Introduction
Maternal nutrition during pregnancy can affect fetal brain development and has been associated with cognitive outcomes of offspring later in life. Reference Craciunescu, Johnson and Zeisel1–Reference Nyaradi, Li, Hickling, Foster and Oddy3 Two essential nutrients that have been linked to the prevention of neural tube defects and children’s neurodevelopment are folate and choline. Reference Nyaradi, Li, Hickling, Foster and Oddy3,Reference Irvine, England-Mason, Field, Dewey and Aghajafari4 Folate and choline are both methyl donors and may play similar roles in fetal brain development, Reference Nyaradi, Li, Hickling, Foster and Oddy3 including DNA and RNA synthesis, epigenetic DNA modification, regulation of homocysteine concentrations, and the synthesis of numerous neurotransmitters. Reference Nyaradi, Li, Hickling, Foster and Oddy3,Reference Blusztajn, Slack and Mellott5–Reference Sarter and Parikh9 Thus, it is possible that maternal folate and choline levels during pregnancy may have an interactive effect on children’s neurodevelopment.
Folate cannot be synthesized by the body and must be supplied from the diet (e.g., leafy green vegetables, legumes, citrus fruits). Folate nutritional status is assessed by measuring folate concentrations in serum/plasma, or red blood cells (RBCs). The World Health Organization (WHO) recommends that RBC folate concentrations should be above 906 nmol/L in women of reproductive age. 10 Choline is produced endogenously in the liver; however, the amount is insufficient to meet metabolic demands. Therefore, dietary intake of foods such as eggs, red meat, fish, nuts and cruciferous vegetables is required. Reference Wiedeman, Barr, Green, Xu, Innis and Kitts11 Choline levels in blood are not routinely measured as there is no standardized method of measurement and no reference levels for blood during pregnancy. Reference Holm, Ueland, Kvalheim and Lien12 Therefore, choline intake is typically measured. The Institute of Medicine has established the adequate intake (AI) value of choline during pregnancy to be 450 mg/day. 13
In animal models, higher maternal levels of folate and choline have been found to influence fetal brain development and are associated with improved cognitive and behavioral outcomes in offspring. Reference Craciunescu, Johnson and Zeisel1,Reference Jadavji, Deng, Malysheva, Caudill and Rozen2,Reference Irvine, England-Mason, Field, Dewey and Aghajafari4,Reference Craciunescu, Brown, Mar, Albright, Nadeau and Zeisel14–Reference Glenn, Kirby and Gibson20 Human studies that have examined associations between maternal folate supplementation, intake, and status during pregnancy and children’s neurodevelopmental outcomes have reported contradictory findings. Reference Julvez, Fortuny, Mendez, Torrent, Ribas-Fitó and Sunyer21–Reference Chen, Qin and Gao27 Julvez et al. Reference Julvez, Fortuny, Mendez, Torrent, Ribas-Fitó and Sunyer21 found that prenatal folic acid supplementation was associated with higher verbal, motor, and verbal-executive function scores in 4-year-old children, and a recent meta-analysis concluded that appropriate maternal folic acid supplementation may have positive effects on children’s intelligence and development, and reduce the risk of language problems, ADHD, autism traits, and behavioral problems. Reference Chen, Qin and Gao27 Research using food frequency questionnaires (FFQs) to estimate maternal prenatal folic acid intake have reported conflicting findings in relation to cognitive outcomes, Reference Villamor, Rifas-Shiman, Gillman and Oken22,Reference Boeke, Gillman, Hughes, Rifas-Shiman, Villamor and Oken25 as have studies that have measured maternal folate concentrations (i.e., plasma/serum folate, RBC folate) directly from blood samples collected during pregnancy. Reference Ars, Nijs and Marroun24,Reference Wu, Dyer, King, Richardson and Innis26,Reference Veena, Krishnaveni and Srinivasan28,Reference Tamura, Goldenberg, Chapman, Johnston, Ramey and Nelson29 Veena et al. Reference Veena, Krishnaveni and Srinivasan28 reported a positive association between maternal plasma/serum levels of folate in the third trimester and children’s performance on a test of cognitive function (i.e., Kaufman Assessment Battery for Children) at 9 years, whereas Wu et al. Reference Wu, Dyer, King, Richardson and Innis26 found that maternal plasma/serum levels in the second and third trimester were not associated with scores on the Bayley-III at 18 months of age. Tamura et al. Reference Tamura, Goldenberg, Chapman, Johnston, Ramey and Nelson29 observed that maternal gestational RBC folate status was not associated with children’s cognitive, memory, or motor development at 5 years of age.
Studies that have examined the associations between choline supplementation, intake, and status, and children’s cognitive outcomes have also reported conflicting results. Caudill et al. Reference Caudill, Strupp, Muscalu, Nevins and Canfield23 found that choline supplementation with 930 mg choline/day compared to 480 mg/day was associated with higher information processing speed in infants up to 13 months of age. Boeke et al. Reference Boeke, Gillman, Hughes, Rifas-Shiman, Villamor and Oken25 reported that first and second trimester maternal choline intake was positively associated with memory performance in 7-year-old children, and Wu et al. Reference Wu, Dyer, King, Richardson and Innis26 observed a positive association between maternal plasma free choline concentrations in the second and third trimester and infant cognitive development at 18 months of age. In contrast, Signore et al. Reference Signore, Ueland, Troendle and Mills30 reported that maternal gestational serum concentrations of free and total choline in the second and third trimesters were not associated with intelligence, visuospatial processing, or memory in children at 5 years of age. Given the contradictory results, additional research is needed.
Previous research is limited by the lack of consideration of sociodemographic factors and maternal levels of other nutrients (e.g., iron, vitamin B12, fatty acids), which could influence the relationship between maternal folate and choline levels and children’s neurodevelopmental outcomes. Further, no human studies have investigated the interactive effect of folate and choline on children’s cognitive and motor development. Using data from 309 maternal–child pairs in the Alberta Pregnancy Outcomes and Nutrition (APrON) study, we examined the associations between maternal RBC folate and choline intake during the second trimester pregnancy, and children’s intelligence, language, memory, executive function, and motor skills at 3–5 years of age. We hypothesized that higher maternal RBC folate status and choline intake would be associated with better neurodevelopmental outcomes in children. We also investigated whether there was an interactive effect of maternal folate status and choline intake on children’s neurodevelopmental outcomes.
Method
Cohort
The present study included a subset of maternal-child pairs (n = 309) from the APrON study (N = 2189) Reference Kaplan, Giesbrecht and Leung31 who met the following inclusion criteria: (1) maternal folate status assessed during the second trimester of pregnancy, (2) maternal choline intake estimated during the second or third trimester of pregnancy, and (3) children participated in a neurodevelopmental assessment at 3–5 years of age. Supplemental Figure S1 shows how this sub-sample was selected.
Exposures
Maternal RBC folate and dietary choline were assessed using methods previously described. Reference Kaplan, Giesbrecht and Leung31–Reference Wahlen, Evans, Turner and Hearn34 In brief, second trimester non-fasting blood samples were taken from the women and a hemolysate was prepared. An ion-capture method was used to analyze the hemolysate to determine RBC folate levels. Dietary choline intake was estimated from dietary recall questionnaires that asked women to describe the quantities, types of foods and beverages, and dietary supplements consumed in the previous 24 h. Reference Lewis, Subhan and Bell32 Second trimester choline data was used if available (n = 299), if not, third trimester choline data was used (n = 10; 3% of the overall data); herein, ‘second trimester’ is used to refer to the choline data. Approximately one-third of the participants completed the 24 dietary recall questionnaires in a face-to-face interview with a trained nutrition education research assistant using a ‘multiple-pass method Reference Lewis, Subhan and Bell32 and two-thirds completed the ‘Food Behaviour Questionnaire’ online. Reference Hanning, Royall, Toews, Blashill, Wegener and Driezen35 A comprehensive Alberta choline database was developed for use with the 24 h dietary intake recall data to estimate the choline content of foods consumed by the APrON participants. Reference Lewis, Subhan and Bell32 The database contained information on total choline content in foods, as well as on the five most common dietary forms of choline (free choline, glycerophosphocholine, phosphocholine, phosphatidylcholine, and sphingomyelin) and betaine. Choline values for food items from the USDA Database for the Choline Content of Common Foods Release 2 (634 foods) were used. Reference Shaw, Carmichael, Yang, Selvin and Schaffer36,Reference Patterson, Bhagwat, Williams, Howe and Holden37 Foods not included in the USDA choline database were substituted with nutritionally comparable foods. The Alberta database that was developed included choline content values for 2707 foods that were consumed by the APrON participants. Questionnaire data was entered into Food Processor Standard Query Language (ESHA Research) to estimate macronutrient intake. Reference Lewis, Subhan and Bell32 These methods have been shown to be reliable and valid for estimating choline intake in pregnant women. Reference Lewis, Subhan and Bell32,Reference Conway, Ingwersen, Vinyard and Moshfegh38
Outcomes
The Wechsler Preschool and Primary Scales of Intelligence – Fourth EditionCND (WPPSI-IVCND), a comprehensive measure of intelligence for children 2:6 (years: months) to 7:7 years of age was used to measure intelligence. Reference Wechsler39 For children under 4:0, full scale IQ (FSIQ) and three composite index scores were calculated (i.e., Verbal Comprehension index (VCI), Visual Spatial index (VSI), and Working Memory index (WMI)); in children ages 4:0 to 7:7, two additional composite index scores were calculated (i.e., Fluid Reasoning Index (FRI), Processing Speed Index (PSI)). WPPSI-IVCND FSIQ and index scores are age-adjusted and have a mean of 100 (SD = 15; range: 40–160); higher scores indicate better performance. The WPPSI-IVCND FSIQ has excellent reliability and validity (r = 0.96), and the reliability and validity of the indices for both age bands are acceptable (r ≥ 0.75). Reference Syeda and Climie40
The NEPSY-II is a multi-domain measure suitable for children between the ages of 3:0 to 16:11. Reference Korkman, Kirk and Kemp41 Language skills were measured using the Phonological Processing and Speeded Naming subtests, and memory was measured using the Memory for Designs, Narrative Memory, and Sentence Repetition subtests. NEPSY-II age-adjusted scaled scores have a mean of 10 (SD = 3; range: 1–19), with higher scores indicating better performance. The NEPSY-II subtests show adequate to high reliability in 3–5-year-olds (r ≥ 0.60). Reference Brooks, Sherman and Strauss42
The Movement Assessment Battery for Children, Second Edition (MABC-2) was used to assess motor skills. Reference Henderson and Sugden43 Motor skills were measured in three areas (i.e., Manual Dexterity, Aiming and Catching, Balance). A Total Test score and MABC-2 standard scores are calculated for each area (M = 10; SD = 3; range: 1–19), with higher scores indicating better performance. The reliability of the Total Test score (r = 0.80) and the area scores (Manual Dexterity, r = 0.77; Aiming and Catching, r = 0.84; and Balance, r = 0.73) is adequate. Reference Henderson and Sugden43
In early childhood, three components of executive function are working memory, inhibitory control, and cognitive flexibility. Reference Anderson and Reidy44 Working memory was assessed using the Spatial Span Task. Reference Hammond, Müller, Carpendale, Bibok and Liebermann-Finestone45 Scores ranged from 0-6, which indicated the number of trials the child completed successfully. Children’s inhibitory control was measured using the Boy-Girl Stroop Task, which was adapted from the Day/Night Task. Reference Carlson46 On the Boy-Girl Stroop, one point was given for each correct response up to a maximum score of 16. Children’s inhibitory control was also assessed using the NEPSY-II Statue subtest. Reference Korkman, Kirk and Kemp41 The NEPSY-II Statue subtest gives an age-adjusted scaled score (M = 10, SD = 3, range: 1–19), with higher scores indicating better performance; the reliability of this subtest is relatively high (r = 0.81). Reference Brooks, Sherman and Strauss42 Children’s cognitive flexibility was examined using the Dimensional Change Card Sort (DCCS), which evaluated children’s ability to learn a card sorting rule and then demonstrate flexibility when the sorting rule was switched. Reference Carlson46 Children’s performance was scored as either a pass (i.e., correct performance on at least 5 of 6 post-switch trials (1 = pass)) or fail (0 = fail).
Covariates
We considered relevant covariates that have been reported to be associated with maternal levels of folate and choline and/or neurodevelopmental outcomes in children as they could possibly explain some of the variability in the outcomes. Reference de Neubourg, Borghans, Coppens and Jansen47–Reference Helland, Smith, Saarem, Saugstad and Drevon58 Information on potential covariates was collected using various methods. At enrollment, women completed questionnaires on sociodemographic factors. Maternal pre-pregnancy body mass index (BMI) was calculated using measured height and self-reported pre-pregnancy weight. Information on child gestational age at birth and child birthweight was obtained from Alberta Health Services birth records. Women provided blood samples that were used to measure hemoglobin concentrations and serum concentrations of vitamin B12 (holotranscobalamin), phospholipid fatty acids (i.e., docosahexaenoic acid (DHA), arachidonic acid (ARA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA)), copper, magnesium, selenium, and zinc. Serum ferritin and plasma vitamin B12 were analyzed in maternal second trimester blood using an AXSYM analyzer (Abbott, Mississauga, ON, Canada). Reference Kaplan, Giesbrecht and Leung31 Serum phospholipid content of DHA, ARA, EPA, and DPA were analyzed in maternal second trimester blood using a modified Folch method to extract lipids from the blood samples; thin-layer chromatography was used to separate phospholipids from other major lipid classes, and fatty acids were separated by automated gas liquid chromatography. Reference Kaplan, Giesbrecht and Leung31,Reference Field, Ryan, Thomson and Clandinin33 Serum phospholipid total omega-3 fatty acids, total omega-6 fatty acids, and total long-chain polyunsaturated fatty acids were determined by summing the relevant fatty acid variables. See Field et al. Reference Field, Ryan, Thomson and Clandinin33 for more detail regarding the methods used to measure fatty acid concentrations. Lastly, to assess maternal copper, magnesium, selenium, and zinc status, maternal blood collected during the second trimester of pregnancy was analyzed at the Alberta Centre for Toxicology, University of Calgary using an inductively coupled plasma-triple quadrupole mass spectrometer. Reference Wahlen, Evans, Turner and Hearn34
Multicollinearity among potential covariates was examined using Pearson and Spearman correlations. Gestational age and child birthweight were highly correlated (r = 0.60, p < 0.001); therefore, only birthweight was included in our models. Similarly, maternal race and ethnicity, and maternal birthplace (i.e., “mother born in Canada”) were highly correlated (r s = 0.58, p < 0.001), thus only race and ethnicity was included. High correlations were also found among fatty acids (see Supplemental Tables S1 and S2); therefore, only the serum total omega-3 fatty acid variable was included as it has been found to be associated with children’s neurodevelopment. Reference Helland, Smith, Saarem, Saugstad and Drevon58 The maternal covariates included in the models were maternal age, race and ethnicity, education, income, parity, delivery mode, pre-pregnancy BMI and maternal levels of iron (i.e., serum ferritin and hemoglobin), vitamin B12, serum total omega-3 fatty acid, magnesium, copper, zinc, and selenium during pregnancy. The child covariates that were included were child birthweight, sex, and age at neurodevelopmental assessment.
Statistical Analyses
Multivariable regression models
Statistical analyses were performed using IBM SPSS Statistics (version 27.0; IBM Corp, Armonk, NY). All assumptions for regression analysis (i.e., linear relationships, normality, homoscedasticity, multicollinearity) were met. Linear regression models examined the associations between RBC folate status and estimated choline intake, and children’s scores on the WPPSI-IVCND, NEPSY-II subests, MABC-2, Boy-Girl Stroop, and Spatial Span. Logistic regression models examined the associations between RBC folate status and estimated choline intake and children’s scores on the DCCS. Initially, unadjusted models examined associations between folate status and choline intake separately and each of the child neurodevelopmental outcomes. Then adjusted multivariable models that included the covariates were used to investigate these associations. Consistent with a method used in previous studies, Reference Thiese, Ronna and Ott59,Reference Nyanza, Bernier, Martin, Manyama, Hatfield and Dewey60 covariates associated with the relevant neurodevelopmental outcome at p < 0.20 were included in the final multivariable model. Child age at the time of the neurodevelopmental assessment was included as a covariate for the executive function tasks that were not age standardized (i.e., Boy-Girl Stroop, Spatial Span, DCCS). Moderation models were used to investigate whether there was a significant interaction between folate status and choline intake on children’s neurodevelopmental outcomes. Moderation models included the following predictors: folate status, choline intake, an interaction term (i.e., product term of RBC folate and estimated choline intake), and relevant covariates. To correct for multiple comparisons, the Benjamini–Hochberg procedure was used to control for false discovery rate (FDR). Reference Benjamini and Hochberg61 We computed adjusted p-values (i.e., q-values), and considered q values from 0.05 to 0.10 as significant.
Power analysis
A power analysis was conducted to determine if our sample size was sufficient to detect a significant interaction between maternal prenatal folate status and estimated choline intake on children’s neurodevelopment. We used G * Power 3.1.9.7 Reference Faul, Erdfelder, Buchner and Lang62 ; linear multiple regression: fixed model, R2 increase, with a medium effect size of 0.15, an α of 0.05, a power of 0.95, 3 tested predictors (i.e., folate status, choline intake, interaction term), and 21 total predictors (i.e., the 3 tested predictors and 18 possible covariates). A medium effect size was chosen as previous studies investigating associations between folate or choline and child neurodevelopmental outcomes have reported medium effect sizes. Reference Ars, Nijs and Marroun24,Reference Veena, Krishnaveni and Srinivasan28 This analysis indicated that a sample size of 120 was sufficient. As the APrON sub-sample included in the present study consisted of 309 maternal–child pairs, this study was well-powered to detect a medium effect.
Post-hoc analyses
Simple slopes analysis
In moderation analysis, the inclusion of an interaction term is known to affect the values of regression coefficients, so further probing is needed to understand the nature of the interaction effect in order to interpret the results. Reference Jaccard, Wan and Turrisi63 Simple slopes analysis was used to probe significant interaction effects using the PROCESS macro (version 3.5.3). Reference Hayes64 Simple slopes analysis examines the significance of conditional effects Reference Fairchild and MacKinnon65 ; in the present study, simple slopes analysis was used to examine the association between continuous folate status and the neurodevelopmental outcome at high (i.e., one standard deviation (SD) above the mean) and low (i.e., one SD below the mean) levels of choline intake. Thus, unlike the main analyses, the simple slopes analysis used a dichotomous version (i.e., high, low) of maternal choline intake.
Sensitivity analysis
The E-value is a new measure related to evidence for causality. It is the minimum strength of association that unmeasured confounders would need to have with both the predictor and the outcome to fully explain away a specific predictor-outcome association, conditional on the measured covariates. Reference VanderWeele and Ding66 One is the lowest possible E-value and indicates that no unmeasured confounding is needed to explain away the observed association. The higher the E-value, the stronger confounder associations must be to explain away the effect. E values for the associations found in the adjusted models were determined using an E value calculator. Reference Mathur, Ding, Riddell and VanderWeele67
Missing data
There was no missing data for the predictor variables (e.g., maternal folate and choline). Among all outcome variables, the percent missing data was less than 5%. Therefore, we excluded participants with missing data in the unadjusted and adjusted models and report results based on participants with complete data.
Ethics approval
The APrON study was approved by health research ethics boards at the University of Calgary (Ethics ID: REB14-1702) and University of Alberta (Study ID: Pro00002954). Women provided informed consent at time of recruitment and provided consent for neurodevelopmental assessment of their children.
Results
Population characteristics
Women in the study were mainly white (90%), well-educated (80% university degree), and had a yearly family income of ≥$70,000CAD (90%). The mean maternal age was 32.3 years (SD: ±3.9), and women had a mean pre-pregnancy BMI of 24.9 (SD: ±5.6). The mean maternal RBC folate concentration was 1366.3 nmol/L (SD: ±455.1, range: 170.6–2931.2). Mean calorie adjusted daily choline intake was 169 mg/day (SD: ±65, range: 54–460). Children were 50.2% (n = 155) female. The mean gestational age at birth was 39.3 weeks (SD: ±1.5), the mean birthweight was 3386.4 g (SD: ±499.6), and the average age of the children at time of assessment was 50.9 months (SD: ±6.1) (Table 1).
Table 1. Maternal and child descriptive characteristics, Alberta, Canada, 2009–2017

a BMI = Body Mass Index.
b RBC = Red Blood Cell.
3.2 Unadjusted and Adjusted Multivariable Models
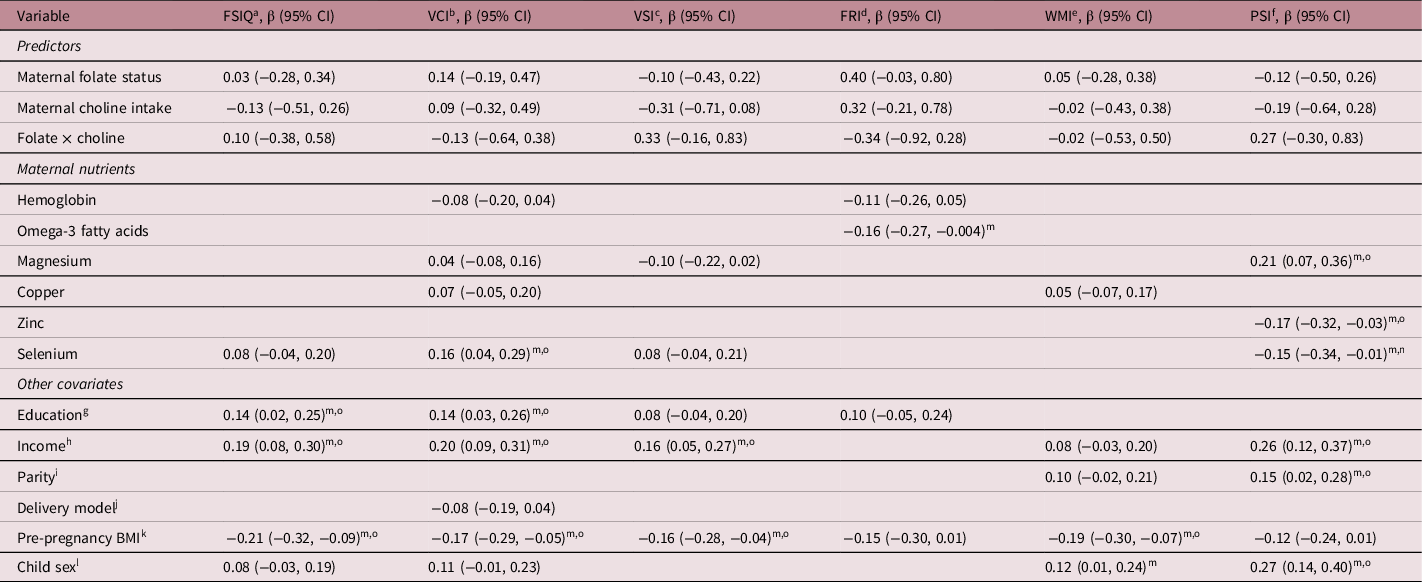
Children’s scores (i.e., means/SDs, percentage pass/fail) on the neurodevelopmental outcome measures are presented in Supplemental Table S4. Unadjusted regression analyses revealed that higher folate status was associated with higher scores on the WPPSI-IVCND FRI (β = 0.21; 95%CI 0.04, 0.37, q = 0.03) and NEPSY-II Phonological Processing (β = 0.20; 95%CI 0.07, 0.33, q = 0.01). Higher choline intake was associated with lower scores on NEPSY-II Speeded Naming (β = −0.13; 95%CI −0.25, −0.01, q = 0.08) (Supplemental Tables S5 and S6). Examination of covariates revealed that several nutrients were associated with neurodevelopmental outcomes, and that higher maternal pre-pregnancy BMI was associated with lower scores on several of the outcome measures (Supplemental Tables S5–S8). In the final adjusted models, no associations were found between folate status, choline intake, or their interaction and children’s outcomes on the WPPSI-IVCND, NEPSY-II, MABC-2, and most of the executive function measures (Tables 2–5). The exception was the DCCS executive function measure, where a significant interaction was found (Table 4). Specifically, maternal folate status (OR = 2.36, 95%CI 1.02, 5.44, q = 0.05), maternal choline intake (OR = 3.14, 95%CI 1.10, 8.97, q = 0.04), and their interaction (OR = 0.20, 95%CI 0.05, 0.76, q = 0.03) were associated with the odds of the children receiving a passing score on the DCCS.
Table 2. Adjusted linear regression models (95% Confidence Intervals) for the associations between maternal folate status and choline intake and WPPSI-IVCND scores in children 3–5 years of age, Alberta, Canada, 2009–2017.*

* Only covariates associated with one of the neurodevelopment test scores in the bivariate analysis at p < 0.20 were included in the adjusted models.
a FSIQ = Full Scale IQ.
b VCI = Verbal Comprehension Index.
c VSI = Visual Spatial Index.
d FRI = Fluid Reasoning Index.
e WMI = Working Memory Index.
f PSI = Processing Speed Index.
g Less than high school diploma/completed high school diploma/trade/technical as reference group.
h Income <$70K as reference group.
i No prior children as reference group.
j Vaginal delivery as reference group.
k BMI = Body Mass Index; All models adjusted for pre-pregnancy BMI.
l Male as reference group.
m p ≤ 0.05.
n q < 0.10.
o q < 0.05.
Table 3. Adjusted linear regression models (95% Confidence Intervals) for the associations between maternal prenatal folate status and choline intake and language and memory scores on the NEPSY-II in children 3–5 years of age, Alberta, Canada, 2009–2017.*

* Only covariates associated with one of the neurodevelopment test scores in the bivariate analysis at p < 0.20 were included in the adjusted models.
a White as reference group.
b Less than high school diploma/completed high school diploma/trade/technical as reference group.
c Income <$70K as reference group.
d No prior children as reference group.
e Vaginal delivery as reference group.
f BMI = Body Mass Index.
g Male as reference group.
h p ≤ 0.05.
i q < 0.10.
j q < 0.05.
Table 4. Adjusted linear and logistic regression models (95% Confidence Intervals) for the associations between maternal prenatal folate status and choline intake and executive functioning tasks in children 3–5 years of age, Alberta, Canada, 2009–2017.*

* Only covariates associated with one of the neurodevelopment test scores in the bivariate analysis at p < 0.20 were included in the adjusted models.
a DCCS = Dimensional Change Card Sort.
b White as reference group.
c Income <$70K as reference group.
d No prior children as reference group.
e Vaginal delivery as reference group.
f BMI = Body Mass Index; All models adjusted for pre-pregnancy BMI.
g Male as reference group.
h p ≤ 0.05.
i q < 0.10.
j q < 0.05.
Table 5. Adjusted linear regression models (95% Confidence Intervals) for the associations between maternal prenatal folate status and choline intake and motor outcomes on the MABC-2 in children 3–5 years of age, Alberta, Canada, 2009–2017.*

* Only covariates associated with one of the neurodevelopment test scores in the bivariate analysis at p < 0.20 were included in the adjusted models.
a Income <$70K as reference group.
b No prior children as reference group.
c All models adjusted for pre-pregnancy BMI.
d Male as reference group.
e p ≤ 0.05.
f q < 0.05.
Post hoc analyses
Simple slopes analysis of the interaction effect
Simple slopes analysis probed the conditional effects of continuous maternal folate status on children’s odds of passing the DCCS at high and low levels of choline intake. This analysis revealed that at low levels of maternal choline intake (i.e., 1SD below the mean; 110.79 mg/day), there was a nonsignificant effect (β = 0.15; 95% CI: −0.17, 0.47, q = 0.35); however, at high levels of maternal choline intake (i.e., 1 SD above the mean; 223.03 mg/day), there was a significant effect of maternal folate status on children’s odds of receiving a passing score on the DCCS (β = −0.44; 95%CI: −0.81, −0.06, q = 0.04). Specifically, for mothers with high levels of choline intake, higher maternal folate status was associated with lower odds of children receiving a passing score on the DCCS (Fig. 1).

Fig. 1. Interaction graph showing children’s odds of passing the Dimensional Change Card Sort (DCCS) as a function of maternal folate status and maternal choline intake at high (i.e., 1 SD above the mean; 223.03 mg/day) and low (i.e., 1 SD below the mean;110.79 mg/day) levels. The interaction effect was only significant at high levels of maternal choline (denoted by an asterisk); at high levels of maternal choline intake, higher maternal folate status was associated with lower odds of children receiving a passing score on the DCCS.
Sensitivity analysis
Our analyses revealed that relatively modest unmeasured confounding could explain away the effects of RBC folate on WPSSI-IV FRI and NEPSY-II Phonological Processing, and the effect of choline intake on Speeded Naming. However, to nullify the interactive effect of folate and choline on children’s performance on the DCCS, considerable unmeasured confounding would be needed. Specifically, a confounder or set of confounders would have to be associated with an almost 4-fold increase in the odds ratio above the measured confounders to explain away this observed effect. See supplemental Table S3 for the E-values (Supplemental Table S3).
Discussion
We found few associations between maternal RBC folate status and choline intake in the second trimester of pregnancy and children’s neurodevelopmental outcomes. These findings are consistent with a study by Tamura et al. Reference Tamura, Goldenberg, Chapman, Johnston, Ramey and Nelson29 that reported no associations between maternal RBC folate levels and child neurodevelopmental outcomes. Wu et al. Reference Wu, Dyer, King, Richardson and Innis26 also reported no associations between maternal plasma/serum folate and children’s cognitive outcomes. Similar to the present study, Boeke et al. Reference Boeke, Gillman, Hughes, Rifas-Shiman, Villamor and Oken25 found that choline intake was not associated with children’s IQ. However, other research has reported associations between maternal folate or choline levels and child neurodevelopmental outcomes. Reference Julvez, Fortuny, Mendez, Torrent, Ribas-Fitó and Sunyer21,Reference Caudill, Strupp, Muscalu, Nevins and Canfield23,Reference Wu, Dyer, King, Richardson and Innis26,Reference Chen, Qin and Gao27,Reference Signore, Ueland, Troendle and Mills30,Reference Wehby and Murray68–Reference Villamor, Rifas-Shiman, Gillman and Oken71 This lack of consistency could be due to the different methods that were used to assess prenatal maternal folate and choline levels (i.e., maternal self-reports on supplement use, estimation of intake from food frequency or 24-h food intake questionaries, measurement of folate or choline status from blood). Further, many of the studies that reported positive associations between prenatal folate and choline levels and children’s neurodevelopmental outcomes did not examine the influence of relevant covariates (e.g., maternal levels of nutrients such as magnesium or selenium, pre-pregnancy BMI). Rigorously designed studies with larger sample sizes may be needed to uncover significant associations between prenatal folate and choline levels and children’s neurodevelopmental outcomes. To better understand the associations between maternal levels of folate and choline during pregnancy and children’s neurodevelopment, future research needs to examine the differential effects of folate and choline status and intake on child outcomes, consider the influence of other nutrients on children’s neurodevelopment, and investigate the effects of supplementation in women, particularly those who have low levels on child outcomes.
When we examined the interaction between folate and choline on children’s neurodevelopmental outcomes, we found a significant interaction for the DCCS, a measure of cognitive flexibility. Specifically, for women with high levels of choline intake during pregnancy (i.e., 1 SD above the mean; 223.03 mg/day), higher folate status during pregnancy was associated with lower odds of children receiving a passing score on the DCCS. It is of note that in the present sample, high levels of maternal choline intake (i.e., 223.03 mg/day) were approximately half the recommended daily intake (i.e., 450–480 mg/day). 72 Further, in 90% (n = 267) of the women, RBC folate status was above the minimum level of 906 nmol/l recommended by the WHO. The interaction effect also shows that at around the minimum level of folate recommended by the WHO (i.e., lower folate intake), “higher” maternal choline intake was associated with higher odds of children passing the DCCS. Thus, this significant interaction effect was found at what could be considered inadequate maternal choline intake levels and maternal folate status that was above the WHO minimum recommended level for pregnant people, suggesting that inadequate choline intake combined with maternal folate status above 906 nmol/L may have teratogenic effects on children’s executive function development. This is consistent with reports that very high folate status is associated with adverse outcomes such as Autism Spectrum Disorder (ASD), reduced birthweight, and asthma. Reference Huot, Dodington and Mollard73–Reference Li, Xu and Cao75 However, further research is needed that examines the levels at which gestational choline and folate are associated with improved neurodevelopment and the upper and/or lower levels at which they may be associated with adverse outcomes. These findings also suggest the need for further research that examines the interactive effects of maternal prenatal folate status and choline intake on children’s neurodevelopmental outcomes, specifically executive function development.
The RBC folate concentrations (M = 1366.3 nmol/L ± 455.1 nmol/L) of most of the women in our study were above the minimum recommended level of 906 nmol/L and many displayed levels well above the minimum level. 76 It is also notable that the mean calorie adjusted choline intake (169 mg/day ± 65 mg/day) of the women was lower than the recommended level (450 mg/day), and that in our sample values at the high end of the observed range (460 mg/day) were only slightly above the Institute of Medicine recommended level. Reference Hinkle, Schieve, Stein, Swan, Ramakrishnan and Sharma77 Choline intake may need to be higher than what was observed in the APrON participants before potential beneficial effects on neurodevelopmental outcomes are observed. This contention is supported by Caudill et al. Reference Caudill, Strupp, Muscalu, Nevins and Canfield23 who reported that increased visual processing speed was observed in children whose mothers consumed over twice the recommended average daily nutrient intake of choline (930 mg/day) compared to those who consumed just slightly over the recommended daily intake (480 mg/day).
In the present study, folate status and choline intake were not examined across pregnancy, but in the second trimester. There may be sensitive periods during pregnancy when exposure to folate and choline are associated with children’s neurodevelopment. For example, Villamor et al. Reference Villamor, Rifas-Shiman, Gillman and Oken22 found that maternal folate concentrations during the first trimester, but not the second trimester, were associated with children’s scores on the Peabody Picture Vocabulary Test-Third Edition at 3 years of age. Future research that examines the associations between maternal concentrations of folate and choline intake at different times during pregnancy and children’s neurodevelopmental outcomes is needed to determine if there are sensitive periods for exposures.
A unique strength of this study was the rich dataset that allowed for the consideration of numerous maternal characteristics and prenatal nutrients as covariates in our regression models; all of which have not been considered in previous studies. It is possible that the associations reported previously may have been due to untested confounders such as pre-pregnancy BMI or prenatal levels of other nutrients. Reference Hinkle, Schieve, Stein, Swan, Ramakrishnan and Sharma77 The results of this study also revealed variability in the nutrients that were associated with various domains of neurodevelopment. Notably, higher maternal selenium was associated with higher scores on the VCI and lower scores on the PSI of the WPPSI-IVCND at 3–5 years. In previous research, we reported that higher maternal selenium levels were associated with poorer outcomes on the Cognitive and Motor scales of the Bayley Scales of Infant and Toddler Development, Third Edition at 2 years of age, suggesting that the effects of maternal prenatal nutrient concentrations on children’s neurodevelopment may vary across age and neurodevelopmental assessment measures. Reference Liu, Martin, Dinu, Field, Dewey and Martin78 In contrast, ferritin, and vitamin B12 were not found to be associated with neurodevelopmental outcomes in any of the final adjusted models unlike previous research; however, this could be because few women in our sample had low levels of these nutrients. Reference Villamor, Rifas-Shiman, Gillman and Oken22,Reference Tamura, Goldenberg and Hou56,Reference del Río Garcia, Torres-Sánchez and Chen57 These findings suggest the need to comprehensively examine associations between combinations of maternal nutrients during pregnancy and children’s neurodevelopmental outcomes, rather than the influence of individual nutrients only.
Limitations of the present study were that maternal folate status was measured in the second trimester of pregnancy and maternal choline intake was measured predominantly in the second trimester (i.e., 3% of the sample had choline intake measured in the third trimester). However, previous research, including research conducted on the APrON cohort, found that folate levels increase throughout pregnancy, whereas choline levels remain relatively constant. Reference Signore, Ueland, Troendle and Mills30,Reference Lewis, Subhan and Bell32,Reference Fayyaz, Wang and Jacobs79 Further, we did not have data on children’s folate and choline levels at 3–5 years. It is possible that maternal levels of these nutrients during the first or third trimester or children’s levels may be more highly associated with children’s neurodevelopmental outcomes. Previous research in rats has found that choline supplementation during embryonic days 12–17 as well as during postnatal days 16–30 was associated with better spatial memory. Reference Meck, Williams, Cermak and Blusztajn80 In children, it has also been observed that dietary folate intake measured at 30 months of age was associated with higher scores on the Mental Development Index of the Bayley Scales of Infant and Toddler Development, Second Edition. Reference Gatica-Domínguez, Rothenberg, Torres-Sánchez, de Schnaas, Schmidt and López-Carrillo81 Thus, future studies, which examine the effects of both maternal and child levels of these nutrients on children’s neurodevelopmental outcomes, are needed. Another limitation is that we did not assess choline status. However, there is no standardized method of measuring choline status and there are no reference levels for blood during pregnancy, which limits the utility of blood tests to detect choline deficiency. Reference VanderWeele and Ding66 The fact that recommended average daily nutrient intake values for choline during pregnancy have been established by the WHO and the European Food Safety Authority (EFSA), allowed us to estimate whether pregnant women in the APrON were consuming foods that provided sufficient choline. In the present study, children’s neurodevelopment was assessed at 3–5 years of age. It is well known that neurodevelopment continues to change throughout childhood and adolescence, and associations between prenatal maternal folate status and choline intake, and neurodevelopment may not become evident until later ages, so future research is encouraged that examines these associations in older children. Lastly, the women who participated in the present study were of relatively high SES (i.e., predominantly white, married, well-educated, and with high household incomes) and most had RBC folate levels above the minimum recommended level, although the majority had choline intake below the daily recommended level. 13 It is possible that women of lower SES may have folate and choline levels that are lower, which could be associated with poorer neurodevelopmental outcomes in their children. It is also possible that other unmeasured variables, such as maternal IQ or maternal–child relationship quality, contributed to the neurodevelopmental outcomes of the children who participated in the present study, and it is currently unknown how these and other unmeasured confounders may affect the present associations. These are important questions to be addressed in future research.
In conclusion, maternal folate status and choline intake during the second trimester of pregnancy were not associated with most neurodevelopmental outcomes at 3–5 years of age in children in the APrON cohort. Future studies should consider the levels of these nutrients, in addition to other nutrients, in women across pregnancy and in infants and young children to determine their associations with children’s neurodevelopmental outcomes. Research is also warranted that investigates the relationships between high RBC folate status and whether there is a conditional effect of low gestational choline intake and high RBC folate status on neurodevelopmental outcomes. Further, research investigating very high levels of maternal exposure to nutrients such as folate during pregnancy is needed, as it is possible that such levels of exposure may have teratogenic effects on children’s neurodevelopment. Reference Colapinto, O’Connor, Dubois and Tremblay82 Also, as both folate and choline are methyl donor nutrients that influence neurogenesis and apoptosis, it is possible that choline supplementation might mitigate the negative effects of folate deficiency on brain development. Future research is needed that investigates this. Finally, due to the interrelationships among choline and folate and their effects on the brain development, their role in epigenetic variation (e.g., DNA methylation, histone modification) through one-carbon (1C) metabolism and their influence on children’s neurodevelopment outcomes requires further investigation.
Supplementary materials
For supplementary material for this article, please visit https://doi.org/10.1017/S2040174423000041
Acknowledgements
We are extremely grateful to all the families who took part in this study and the whole APrON team (89) including the investigators, research assistants, graduate and undergraduate students, volunteers, clerical staff, and managers.
We acknowledge the significant contributions of the APrON Study Team whose individual members are: B.J. Kaplan, C.J. Field, R.C. Bell, F.P. Bernier, M. Cantell, L.M. Casey, M. Eliasziw, A. Farmer, L. Gagnon, G.F. Giesbrecht, L. Goonewardene, D. Johnston, L. Kooistra, N. Letourneau, D.P. Manca, J.W. Martin, L.J. McCargar, M. O’Beirne, V.J. Pop, A.J. Deane, and N. Singhal, and the APrON Management Team who include: N. Letourneau (current PI), R.C. Bell, D. Dewey, C.J. Field, L. Forbes, G. Giesbrecht, C. Lebel, B. Leung, C. McMorris, K. Ross.
Financial support
This cohort was established by an interdisciplinary team grant from Alberta Innovates Health Solutions (formally the Alberta Heritage Foundation for Medical Research). Additional funding from the Canadian Institutes of Health Research (MOP-123535), the U.S. National Institutes of Health (Exploration/Development Grant 1R21ES021295-01R21), and the Alberta Children’s Hospital Foundation allowed for the collection and analysis of data presented in this manuscript. Salary support was provided to G. England-Mason through a Postgraduate Fellowship in Health Innovation provided by Alberta Innovates, the Ministry of Economic Development, Trade and Tourism, and the Government of Alberta. The funding sources were not involved in the study design, collection, analysis, and interpretation of data; writing of the manuscript; or in the decision to submit this article for publication.
Conflicts of interest
The authors have no conflicts of interest to declare.
Ethical standards
The APrON protocol was approved by health research ethics boards at the University of Calgary (Ethics ID: REB14-1702) and University of Alberta (Study ID: Pro00002954). Women provided informed consent at time of recruitment and provided consent for neurodevelopmental assessment of their children.