Introduction
Cystic echinococcosis (CE) is a severe zoonosis distributed worldwide caused by species of the Echinococcus granulosus sensu lato (s.l.) complex (Deplazes et al., Reference Deplazes, Rinaldi, Alvarez Rojas, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson and Jenkins2017). The current taxonomy of E. granulosus s.l. establishes strong delimitations between Echinococcus granulosus sensu stricto (s.s.) (which includes the genotypes G1/G3 and related variants), Echinococcus equinus (G4), Echinococcus ortleppi (G5) and the group comprising the G6/7/8/10 genotypes for which the nomenclature remains controversial (Lymbery et al., Reference Lymbery, Jenkins, Schurer and Thompson2015; Nakao et al., Reference Nakao, Lavikainen and Hoberg2015). Furthermore, the G6 and the G7 genotypes show clear differences in intermediate host specificity; G6 is commonly associated with camels and goats and G7 with pigs. Additionally, molecular data comparing sequences of the full length of the cox1 and nad genes (Addy et al., Reference Addy, Wassermann, Kagendo, Ebi, Zeyhle, Elmahdi, Umhang, Casulli, Harandi, Aschenborn, Kern, Mackenstedt and Romig2017) and the complete mitochondrial genome (Laurimäe et al., Reference Laurimäe, Kinkar, Romig, Omer, Casulli, Umhang, Gasser, Jabbar, Sharbatkhori, Mirhendi, Ponce-Gordo, Lazzarini, Soriano, Varcasia, Rostami Nejad, Andresiuk, Maravilla, González, Dybicz, Gawor, Šarkūnas, Šnábel, Kuzmina and Saarma2018) confirmed the differentiation of both genotypes.
Echinococcus granulosus s.s. is accountable for the majority of human CE cysts genotyped worldwide (88.4%) followed by the G6 genotype (7.3%) (Alvarez Rojas et al., Reference Alvarez Rojas, Romig and Lightowlers2014). However, in some countries/geographic areas, the G6 genotype is the most commonly found variant of the parasite in humans including Egypt (Abdel Aaty et al., Reference Abdel Aaty, Abdel-Hameed, Alam-Eldin, El-Shennawy, Aminou, Makled and Darweesh2012; Alam-Eldin et al., Reference Alam-Eldin, Aaty and Ahmed2015), Mauritania (Bardonnet et al., Reference Bardonnet, Piarroux, Dia, Schneegans, Beurdeley, Godot and Vuitton2002, Reference Bardonnet, Benchikh-Elfegoun, Bart, Harraga, Hannache, Haddad, Dumon, Vuitton and Piarroux2003; Bart et al., Reference Bart, Bardonnet, Elfegoun, Dumon, Dia, Vuitton and Piarroux2004; Maillard et al., Reference Maillard, Benchikh-Elfegoun, Knapp, Bart, Koskei, Gottstein and Piarroux2007, Reference Maillard, Gottstein, Haag, Ma, Colovic, Benchikh-Elfegoun, Knapp and Piarroux2009) and Sudan (Omer et al., Reference Omer, Dinkel, Romig, Mackenstedt, Elnahas, Aradaib, Ahmed, Elmalik and Adam2010). The G6 genotype is also present, but is not the most commonly found variant of E. granulosus, in other countries/geographic areas including Iran (Shahnazi et al., Reference Shahnazi, Hejazi, Salehi and Andalib2011; Sadjjadi et al., Reference Sadjjadi, Mikaeili, Karamian, Maraghi, Sadjjadi, Shariat-Torbaghan and Kia2013); Russia (Konyaev et al., Reference Konyaev, Yanagida, Ingovatova, Shoikhet, Nakao, Sako, Bondarev and Ito2012); Mongolia (Jabbar et al., Reference Jabbar, Narankhajid, Nolan, Jex, Campbell and Gasser2011) and the Turkana region in Kenya (Casulli et al., Reference Casulli, Zeyhle, Brunetti, Pozio, Meroni, Genco and Filice2010). In South America, the G6 genotype has been identified in a single human cyst in Chile (Manterola et al., Reference Manterola, Benavente, Melo, Vial and Roa2008) and in 2 humans from Peru (Santivañez et al., Reference Santivañez, Gutierrez, Rosenzvit, Muzulin, Rodriguez, Vasquez, Rodriguez, Gonzalez, Gilman and Garcia2008; Moro et al., Reference Moro, Nakao, Ito, Schantz, Cavero and Cabrera2009). In the Neuquén province of Argentina, the G6 genotype has been described in 57.1% (Kamenetzky et al., Reference Kamenetzky, Gutierrez, Canova, Haag, Guarnera, Parra, García and Rosenzvit2002), 36.5% (Guarnera et al., Reference Guarnera, Parra, Kamenetzky, García and Gutiérrez2004) and 53.8% (Debiaggi et al., Reference Debiaggi, Soriano, Pierangeli, Lazzarini, Pianciola, Mazzeo, Moguillansky and Farjat2017) of human cysts genotyped.
Since the beginning of the study of the variability of E. granulosus s.l., it has been suggested that species/genotypes of E. granulosus s.l. complex could show biological differences for example in the development of cysts. Previous studies have suggested a higher growth rate in cysts attributed to G6 compared with E. granulosus s.s. (Guarnera et al., Reference Guarnera, Parra, Kamenetzky, García and Gutiérrez2004) and an apparent tropism of the G6 genotype for brain tissue (Sadjjadi et al., Reference Sadjjadi, Mikaeili, Karamian, Maraghi, Sadjjadi, Shariat-Torbaghan and Kia2013). More recently, Örsten et al. (Reference Örsten, Çiftçi, Azizova, Yüce, Uysal, Imamoğlu, Karaağaoğlu, Akinci, Akyön, Casulli and Akhan2020) showed that cysts from the cluster G6/7 (6 samples) were detected more frequently in non-liver locations and usually had a smaller diameter compared with 110 cysts genotyped as E. granulosus s.s. In the present study, we analyse differences in biological features of a large number of human cysts in CE-confirmed cases from Neuquén province caused by genotypes of E. granulosus s.s. and the G6 genotype of E. granulosus s.l.
Materials and methods
Parasite material
One hundred twenty-four human E. granulosus cysts were acquired from 90 CE-confirmed patients who underwent surgery at different public and private hospitals in Neuquén province in Argentina between May 2014 and July 2018. These 90 individuals represent 37.7% of all CE cases reported from Neuquén in the studied period according to the National System for Health Surveillance (Surveillance, Reference Surveillance2019). Data about the origin, age, gender of the patient, infected organ/tissue, diameter and number of cysts per patient were recorded. Cysts from patients with hepatic CE were classified according to the WHO-IWGE classification (Brunetti et al., Reference Brunetti, Kern and Vuitton2010). Cyst fertility was determined by detecting the presence of protoscoleces in the hydatid fluid. The viability of protoscoleces was assessed in a dye-exclusion test using 0.4% Trypan Blue and by the motility of flame cells. Protoscoleces or a piece of the germinal layer were collected from each cyst and fixed in 70% ethanol and stored at −20°C for molecular analysis, each cyst was considered a sample.
Molecular analysis
DNA was isolated from each sample using the QIAamp DNA mini kit (QIAGEN, Germany) according to the manufacturer's instructions and stored at −20°C. To determine the cyst genotype a fragment of 366 bp of the cox1 gene of E. granulosus s.l. was amplified and sequenced as previously described (Bowles et al., Reference Bowles, Blair and Mcmanus1992; Debiaggi et al., Reference Debiaggi, Soriano, Pierangeli, Lazzarini, Pianciola, Mazzeo, Moguillansky and Farjat2017). The sequences were aligned and compared with reference sequences for E. granulosus s.s. G1 (accession number: U50464) and G3 (M84663), E. equinus G4 (M84664), E. ortleppi G5 (M84665) and the genotypes G6 (M84666) and G7 (M84667) and used as input in BLAST for comparison.
Data analysis
Statistical analysis was carried out using the package InfoStat (v2017e). Univariate analysis was applied to quantitative variables (age, number of cysts per patient and cyst size), and the results were expressed as mean, one standard deviation and range. Differences in cyst size were analysed using the Student's t-test; Chi-square (χ 2) test was used to compare categorical data. Differences were considered statistically significant when the P value < 0.05.
Results
Genetic characterization of cysts
Echinococcus granulosus s.s. was identified in 81/124 (65.3%) cysts available for this study; these 81 cysts were acquired from 51/90 (56.7%) patients. The G6 genotype of E. granulosus s.l. was identified in 43/124 (34.7%) cysts; these 43 cysts were acquired from 39/90 (43.3%) patients available for the present study (Table 1). From the 81 sequences identified as E. granulosus s.s., 67 showed 100% homology with the reference sequence for the genotype G1 (U50464), 4 were identical to the reference sequence for G3 (M84663); while other 10 sequences were identical to GenBank entries previously identified in Neuquén including 8 sequences identical to G1nqnC (GU980911), 1 to G1nqnD (KC954601) and 1 to G1nqnE (JN176929). All the sequences from the 43 cysts identified as G6 shared 100% homology with the reference sequence for G6 (M84666).
Table 1. Distribution of genotypes of E. granulosus sensu lato on cysts included in this study and classification of CE patients according to the gender and age group

* P < 0.05.
**P < 0.005.
Clinical characteristics of the patients: age and gender
The mean age for CE patients was 35.7 ± 22 years (range: 3–81, median = 33). There were 29/90 (32.2%) patients ≤18 years, and 61/90 (67.8%) >18 years of age out of the total of 90 CE patients included in this study (Table 2). The mean age of patients under 18 years was 11 ± 4.3 years (median = 14), and 44.1 ± 16.7 years (median = 45) for patients >18 years. Males accounted for 62.2% (56/90) of the total number of patients (χ 2 = 0.1; P = 0.75). In the group of patients ≤18 years of age, males represented 82.8% (23/29), showing a significant difference with female cases of the same age group (χ 2 = 14.23; P = 0.002). E. granulosus s.s. infected 69% (20/29) of the patients from 0 to 18 years old, while the G6 genotype was responsible for 31% (9/29) of the same age bracket showing significant differences (χ 2 = 4.2; P < 0.05) (Table 1).
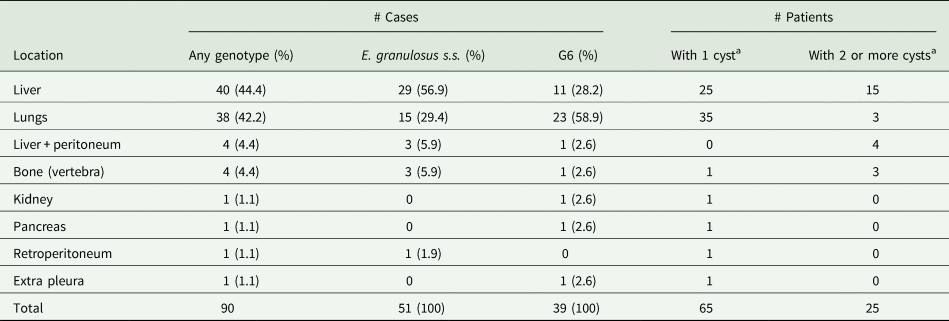
Table 2. Frequency of cyst location and number of cysts per patient in 90 confirmed human cystic echinococcosis patients from Neuquén, Argentina, between 2014 and 2018
a P < 0.05.
Cyst localization and number of cysts per patient
The liver was the most frequently affected organ with 44.4% of the 90 CE cases, followed by the lungs with 42.2% showing a liver/lung ratio of 1.16/1 (Table 2). The rest of the Echinococcus cysts were found in the peritoneum, retroperitoneum, bone, kidney, pancreas and extra pleura. The number of cysts per patient ranged from 1 to 6. In total, 65/90 patients harboured only 1 cyst (72.2%) and 25 had 2 or more cysts (27.8%) (Table 2).
Of the 124 cysts analysed in this study, 63 were in the liver, from which 50 cysts were identified as E. granulosus s.s. (79.4%) and 13 as the G6 genotype (20.6%). Forty-one cysts were in the lungs, from which 17 were identified as E. granulosus s.s. (41.5%) and 24 as the G6 genotype (58.5%) (Table 3). The liver/lung ratio for E. granulosus s.s. was 2.13/1, while for the G6 genotype, the ratio was 0.52/1. Most of the cysts identified with the G6 genotype (30/43) were detected in extra-hepatic localization, while most of the cysts were identified as E. granulosus s.s. showed hepatic localization (50/81) (χ 2 = 10.7; P = 0.001) (Table 3). Patients infected with the G6 genotype presented a maximum of 2 cysts while those infected with E. granulosus s.s. presented up to 6 cysts (χ 2 = 11.34; P < 0.001).
Table 3. Distribution of genotypes, diameter and fertility of E. granulosus sensu lato cysts concerning the anatomical localization

*P < 0.05.
*** P = 0.001.
Cyst size
The mean diameter of the 124 cysts was 7.0 ± 4.2 cm (range = 0.3–20 cm). For hepatic cysts, the mean diameter was 7.9 cm (1.8–20 cm), for pulmonary cysts 7.2 cm (2.5–15 cm), for vertebral bone 0.5 cm (0.3–0.5 cm) and peritoneum cysts 4.3 cm (3–8 cm). Statistical significance was found in differences in the diameter of lung cysts attributed to E. granulosus s.s, compared with those of the G6 from the same organ (P < 0.05) (Table 3).
Fertility and viability of Echinococcus cysts
We observe protoscoleces in 79 out of 124 cysts (63.7%); viable protoscoleces were observed in 30.3% of those cysts. Fertile cysts were found in all locations except in the single cyst found in the pancreas. Fertility did not show significant differences between species/genotypes: 50/81 cysts attributed to E. granulosus s.s (61.7%) were fertile, while 29/43 cysts attributed to the G6 genotype had protoscoleces (67.4%) (P = 0.665) (Table 3).
Stage of liver cysts using the WHO criteria
WHO classification was used for the ultrasound examination of 31 patients who harboured a total of 48 hepatic cysts: 20 cysts were active (CE1 and CE2), 17 were transitional cysts (CE3) and 11 were inactive cysts (CE4). Echinococcus granulosus s.s. was identified in cysts in the 4 stages found CE1, CE2, CE3 and CE4; the G6 genotype was characterized in cysts CE1, CE3 and CE4. In total, 84.6% (33/39) of cysts characterized as E. granulosus s.s. were active and transitional and 6/39 (15.3%) were inactive. In the case of G6 cysts, 55.6% (5/9) were inactive, observing significant differences with E. granulosus s.s. cysts (χ 2 = 6.94; P = 0.0084).
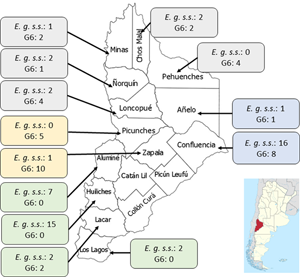
Geographic distribution of CE patients in Neuquén
The province was divided into 4 areas (North, East, South and West) and the patients were assigned to each area according to their origin. The distribution of patients in each region was: North (20 patients), South (30), East (24) and West (16) (Fig. 1). Aggregation of human cases caused by E. granulosus s.s. was observed in the Southern area of Neuquén with 27/30 patients (χ 2 = 18.4; P < 0.00001). For patients infected with genotype G6, aggregation was observed in 15/16 individuals in the Western zone (χ 2 = 17.7; P < 0.0001) and in 13/20 patients from the Northern part of Neuquén (χ 2 = 3.8; P < 0.05).
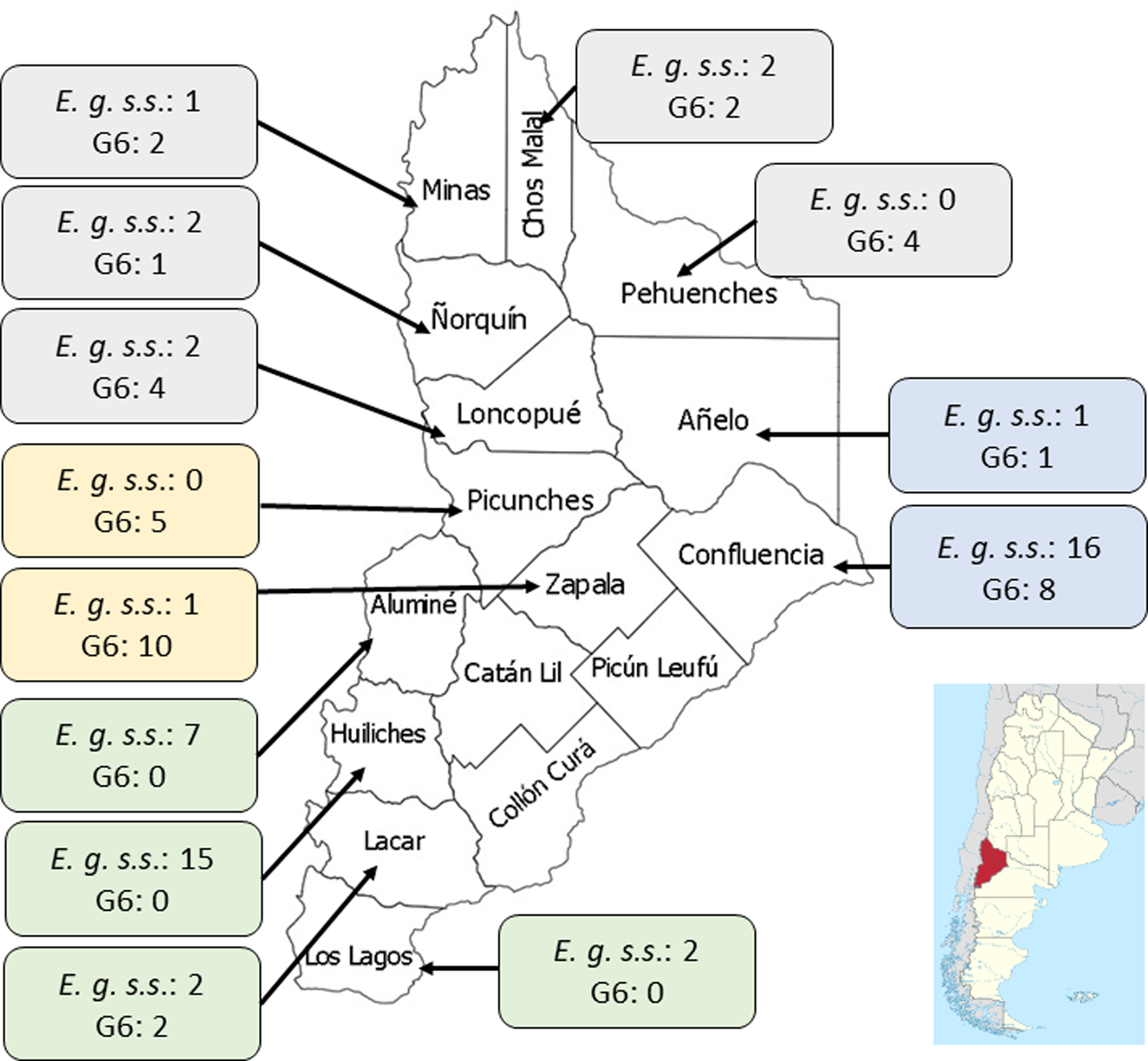
Fig. 1. Map of the Neuquén province showing the distribution of E. granulosus s.s. (E.g. s.s.) and the G6 genotype identified in 90 patients from 13 districts of this province. The number of cases for each species/parasite is indicated within the boxes. District names belonging to each geographic region: (1) Northern region (grey boxes): Minas, Chos Malal, Pehuenches, Loncopue, Ñorquin; (2) Eastern region (blue boxes): Confluencia, Añelo; (3) Western region (yellow boxes): Zapala, Picunches; (4) Southern region (green boxes): Aluminé, Huiliches, Lacar, Los Lagos.
Discussion
The Neuquén province is highly endemic for CE; livestock raising focused on goats is an important activity in rural areas which has favoured the presence of the G6 genotype of E. granulosus s.l. Furthermore, socioeconomic characteristics of the population have perpetuated the transmission of the parasite in the province despite the control programme that has been carried out since 1970. In Argentina, the mean annual incidence of human CE has been estimated to be 0.7/105 inhabitants in 2018. Neuquén province has the second highest incidence of CE in the country estimated at 6.18/105 (Surveillance, Reference Surveillance2019).
Data from the present study showed that 81/124 (65.3%) of the cysts analysed from 90 patients were characterized as E. granulosus s.s. and 43/124 (34.7%) human cysts were identified as genotype G6. The percentage of G6 cysts found in this study is lower than the one reported previously in 2 previous studies from Neuquén in which most human cysts belonged to the G6 genotype of E. granulosus s.l.: 57.1% (12/21) (Kamenetzky et al., Reference Kamenetzky, Gutierrez, Canova, Haag, Guarnera, Parra, García and Rosenzvit2002) and 53.8% (14/26) (Debiaggi et al., Reference Debiaggi, Soriano, Pierangeli, Lazzarini, Pianciola, Mazzeo, Moguillansky and Farjat2017). This difference could be attributed to the fact that a greater number of cysts from almost all districts of Neuquén were province were included in this study. Nevertheless, in the current report, the percentage of E. granulosus human cysts attributed to the G6 genotype is much higher than the worldwide situation (7.34%) (Alvarez Rojas et al., Reference Alvarez Rojas, Romig and Lightowlers2014).
There are 751 853 goats in the Neuquén province which represent 17.5% of goats in the Argentinian territory (Census, Reference Census2020). Goats are raised in a transhumance model which comprises a close contact human-dog (Soriano et al., Reference Soriano, Pierangeli, Pianciola, Mazzeo, Lazzarini, Saiz, Kossman, Bergagna, Chartier and Basualdo2010; Debiaggi et al., Reference Debiaggi, Soriano, Pierangeli, Lazzarini, Pianciola, Mazzeo, Moguillansky and Farjat2017; Census, Reference Census2020). The geographical distribution of E. granulosus s.s and the G6 genotype in humans from the province of Neuquén was markedly heterogeneous (Fig. 1). The largest number of cysts belonging to E. granulosus s.s was found in patients from the South and East regions, which is characterized by the breeding of sheep and cattle and very low raising of goats (Census, Reference Census2020). The highest number of cysts belonging to the G6 genotype was found in the North and West region, which are mostly characterized by the transhumant breeding of goats.
The analysis of the distribution of CE cases according to gender and age showed that there were more male than female patients under 18 years of age, mainly in the age range between 10 and 18 years. This difference could be explained by the fact that most boys accompany their parents in herding livestock, therefore having closer contact with dogs. We found that 32.2% of the CE cases studied corresponded to children under 18 years, which suggests active transmission of the parasite exists in Neuquén despite a long-standing control programme for CE.
The most frequent anatomical localization of cysts was in the liver, followed by the lungs while some cysts were found in unusual localizations like bones (vertebra), peritoneum, kidney, pancreas, retroperitoneum and extra pleura. The total liver/lung ratio in this study was 1.16/1; this value is lower compared with the reported in a review based on 9,970 hospital records from South America, Africa, Europe, Australia and Asia (Larrieu and Frider, Reference Larrieu and Frider2001) which showed a ratio of 2.5/1. In a previous study analysing CE patients from Neuquén, the liver/lung ratio was 4.1/1 (Pierangeli et al., Reference Pierangeli, Soriano, Roccia, Giménez, Lazzarini, Grenóvero, Menestrina and Basualdo2007). The factors that determine the final anatomic localization of cysts are still unknown. A systematic review of the literature on human CE indicated that E. granulosus s.s. is located preferentially in the liver (73.4%), followed by the lungs (19.6%). The G6 genotype is reported to affect the liver (54.3%), lungs (25.7%), brain (12.9%) and other organs (7.1%) (Cucher et al., Reference Cucher, Macchiaroli, Baldi, Camicia, Prada, Maldonado, Avila, Fox, Gutiérrez, Negro, López, Jensen, Rosenzvit and Kamenetzky2016). In this study, we observed that 78.1% of the hepatic cysts corresponded to E. granulosus s.s. and 21.9% corresponded to the G6 genotype, while 58.5% of the pulmonary cysts corresponded to the G6 genotype (P = 0.0001). Although it was observed that most G6 genotype cysts were found in the lungs, this genotype also infected other organs such as the liver, vertebrae, kidneys, peritoneum, pancreas and extra pleura. The localization of G6 genotype cysts in humans seems to be similar to that observed in goats of Neuquén and in previous studies that suggested a tropism of the G6 genotype for the lung (Bardonnet et al., Reference Bardonnet, Piarroux, Dia, Schneegans, Beurdeley, Godot and Vuitton2002; Soriano et al., Reference Soriano, Pierangeli, Pianciola, Mazzeo, Lazzarini, Saiz, Kossman, Bergagna, Chartier and Basualdo2010). Sadjjadi et al. (Sadjjadi et al., Reference Sadjjadi, Mikaeili, Karamian, Maraghi, Sadjjadi, Shariat-Torbaghan and Kia2013) also suggested an extrahepatic localization of cysts attributed to G6, in this case, the brain. However, they did not include CE patients with cysts in lungs in their study (Sadjjadi et al., Reference Sadjjadi, Mikaeili, Karamian, Maraghi, Sadjjadi, Shariat-Torbaghan and Kia2013). Our results are in agreement with the recently published work by Örsten et al. (Reference Örsten, Çiftçi, Azizova, Yüce, Uysal, Imamoğlu, Karaağaoğlu, Akinci, Akyön, Casulli and Akhan2020), who suggested that the cluster G6/G7 can be detected in extra-hepatic localizations more frequently than E. granulosus s.s. However, Örsten et al. (Reference Örsten, Çiftçi, Azizova, Yüce, Uysal, Imamoğlu, Karaağaoğlu, Akinci, Akyön, Casulli and Akhan2020) compared 104 E. granulosus s.s. with only 6 cysts belonging to the G6/7 cluster that were not differentiated between G6 and G7. The present study shows data from a more robust number of samples including 81 cysts attributed to E. granulosus s.s. and 43 to the G6 genotype. Our results also suggest that cysts' localization could be related to the infecting species/genotypes of E. granulosus s.l. Data analysing the relationship between genetic diversity and the number of cysts per patient is scarce. The analysis of the relationship between the species/genotypes of E. granulosus s.l. and the number of cysts per patient showed that 42.9% of the patients infected with E. granulosus s.s. had more than one cyst (with a maximum of 6 cysts in 1 patient), while 9.8% of the patients infected with the G6 genotype presented up to 2 cysts (P < 0.001). In this study, we also found that E. granulosus s.s. lung cysts size was larger than lung cysts from G6 genotype (P < 0.05). While Guarnera et al. (Reference Guarnera, Parra, Kamenetzky, García and Gutiérrez2004) suggested that G6 genotype lung cysts were larger than E. granulosus s.s. lung cysts. These differences may be due to the fact that in this study we analysed more samples. However, it is important to note that cyst size depends on the time of surgery performed, which usually occurs when the patient present symptoms, while the time of infection remains unknown. These data are relevant if, for example, abdominal ultrasound screening would be used in Neuquén where several cases would be considered as false negatives since most cysts of G6 would be infecting the lungs.
No significant differences were observed in the fertility and viability of cysts between species/genotypes in this study. Few reports relate fertility to the genotype of the cyst, E. granulosus s.s. shows high percentages of fertility ranging between 50 and 96% (Bart et al., Reference Bart, Abdukader, Zhang, Lin, Wang, Nakao, Ito, Craig, Piarroux, Vuitton and Wen2006; Lahmar et al., Reference Lahmar, Rebaï, Boufana, Craig, Ksantini, Daghfous, Chebbi, Fteriche, Bedioui, Jouini, Dhibi, Makni, Ayadi, Ammous, Kacem and Ben Safta2009; Omer et al., Reference Omer, Dinkel, Romig, Mackenstedt, Elnahas, Aradaib, Ahmed, Elmalik and Adam2010; Macin et al., Reference Macin, Orsten, Samadzade, Colak, Cebeci and Findik2021). Patients with an indication for CE surgery usually receive pharmacological treatment with benzimidazoles to reduce the risk of secondary CE after surgery. This treatment is adjusted to the patient's characteristics (e.g., age, comorbidities) and clinical disease aspects (e.g., location and stage of the cyst, presence of complications). Thus, pharmacological treatment before surgery could affect the fertility of cysts regardless of genotype.
Despite the efforts to control parasite transmission in Neuquén since 1970 (Craig et al., Reference Craig, Hegglin, Lightowlers, Torgerson, Wang, Thompson, Deplazes and Lymbery2017) CE remains endemic. Vaccination of livestock has not been included in control activities in Neuquén as in other parts of Argentina like Rio Negro Province (Labanchi et al., Reference Labanchi, Poggio, Gutiérrez, Mujica, Araya, Grizmado, Calabro, Crowley, Arezo, Seleiman, Herrero, Sepulveda, Talmon, Diaz and Larrieu2022). It remains unknown if the current EG95 vaccine would protect against the infection with the G6 genotype of E. granulosus s.l. (Alvarez Rojas et al., Reference Alvarez Rojas, Gauci, Nolan, Harandi and Lightowlers2012, Reference Alvarez Rojas, Gauci and Lightowlers2013). Considering the high prevalence of the G6 genotype in Neuquén province, it offers the perfect scenario to elucidate that question in the near future.
In conclusion, we provide strong support for the idea that E. granulosus s.s. presents a tropism for the liver, while the G6 genotype is more commonly found in extra-hepatic localizations (i.e., lungs). It was also observed that genotypes would influence the number of cysts per patient and the size of the lung cysts. More studies should be carried out in endemic regions worldwide to increase knowledge on clinical aspects of human CE caused by different species and genotypes of E. granulosus sensu lato. These facts could be considered in patient care guidelines and in CE control programs, to contribute to the improvement of the epidemiological situation of this zoonosis.
Data availability
There is no extra data for this paper.
Acknowledgements
The authors are very grateful to all the members of the Neuquén Hidatidosis Group who collaborated in this study: surgeons who provided the cysts; infectious disease doctors who attended to the patients and recorded their data; physicians who performed the diagnostic imaging; nurses and technicians.
Author's contributions
NBP, LC and SVS conceived and designed the study. DC, PT and MI collected and coordinated clinical data and the informed consent of patients. MFD and LEL performed all tests. MFD, CAAR, PD and NBP wrote the article.
Financial support
This study was supported by grants 04/N018 and 04/N029 from the Research Department of Universidad Nacional del Comahue and partially supported by the Institute of Parasitology, University of Zurich, Switzerland.
Conflicts of interest
The authors declare there are no conflicts of interest.
Ethical standards
The Advisory Committee on Human Biomedical Research, from the Sub-secretary of Health in Neuquén, approved the present study (CAIBSH No. file: 4420-138451/2013). All adult subjects and parents/tutors of the patients under 18 years gave their written informed consent to be included in this study and to authorize the anonymous use of their clinical data.