity(Reference Hirayama, Hamazaki and Terasawa157, Reference Voigt, Llorente, Jensen, Fraley, Berretta and Heird175) or impulsivity alone(Reference Hallahan, Hibbeln, Davis and Garland126), and one study found no benefits of n-3PUFA compared with current medication (Ritalin)(Reference Harding, Judah and Gant176).
Table 6 Trial evidence investigating a role for n-3 long-chain PUFA (n-3PUFA) in inattention, hyperactivity, impulsivity and attention deficit hyperactivity disorder: placebo-controlled trials
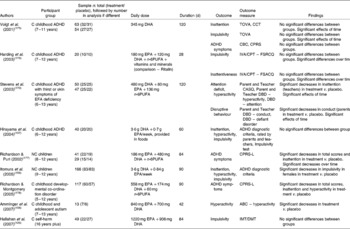
C, clinical; ADHD, attention deficit hyperactivity disorder; TOVA, Test of Variables of Attention(Reference Greenberg and Kindschi179); CCT, Children's Color Trials Test(Reference D'Elia, Satz, Uchiyama and White180); CBC, Child Behaviour Checklist(Reference Achenbach and Edenbrock181); CPRS, Conners' Parent Rating Scales(Reference Conners182); IVA/CPT – FSRCQ, Intermediate Visual and Auditory/Continuous Performance Test – Full Scale Response Control Quotient(Reference Seckler, Burns, Montgomery and Sandford183); IVA/CPT – FSACQ, Intermediate Visual and Auditory/Continuous Performance Test – Full Scale Attention Control Quotient(Reference Seckler, Burns, Montgomery and Sandford183); EFA, essential fatty acid; n-6PUFA, n-6 long-chain PUFA; CASQ, Conners' Abbreviated Symptom Questionnaires(Reference Conners184); DBD, Disruptive Behaviour Disorders Rating Scale(Reference Pelham, Gnagy, Greenslade and Milich185); NC, non-clinical; CPRS-L, Conners' Parent Rating Scales (long version)(Reference Conners182); ABC, Aberrant Behaviour Checklist(Reference Aman, Singh, Stewart and Field166); IMT/DMT, Immediate and Delayed Memory Tasks(Reference Dougherty, Marsh and Mathias186).
Evaluation
The one epidemiological study provides no evidence of a role for n-3PUFA in inattention, hyperactivity and impulsivity, once confounders are taken into consideration. The findings from clinical studies suggest that n-3PUFA and particularly DHA may be important in inattention, impulsive and disruptive behaviours, although evidence is currently very limited. The findings from trials are currently equivocal, and the evidence overall is far from conclusive. The majority of studies have found benefits from n-3PUFA supplementation on selected aspects of mood or behaviour, although no benefits are found for other aspects of mood or behaviour, and studies that fail to find effects are also available.
Schizophrenic disorders
Schizophrenia is defined by a mixture of characteristic (positive and negative) signs and symptoms which have been present for a significant proportion of time during a 1-month period with indications of the disorder persisting for at least 6 months. Positive symptoms reflect an extension or distortion of normal functions, for example, delusions, hallucinations, and disorganised speech or behaviour. Negative symptoms reflect a diminution or loss of normal functions, for example, restrictions in the range or intensity of emotional expression, restrictions in the fluency or productivity of thought or speech, and restrictions in the initiation of goal-directed behaviour(33). Epidemiological, clinical and trial evidence investigating a role for n-3PUFA in schizophrenia and schizophrenic disorders is available.
Epidemiological evidence
Three studies have investigated associations between n-3PUFA intake and schizophrenia. One ecological study investigated the association between total fat, fat from animals and birds and fat from fish and vegetables in the diet and course and outcome of schizophrenia in eight countries. This study found a positive association between poorer course and outcome for schizophrenia and total fat consumption and consumption of fat from animals and birds, and no association between schizophrenia course or outcome and fat from fish and vegetables, although in a regression model both a high consumption of fat from animals and birds and a low consumption of fat from fish and vegetables were predictive of poorer schizophrenia outcome(Reference Christensen and Christensen187). A second ecological study conducted on data from fourteen countries found no association between seafood consumption and prevalence rates of schizophrenia(Reference Noaghiul and Hibbeln37). One study in the USA investigated the relationship between the whole diet and a clinical diagnosis of schizophrenia in 146 schizophrenic patients compared with population norms. This study also found positive associations between schizophrenia and consumption of saturated fat and polyunsaturated fats, but no association with n-3PUFA(Reference Strassnig, Singh Brar and Ganguli188).
Clinical evidence
Associations between n-3PUFA status and schizophrenia or schizophrenic symptoms, assessed by comparison of individuals with schizophrenia compared with controls, are shown in Table 7(Reference Ranjekar, Hinge, Hegde, Ghate, Kale, Sitasawad, Wagh, Debsikdar and Mahadik81, Reference Kaiya, Horrobin, Manku and Morse-Fisher83, Reference Assies, Lieverse, Vreken, Wanders, Dingemans and Linszen91, Reference Obi and Nwanze189–Reference McNamara, Jandacek, Rider, Tso, Hahn, Richtand and Stanford201). Patterns in n-3PUFA status are again inconclusive. Ten studies show decreased levels of n-3PUFA in schizophrenics compared with controls, while four studies show elevated levels of n-3PUFA in schizophrenics compared with controls(Reference Kaiya, Horrobin, Manku and Morse-Fisher83, Reference Obi and Nwanze189, Reference Horrobin, Manku, Morse-Fisher, Vaddadi, Courtney, Glen, Glen, Spellman and Bates190–Reference Peet, Shah, Selvam and Ramchand198). Two studies show increases in n-6PUFA:n-3PUFA (arachidonic acid:DHA) ratios in schizophrenics compared with controls(Reference Assies, Lieverse, Vreken, Wanders, Dingemans and Linszen91, Reference McNamara, Jandacek, Rider, Tso, Hahn, Richtand and Stanford201) and two studies show decreases in n-6PUFA:n-3PUFA ratios(Reference Horrobin, Manku, Morse-Fisher, Vaddadi, Courtney, Glen, Glen, Spellman and Bates190, Reference Peet, Shah, Selvam and Ramchand198). All studies except five(Reference Assies, Lieverse, Vreken, Wanders, Dingemans and Linszen91, Reference Obi and Nwanze189, Reference Landen, Davidsson, Gottfries, Mansson and Blennow195–Reference Evans, Parikh, Khan, Coussons, Buckley and Mahadik197) also found decreased n-6PUFA in schizophrenics compared with controls.
Table 7 Clinical evidence investigating a role for n-3 long-chain PUFA (n-3PUFA) in schizophrenia: comparisons between cases and controls

ALA, α-linolenic acid; DPA, docosapentaenoic acid; LA, linoleic acid; AA, arachidonic acid; C, clinical; PL, plasma phospholipids; C0, untreated clinical population; ↑ , higher PUFA in cases compared with comparison; C24, clinical population treated for 24 weeks; EM, erythrocyte membranes; x, no association; ↓ , lower PUFA in cases compared with comparison; TD, Tardive dyskinesia; CE, plasma cholesteryl esters; CV, cardiovascular.
* Other n-3PUFA tested but no associations found.
† Other n-3PUFA tested and associations found.
Studies that investigate relationships between levels of n-3PUFA and severity of symptoms show similar inconsistencies. Assies et al. (Reference Assies, Lieverse, Vreken, Wanders, Dingemans and Linszen91) found negative associations between EPA status and schizophrenic symptoms, Reddy et al. (Reference Reddy, Keshavan and Yao199) found no associations between n-3PUFA levels and schizophrenic symptoms, Mellor et al. (Reference Mellor, Laugharne and Peet202) found no associations between n-3PUFA levels and schizophrenic symptoms, and positive associations with involuntary movement, but Peet et al. (Reference Peet, Shah, Selvam and Ramchand198) found positive associations between DHA status and positive schizophrenic symptoms, and Richardson et al. (Reference Richardson, Cyhlarova and Ross203) found positive associations between n-3PUFA levels and positive schizotypal trait measures in healthy adults, and no associations with negative schizotypal trait measures. Three studies also found positive associations between n-6PUFA levels and schizophrenic symptoms(Reference Assies, Lieverse, Vreken, Wanders, Dingemans and Linszen91, Reference Mellor, Laugharne and Peet202) or schizotypal trait measures(Reference Richardson, Cyhlarova and Ross203), although Peet et al. (Reference Peet, Shah, Selvam and Ramchand198) found negative associations between linoleic acid status and negative schizophrenic symptoms.
Trial evidence
Several open-label studies have investigated the effects of n-3PUFA on schizophrenic symptoms. These studies found improvements in schizophrenic symptoms following supplementation with EPA(Reference Mellor, Laugharne and Peet202, Reference Shah, Vankar, Telang, Ramchand and Peet204) and improvements in schizophrenic symptoms and quality of life following supplementation with EPA+DHA(Reference Arvindakshan, Ghate, Ranjekar, Evans and Mahadik196). Case reports of treatment with n-3PUFA for schizophrenia have also yielded benefits(Reference Rudin205, Reference Puri and Richardson206).
Five placebo-controlled studies of which we are aware have investigated the effects of supplementation with n-3PUFA on schizophrenic symptoms. Peet et al. (Reference Peet, Brind, Ramchand, Shah and Vankar207) found decreases in symptoms following EPA supplementation at 2 g/d for 3 months compared with placebo in two studies, and Emsley et al. (Reference Emsley, Myburgh, Oosthuizen and van Rensburg208) found decreases in symptoms following E-EPA supplementation at 2 g/d compared with placebo for 12 weeks. Peet & Horrobin(Reference Peet and Horrobin123) also found decreases in symptoms following supplementation with 2 g E-EPA/d compared with placebo for 12 weeks in patients with adjunctive treatment with clozapine, although limited effects were found for 1 g/d and 4 g/d doses and in patients with other adjunctive medication, and no effects of any dose or in any group were found on involuntary movement. Fenton et al. (Reference Fenton, Dickerson, Boronow, Hibbeln and Knable122) also found no improvements in schizophrenic symptoms or in involuntary movement following supplementation with 3 g E-EPA/d compared with placebo for 16 weeks and Peet et al. (Reference Peet, Brind, Ramchand, Shah and Vankar207) found no effects on schizophrenic symptoms from supplementation with 2 g DHA/d compared with placebo for 3 months.
Evaluation
Epidemiological evidence suggests that n-3PUFA intakes may be unimportant in schizophrenia and schizophrenic conditions, although total PUFA or total fat intake may be important. Clinical studies also suggest that schizophrenia may not be associated with biochemical concentrations of n-3PUFA, but that levels of total PUFA or total fat may be more important. Studies of n-3PUFA supplementation have found some benefits of n-3PUFA for schizophrenia, although the results of one meta-analysis, to date, suggest no benefits of n-3PUFA supplementation compared with placebo (combined mean difference of − 2·61 (95 % CI − 6·37, 1·15) Positive and Negative Syndrome Scale scores(Reference Freeman, Hibblen and Wisner139). Only very limited evidence, however, is clearly currently available.
Other mood and behavioural disorders
In investigation of other mood or behavioural disorders, a potential role for n-3PUFA has also been suggested in autism and Asperger's syndrome. Autism is defined by the presence of markedly abnormal or impaired development in social interaction and communication, and a markedly restricted repertoire of activity and interests(33). Asperger's syndrome is defined by severe and sustained impairments in social interaction and the development of restricted and repetitive patterns of behaviour, interests and activities(33).
In support of an association between n-3PUFA and autism and/or Asperger's syndrome, Vancassel et al. (Reference Vancassel, Durand, Barthelemy, Lejeune, Martineau, Guilloteau, Andres and Chalon209) found low levels of n-3PUFA and higher ratios of n-6PUFA:n-3PUFA in individuals with autism compared with mentally retarded individuals, Bell et al. (Reference Bell, MacKinley, Dick, MacDonald, Boyle and Glen210) found lower levels of n-3PUFA, and particularly docosapentaenoic acid n-3 in individuals with autism and individuals with Asperger's syndrome compared with controls, and Johnson & Hollander(Reference Johnson and Hollander211) found beneficial effects of supplementation with EPA in one individual with autism. However, in a placebo-controlled trial conducted in thirteen children and adolescents with diagnosed autism, no benefit of EPA+DHA was found(Reference Amminger, Berger, Schafer, Klier, Friedrich and Feucht158). Evidence in this area is clearly too limited to draw reliable conclusions.
n-3 Long-chain polyunsaturated fatty acids in mood and behaviour – evaluation
Evidence available investigating a role for n-3PUFA in mood and behaviour is highly inconsistent. The greatest available evidence investigates a role for n-3PUFA in depression and depressive disorders, but this evidence provides no clear picture of the role of n-3PUFA in these conditions. Evidence suggesting a role for n-3PUFA in anxiety and fatigue is much more limited but equally equivocal. Evidence suggesting a role for n-3PUFA in aggression, anger and hostility is also equivocal. Studies investigating a role for n-3PUFA in inattention, hyperactivity, impulsivity and ADHD do suggest some associations, although evidence is far from conclusive. Studies investigating schizophrenia and schizophrenic disorders suggest no clear role for n-3PUFA in these conditions, but evidence again is far from conclusive.
While findings are inconsistent, so too is the methodology used to attain these findings. Evidence is provided from epidemiological and clinical studies and from trials. Large epidemiological studies have the advantage of investigation of the population as a whole, but their cross-sectional and observational nature, and their lack of precision and detail severely limit the conclusions that can be drawn from them. While associations between n-3PUFA intake and various aspects of mood and mood disorders may be found, a direct association is far from necessary, and a causal association, even less so. All associations are bidirectional or may be explained by some third party. Behavioural evidence suggests various mood disorders to be associated with subsequent decreases in self-care and healthful behaviours, such as consumption of a healthy diet(Reference DiMatteo, Lepper and Croghan212). Behavioural and lifestyle variables have also been associated both with mood disorders and with dietary intake, and may explain any relationship between the two(Reference Barberger-Gateau, Jutand, Letenneur, Larrieu and Tavernier48, Reference Appleton, Peters, Hayward, Heatherley, McNaughton, Rogers, Gunnell, Ness and Kessler50, Reference Appleton, Woodside and Yarnell51). The attenuation of relationships between n-3PUFA intake and depression following adjustment for confounding variables suggests that any association between n-3PUFA intake and depression is unlikely to be a genuine association(Reference Appleton, Peters, Hayward, Heatherley, McNaughton, Rogers, Gunnell, Ness and Kessler50, Reference Appleton, Woodside and Yarnell51), and similar explanations may also apply for other aspects of mood or behaviour. Adequate consideration and measurement of potential confounders, however, can be difficult.
Epidemiological evidence is also based on fish or n-3PUFA intakes. Because of the essential nature of n-3PUFA, dietary intakes of n-3PUFA can be suggested to closely reflect n-3PUFA status(Reference Browne, Scott and Silvers78). However, fish intake may not be a good proxy for n-3PUFA status as it is dependent on the type of fish consumed, and plant sources of n-3PUFA, such as nuts and seeds, rely on biological conversion to longer-chain n-3PUFA before affecting longer-chain n-3PUFA function and status(Reference Burdge, Finnegan, Minihane, Williams and Wootton213). n-3PUFA intake may also not be a good proxy for n-3PUFA status, as n-3PUFA status depends on n-3PUFA metabolism and synthesis as well as n-3PUFA intake, and the relationship between intake and incorporation into tissues has been found to be non-linear(Reference Browne, Scott and Silvers78).
Clinical studies are also disadvantaged by their cross-sectional and observational nature, again limiting the conclusions that can be drawn. Clinical studies that find an association between biological status and mood are often used to suggest a biologically mediated effect on mood; yet, again, causal explanations cannot be drawn from cross-sectional studies such as these. Again, relationships are bidirectional or may be explained by a third party. Evidence suggesting that mood affects n-3PUFA status is available from various animal and human studies. Stress is intricately linked with many psychiatric conditions, and 3 weeks of physical and psychological stress has been found to result in decreased neuronal phospholipids and increased lipid peroxidation products in rats(Reference Gulyaeva, Levishina and Obidin214). Isolation stress has also been found to result in decreased activity of the Δ5 and Δ6 desaturase enzymes in rats(Reference Mills, Huang, Nane and Poisson215), and Brenner(Reference Brenner216) reports reductions in the activity of Δ5 and Δ6 desaturase enzymes from a variety of stress-related hormones including adrenalin, adrenocorticotropic hormone, cortisol and steroids in humans. The Δ5 and Δ6 desaturase enzymes are necessary for n-3PUFA elongation and synthesis. Smoking and alcohol consumption, often also associated with psychiatric conditions, have also been found to impact on n-3PUFA synthesis resulting in reductions in levels(Reference Hibbeln, Makino, Martin, Dickerson, Boronow and Fenton217, Reference Reddy and Yao218). Traditional medications for a number of psychiatric conditions may also impact on n-3PUFA status, although work on first-episode schizophrenics suggests that medications are unlikely to explain low levels of n-3PUFA in these individuals(Reference Khan, Evans, Gunna, Scheffer, Parikh and Mahadik194, Reference Reddy, Keshavan and Yao199).
Evidence suggesting that both n-3PUFA status and mood may be explained by a third party is also available. Several nutrients that play a role in the metabolism of n-3PUFA have also been implicated in the regulation of mood. Erythrocyte concentrations of folate in humans have been associated with mania(Reference Hasanah, Khan, Musalmah and Razali219) and plasma concentrations of homocysteine have been associated with hostility, anger(Reference Stoney and Engebretson220) and schizophrenia(Reference Kemperman, Veurink, van der Wal, Knegtering, Bruggeman, Fokkema, Kema, Korf and Muskiet200), but folate-deficient diets in rats have also been found to result in decreases in DHA in rat plasma(Reference Durand, Prost and Blache221) and nervous tissue(Reference Hirono and Wada222). Mg and Zn deficiency have been reported in children diagnosed with ADHD compared with controls(Reference Bekaroglu, Aslan, Gedik, Deger, Mocan, Erduran and Karahan223, Reference Starobrat-Hermelin and Kozielec224), Zn deficiency has been correlated with severity of ADHD symptoms(Reference Arnold, Bozzolo, Hollway, Cook, DiSilvestro, Bozzolo, Crowl, Ramadan and Williams225) and Mg and Zn supplementation has been found to improve ADHD symptoms in children(Reference Starobrat-Hermelin and Kozielec224, Reference Akhondzadeh, Mohammadi and Khademi226), but Mg and Zn deficiencies have also been found to result in decreased synthesis of n-3PUFA, via reduced activity of desaturase enzymes(Reference Galland227, Reference Arnold and DiSilvestro228). Hormones and other biological factors may also affect both n-3PUFA status and mood(Reference Bolton-Smith, Woodward and Tavendale229, Reference Burdge and Calder230). Factors related to n-3PUFA intake may also affect both n-3PUFA status and mood(Reference Appleton, Peters, Hayward, Heatherley, McNaughton, Rogers, Gunnell, Ness and Kessler50).
Clinical studies do offer the precision and detail that cannot be obtained in large epidemiological studies, although they suffer as well, as a result of this precision. The majority of clinical studies are small and conducted on highly selected samples, making confounding factors difficult to adequately control for, and generalisation difficult(Reference Sontrop and Campbell231). Consensus between studies is also difficult due to the use of different biological samples used to measure n-3PUFA status. Studies use assays of plasma, erythrocyte membranes, adipose tissue and brain tissue, each thought to be of different potential relevance to mood-related biochemical processes(Reference McNamara, Hahn, Jandacek, Rider, Tso, Stanford and Richtand72).
Well-conducted blinded, placebo-controlled trials can allow investigation of causal explanations. Trial methodology investigating the effects of n-3PUFA on mood, however, is also inconsistent. Of possibly greatest potential impact, trials are inconsistent in their use of n-3PUFA. Some studies use ALA, some use EPA, some use DHA and others use a combination, yet the suggested potential mechanisms of action of ALA, EPA and DHA on mood differ greatly. Doses of n-3PUFA also vary greatly between studies, yet the bioavailability of n-3PUFA may differ dependent on source(Reference Visioli, Rise, Barassi, Marangoni and Galli232), and PUFA synthesis is affected by PUFA levels, so large intakes of n-3PUFA may inhibit n-3PUFA synthesis(Reference Burdge, Finnegan, Minihane, Williams and Wootton213, Reference Brookes, Chen, Xu, Taylor and Asherson233). Use of n-3PUFA alone, or in conjunction with vitamin E, n-6PUFA, other vitamins and minerals and existing medications also varies greatly between studies, yet interactions between these compounds are rarely considered(Reference James, Gibson and Cleland5). Vitamin E, n-6PUFA and various vitamins and minerals may also affect mood, and can impact on n-3PUFA synthesis and activity(Reference Kemperman, Veurink, van der Wal, Knegtering, Bruggeman, Fokkema, Kema, Korf and Muskiet200, Reference Hasanah, Khan, Musalmah and Razali219, Reference Stoney and Engebretson220, Reference Bekaroglu, Aslan, Gedik, Deger, Mocan, Erduran and Karahan223–Reference Akhondzadeh, Mohammadi and Khademi226, Reference Horrobin, Jenkins, Bennett and Christie234, Reference Kirsch, Deacon, Huedo-Medina, Scoboria, Moore and Johnson235).
Second, aspects of mood investigated have been very varied. However, the biochemistry underlying depression, for example, may be very different to the biochemistry underlying anxiety or aggression. Similarly, DHA deficiency is thought to contribute predominantly to the development of postpartum depression(Reference Freeman, Hibbeln, Wisner, Watchman and Gelenberg98, Reference Freeman, Hibbeln, Wisner, Brumbach, Watchman and Gelenberg99), yet DHA supplementation has not been the focus of study for other forms of depression. Differences between and within trials also exist in their definitions and measurement of outcome mood.
Trials also differ markedly in population studied. Some studies use males, some use females and some use both, yet various hormones impact on n-3PUFA synthesis and degradation, and effects may differ in males and females(Reference McNamara, Hahn, Jandacek, Rider, Tso, Stanford and Richtand72, Reference Burdge and Calder230). Some studies use adults and others use children, yet n-3PUFA metabolism may change with age(Reference Bolton-Smith, Woodward and Tavendale229). Some studies use individuals of low n-3PUFA status before the start of the study whereas other do not, yet there is a suggestion that effects of n-3PUFA may be found only in deficient individuals(Reference Sontrop and Campbell231). Some studies use clinical populations, some use non-clinical populations; again, effects may differ. A recent series of meta-analyses, for example, found evidence of a beneficial effect of n-3PUFA supplementation in individuals with a diagnosed depressive disorder, but found no evidence of a benefit in populations without a depressive diagnosis(Reference Appleton, Hayward, Gunnell, Peters, Rogers, Kessler and Ness138).
Trials also differ in conduct and quality. Trials using poor-quality n-3PUFA preparations and a likely fishy aftertaste can be criticised for poor blinding and the possibility of results due to expectations(Reference Stoll, Damico, Daly, Severus and Marangell236). Trials using olive oil as a placebo have also been criticised due to the potential mood-altering affects of oleamide, a product of oleic acid, a significant component of olive oil(Reference Puri and Richardson237). Trials using short time intervals can also be criticised due to the time interval required for n-3PUFA to be fully incorporated into the biological system(Reference Sontrop and Campbell231).
Differences in methodology currently, however, cannot systematically explain differences in trial outcomes. The benefits of n-3PUFA supplementation in some trials provide some evidence that n-3PUFA may be implicated in the regulation of mood and behaviour. It is thus possible that n-3PUFA do have a role in the regulation of mood and behaviour, but whether that role is direct or indirect is yet to be uncovered.
The absence of effects in all trials, however, also suggests that implication of n-3PUFA in the regulation of mood and behaviour in all individuals seems unlikely, and present theories of a role for n-3PUFA in mood and behaviour fail to explain why supplementation with n-3PUFA does not benefit all individuals with the same conditions. Individual differences in n-3PUFA metabolism, however, have recently been suggested and associated with disruptions to mood and behaviour. Covault et al. (Reference Covault, Pettinati, Moak, Mueller and Kranzler238) have found polymorphisms within the gene encoding long-chain fatty acid-CoA ligase type 4 (FACL4), an enzyme important in the incorporation of PUFA into the cell membrane, and found certain polymorphisms to be associated with depression. Pae et al. (Reference Pae, Yu, Kim, Lee, Lee, Jun, Lee and Paik239) have found polymorphisms in the gene encoding type IV cytosolic phospholipase A2 (cPLA2) – an enzyme important for n-3PUFA uptake, and found certain polymorphisms to be associated with depression. Brookes et al. (Reference Brookes, Chen, Xu, Taylor and Asherson233) found associations between one of the genes involved in fatty acid synthesis (fatty acid desaturase 2) and clinical diagnosis of ADHD. Schizophrenia has also been associated with one of the genes responsible for the activity of PLA2, a group of enzymes responsible for the incorporation of PUFA into the cell membrane and the degradation of PUFA to form eicosanoids(Reference Peet, Brind, Ramchand, Shah and Vankar207). Alterations in n-3PUFA synthesis thus may suggest that certain individuals have a predisposition to disruptions to mood or behaviour. Thus, alterations at different stages of n-3PUFA synthesis and degradation may explain why some individuals are affected by some n-3PUFA, as opposed to others. Alterations in PUFA synthesis may also explain why supplementation with n-3PUFA fails to affect mood in some individuals, dependent on the mechanisms by which n-3PUFA affect mood. It has also been argued that, due to the essential nature of PUFA, a predisposition to disruptions to mood or behaviour may be only exposed under certain environmental conditions(Reference Ross240). Genetic explanations for mood and behaviour, however, fail to explain the recent and continuing increase in mood disorders throughout the world(Reference Hibbeln, Salem and Holick241).
A role for n-3PUFA in the regulation of mood and behaviour is currently far from clear. Furthermore, due to the multi-factorial nature of mood and behaviour, it may be unlikely that any single mechanism is likely to affect or benefit all aspects of mood in all individuals. More work investigating the role of n-3PUFA in the regulation of mood and behaviour is clearly required. The greatest need, however, is for more work based on biochemical mechanisms. A role for n-3PUFA in various neurotransmitter systems and their links with mood and behaviour need to be clearly elucidated. Similarly, a role for n-3PUFA in inflammatory processes and the impact of these on mood and behaviour need to be clearly established. Until the biochemical mechanisms underlying the regulation of different aspects of mood and behaviour are more clearly understood, trials will continue to be designed and explained on an ad hoc basis and differences between trials will remain unexplained. Very limited work currently investigates the mechanisms by which mood is regulated in humans, and the means by which n-3PUFA may exert effects on these mechanisms. Exact n-3PUFA and dose of n-3PUFA for supplementation, for example, ought to be based on proposed mechanisms of action. Traditional medications for various psychiatric conditions were developed based on chance findings, with little understanding of underlying mechanisms, and despite success for about 30–60 % of patients treated, little subsequent progress in development has been achieved(Reference Peet242). We need to ensure a similar pattern of events does not occur for n-3PUFA.
A natural remedy for various mood and behavioural conditions, such as n-3PUFA, with few side effects and other potential health benefits (for example, for CVD, immune function and inflammatory conditions(Reference Ruxton, Calder, Reed and Simpson2, Reference Ruxton, Reed, Simpson and Millington243)) is highly attractive, yet expense, false hope and limited success in all individuals(Reference Shi, Nakamura, Shimbo and Takano244) caution against a blanket recommendation for the use of n-3PUFA for mood or behaviour. Side effects following supplementation with n-3PUFA have also been reported. Gastrointestinal complaints and loose stools are common(Reference Stoll, Severus, Freeman, Rueter, Zboyan, Diamond, Cress and Marangell114, Reference Van Strater and Bouly245), n-3PUFA may adversely affect blood coagulation in individuals treated with anti-coagulants and glucose metabolism in diabetics(Reference Van Strater and Bouly245), and Kinrys(Reference Kinrys246) and Marangell et al. (Reference Marangell, Suppes, Ketter, Dennehy, Zboyan, Kertz, Nierenberg, Calabrese, Wisniewski and Sachs247) report episodes of hypomania following supplementation with n-3PUFA. Until more evidence is available, n-3PUFA cannot be advocated for the treatment of numerous mood and behavioural conditions.
Conclusion
In conclusion, the evidence currently available from epidemiological, clinical and intervention studies investigating the role of n-3PUFA in the regulation of mood and behaviour is limited and highly inconsistent. The field is further compromised by an inadequate understanding of the biochemical mechanisms underlying the regulation of mood and behaviour in humans. There is a clear need for increased high-quality work focusing on understanding the potential mechanisms by which n-3PUFA may impact on mood and behaviour. Work appears most advanced (from publications) in relation to schizophrenia(Reference Yao, Magan, Sonel, Gurklis, Sanders and Reddy19, Reference Skosnik and Yao248), but further work is needed here as well as in relation to other conditions. An important priority for future research is to conduct adequately powered, well-designed randomised controlled intervention trials, but this should be done in tandem with work investigating the mechanisms by which n-3PUFA may affect mood and behaviour.
Acknowledgements
K. M. A. undertook the majority of the research required for and the majority of the writing of this article; P. J. R. and A. R. N. helped with the writing. The work was supported by Queen's University of Belfast (Belfast, UK) and The University of Bristol (Bristol, UK). All authors declare that they have no conflicts of interest.











