On account of the critical role played by folate in preventing neural tube defects (NTD)(1–Reference Berry, Li and Erickson4), health authorities since the early 1990s have recommended that women who could become pregnant should increase their dietary folate intake and take a daily supplement of 400 μg of folic acid, the synthetic form of the vitamin(5–7). Unfortunately, compliance with this advice has been poor(Reference Clark and Fisk8–Reference Forster, Wills and Denning14), and this fact, plus the recognition that as many as 50 % of all pregnancies are unplanned(Reference Grimes15) and unlikely to be protected, has led governments in the USA, Canada, Chile and other countries to introduce mandatory folic acid fortification of grain products(16–Reference Bell and Oakley19). These policies have succeeded in reducing NTD occurrence by approximately 20–50 %(Reference Honein, Paulozzi and Mathews20–Reference Hertrampf and Cortés24) and may also have reduced stroke-related mortality in the general population(Reference Yang, Botto and Erickson25, Reference Wang, Qin and Demirtas26), as well as having had some benefit against CHD through their effect on lowering homocysteine(Reference Wald, Morris and Law27).
In spite of the success of mandatory folic acid fortification for NTD prevention, and despite calls by some experts(Reference Brent and Oakley28–Reference Bentley, Weinstein and Willett31) to increase fortification levels, European governments have yet to introduce such policies(Reference Stoll, Alembik and Dott13, Reference Wald32). Indeed, in some European countries, even voluntary folic acid fortification is prohibited(Reference Buttriss12). This reluctance to legislate for mandatory fortification is due to an ongoing debate about the long-term safety of exposing the general population to high intake of folic acid(Reference Wright, Finglas and Southon33–Reference Lucock and Yates36). The debate centres around two main issues, namely whether high folic acid intake could promote the formation of colorectal tumours in patients with undiagnosed pre-malignant and malignant lesions(Reference Kim37–Reference Hirsch, Sanchez and Albala40), and whether they could mask the appearance of vitamin B12 deficiency anaemia while still allowing the irreversible neurological manifestations of the deficiency to progress(Reference Scott41, Reference Morris, Jacques and Rosenberg42).
On account of this uncertainty, attention has been directed at other strategies that might help increase population intake of natural folates, which do not carry with them the same safety concerns as synthetic folic acid. These strategies include the development of novel foods enriched with natural folates(Reference Buttriss12). Recently, several groups have shown that the folate content of eggs can be increased significantly by supplementing the diet of laying hens with folic acid(Reference Sherwood, Alphin and Saylor43–Reference Hoey, McNulty and McCann48). Most of the additional folate appears in the eggs in the natural form, mainly as 5-methyltetrahydrofolate(Reference Hoey, McNulty and McCann48). It was proposed that folate-enriched eggs could offer a practical means of increasing folate intake in the general population, especially where there was limited or no access to folic acid-fortified foods(Reference Hoey, McNulty and McCann48). The opportunity to specifically market folate-enriched eggs to women of childbearing age was also suggested(Reference House, Braun and Balance44). To date, however, the potential impact of such products on folate intake or supply in the European Union (EU) has not been evaluated.
FAO produces annual food balance sheets that provide data on the overall per capita supply of commodities within countries. These data can be used to investigate the effect of changing the concentration of a nutrient in a commodity on nutrient supply at the national level. In the case of eggs, multiplying the daily per capita supply of eggs by their average folate concentration provides an indication of the amount of folate being provided by eggs. The effect of increasing egg folate concentrations through dietary enrichment can then be estimated. The objectives of the present study are: (i) to evaluate the trends in egg supply across Europe between 1961 and 2003; (ii) to determine the contribution made by normal un-enriched eggs to folate supply in EU countries in 2003; and (iii) to estimate the potential increase in folate supply that could be achieved if normal eggs were replaced by folate-enriched eggs.
Experimental methods
Annual food balance sheets for the European continent for the years 1961–2003 and the latest available (2003) food balance sheets for twenty-six individual EU countries were downloaded from the FAOSTAT database(49). Data for Luxembourg were unavailable. For each country, per capita energy supply (MJ/d), per capita egg supply (g/d) and energy from eggs (% of total energy) were collated on a Microsoft Excel® spreadsheet (Microsoft Corporation, Redmond, WA, USA). The folate concentrations of un-enriched and folate-enriched eggs from three representative hen-feeding studies from the recent literature were used to calculate the potential ability of folate-enriched eggs to increase the daily per capita folate supply in each country. The folate concentrations used in this simulation were: 30·5 and 85·4 μg/100 g (House et al.(Reference House, Braun and Balance44)), 29·6 and 79 μg/100 g (Hebert et al.(Reference Hebert, House and Guenter45)) and 64 and 150 μg/100 g (Hoey et al.(Reference Hoey, McNulty and McCann48)).
Results and discussion
Figure 1 shows the trend in per capita egg supply, expressed as energy (kJ/d) from eggs, across Europe between 1961 and 2003. Per capita egg supply increased from 163 kJ/d in 1961 to a maximum of 224 kJ/d in 1982. Between 1982 and 1996, this figure decreased to 190 kJ/d. However, in recent years, the trend has begun to rise again. Overall, the contribution of eggs to total energy supply in Europe has shown little variation, fluctuating between 1·3 % and 1·6 %.
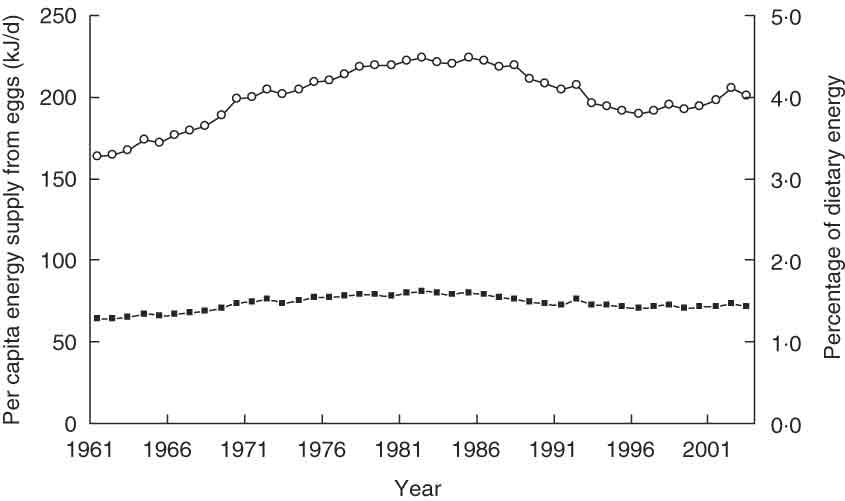
Fig. 1 Daily per capita egg supply on the European continent between 1961 and 2003. Data are expressed as per capita energy (kJ/d) from eggs (![]() ) and as percentage of total dietary energy (
) and as percentage of total dietary energy (![]() ) supply (FAOSTAT(49))
) supply (FAOSTAT(49))
The FAO food balance sheets for 2003 for twenty-six individual EU countries revealed that per capita energy supply ranged from 11·6 MJ/d in Slovakia to 15·7 MJ/d in Portugal, with a mean of 14·2 MJ/d (data not shown). Similarly, per capita egg supply ranged from 18·1 g/d in the Republic of Ireland to 47·8 g/d in Denmark with a mean of 32·8 g/d. This is equivalent to a little over half an egg. The mean contribution of eggs to total energy supply in these twenty-six countries in 2003 was 1·4 %.
Figure 2 shows that if the egg supply in each of these countries consisted entirely of folate-enriched eggs, this would provide approximately 41·5 μg folate/d per capita, compared with 16·2 μg/d per capita from un-enriched eggs, a difference of 25·4 μg/d. For Ireland, which has a relatively high(50) (albeit declining(Reference Cotter and Daly51)) rate of NTD and where a government decision to mandate folic acid addition to bread has been postponed pending further safety evaluations(52, Reference Sweeney, Staines and Daly53), the potential increase in folate supply would be only 14 μg/d. For the UK, where NTD affect 700–900 pregnancies a year and where mandatory fortification, having previously been rejected(54), is back on the political agenda(55), the potential increase would be 25·1 μg/d. The biggest effect would occur in Denmark, where folate supply would increase by some 36·9 μg/d.
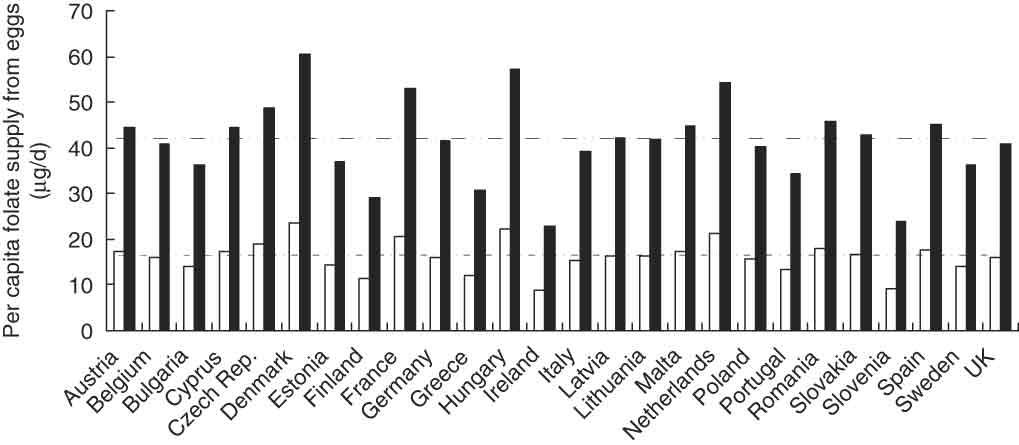
Fig. 2 Potential contribution (μg/d) of un-enriched (□) and folate-enriched (▪) eggs to per capita folate supply in twenty-six European Union countries (FAOSTAT(49))
Data from dietary intake studies provide supportive evidence that folate-enriched eggs would have a relatively minor impact on folate intake at a population level. Mean egg consumption in Irish adults in the late 1990s was only 17 g/d(56). Allowing for the folate concentration of raw eggs (50 μg/100 g)(57), this implies that eggs provided only about 8·5 μg folate/d or 3 % of overall folate intake(Reference O’Brien, Kiely and Harrington58). In the USA, mean dietary folate intake in adults before mandatory folic acid fortification was 283·4 μg/d(Reference Ford and Ballew59). Eggs accounted for 5·1 % of folate intake(Reference Applegate60) or about 14 μg/d. In a small cross-sectional study of ninety-five young Canadian women aged 18–25 years, eggs contributed only 1·9 % of dietary folate intake or about 6 μg/d(Reference Shuaibi, House and Sevenhuysen61). These data suggest that even if the folate content of the entire egg supply could be enriched two- to threefold (the levels that have been achieved experimentally in feeding trials), the overall contribution to folate intake at a population level would only be about 10–40 μg/d. By comparison, the US mandatory folic acid fortification policy was designed to provide an extra 100 μg folic acid/d, although, according to some reports(Reference Rader, Weaver and Angyal62–Reference Quinlivan and Gregory64), it appears to have contributed about twice that amount.
In addition to the effects of folate-enriched eggs on per capita folate supply, it is important to consider their potential contribution if they were marketed specifically towards women of childbearing age. Hoey et al.(Reference Hoey, McNulty and McCann48) stated that consumption each day of one of the enriched eggs from their studies could provide an extra 75 μg of folate. Likewise, Roth-Maier and Böhmer(Reference Roth-Maier and Böhmer46) reported that one enriched egg can provide up to 76 μg folate. The latter authors determined egg folate bioavailability in a pig model system to be 68 %. Assuming that bioavailability is the same in man, then one fortified egg could deliver up to 52 μg of bioavailable folate. However, folic acid bioavailability from fortified foods is about 85 %(Reference Suitor and Bailey65), which suggests that it would be necessary to eat about 1·6 folate-enriched eggs per day to obtain the same amount of folate as would be provided by a mandatory folic acid fortification policy modelled on that of the USA.
The promotion of eggs as a source of folate raises questions about the potential for conflict with consumer attitudes towards cholesterol and with dietary guidelines that emphasise reducing energy, total fat, saturated fat and sodium. Song and Kerver(Reference Song and Kerver66) analysed the data from the National Health and Nutrition Examination Survey (NHANES) III and reported that folate intake in US egg consumers was 22 μg/d higher than in non-consumers. However, egg consumers also had significantly higher intake of cholesterol (>360 mg/d), energy (>1172 kJ/d), fat (>22 g/d), saturated fat (>6·8 g/d) and sodium (>520 mg/d). Eggs are a major source of cholesterol, with an average egg providing about 385 mg(57). Although many countries do not have quantitative thresholds for cholesterol intake(67), the US dietary guidelines continue to recommend limiting cholesterol intake in the general public to less than 300 mg/d and 200 mg/d in the case of individuals with elevated LDL cholesterol(68). A similar recommendation is made by the American Diabetes Association, while the National Cholesterol Education Program and the American Heart Association call for limits of <200 mg/d and <300 mg/d, respectively(Reference Krebs-Smith and Kris-Etherton69). Although these recommendations have been questioned because of the fact that dietary cholesterol has much less of an influence on plasma cholesterol than total fat and especially saturated and trans fat(Reference McNamara70, Reference Kritchevsky and Kritchevsky71), the general public is unaware of this and may tend to regard all high-cholesterol foods, including eggs, with suspicion. This situation may be exacerbated by the high-profile marketing of novel cholesterol-lowering foods, the recent EU approval of a cholesterol-related health claim for plant sterols and stanols(72) and by the increased tendency for doctors to prescribe statin drugs to aggressively lower serum cholesterol(Reference Walley, Folino-Gallo and Stephens73). Thus, it may be difficult to persuade the general public to increase egg consumption appreciably regardless of the purported health benefits.
A limitation of the present study is the fact that the results are estimates of per capita folate supply based on food balance sheet data. However, they are more likely to overstate rather than understate the true potential of folate-enriched eggs because food balance sheets do not take into account food wasted after purchase(Reference Schmidhuber and Traill74). In addition, the estimates are based on the assumption that all eggs available for consumption within each country are folate enriched, whereas in the absence of strong incentives to egg producers it is difficult to imagine this situation ever occurring. Finally, even if poultry feed manufacturers were to add overages to their feeds, it probably would not have any additional benefit because egg folate concentrations appear to be saturable after a critical level(Reference Sherwood, Alphin and Saylor43–Reference Hebert, House and Guenter45, Reference Bunchasak and Kachana47, Reference Hoey, McNulty and McCann48).
In conclusion, the present study has shown that because of the low supply of eggs in the EU, even enriching the folate concentration of the entire egg supply two- to threefold would only increase per capita folate supply by about 25 μg/d. If targeted at individuals (e.g. women of childbearing age) and consumed daily, folate-enriched eggs could provide useful amounts of natural folates. However, the apparently lower folate bioavailability from folate-enriched eggs compared with fortified foods must be considered. Whether the public could be persuaded to consume these novel products in the required amounts is questionable because of the relatively static egg supply patterns that have persisted throughout Europe for over 40 years and because of possible conflicts with dietary guidelines and consumer attitudes regarding cholesterol. In the light of the continuing reluctance of EU governments to introduce mandatory folic acid fortification, further research is urgently required on other ways of increasing natural folate intake within EU countries.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The authors declare that they have no conflicts of interest. The authors express their sincere gratitude to FAOSTAT, Statistics Division, Food and Agriculture Organization of the UN, for allowing them to use their data. T.S. and S.S. conceived the study. T.S. acquired and interpreted the data and drafted the paper. S.S. revised the paper critically for important intellectual content. Both authors approved the final version of the paper.




