Food craving, defined as intense desire or longing to eat a particular food(Reference Kozlowski and Wilkinson1), is a common experience for many individuals(Reference Weingarten and Elston2). Chocolate is the food most frequently craved, especially among women(Reference Rozin, Levine and Stoess3–Reference Kemps, Tiggemann and Hart5), and may become associated with unhealthy behaviours such as frequent snacking, binge eating and other eating disorders, which may lead to overweight, obesity and addiction-like behaviours(Reference Hetherington and MacDiarmid6).
The major problem in the treatment of excessive eating is the high rate of relapse to food seeking and maladaptive eating habits(Reference Kramer, Jeffery and Forster7–Reference Peterson and Mitchell9). Relapse to palatable foods, like that to drugs, is often induced by stress, cues previously associated with the reward and exposure to the drug or food previously sought(Reference Grilo, Shiffman and Wing10–Reference Gorin, Phelan and Wing13).
Animal models indicate that the reinforcing and motivational effects of drugs and palatable foods are mediated by similar neuronal circuitries and molecular mechanisms(Reference Volkow and Wise14–Reference Gearhardt, Yokum and Orr18). However, in contrast to the great deal of research focused on relapse to drug seeking(Reference de Wit and Stewart19–Reference Self26), relatively fewer preclinical studies have been addressed to the identification of neuronal mechanisms of relapse to palatable food seeking(Reference Ghitza, Gray and Epstein27–Reference McGregor, Dam and Mallet30). These studies adopted the same reinstatement models validated for drug relapse and applied eliciting stimuli, including cues previously associated with food, non-contingent exposure to food and physical or pharmacological stresses(Reference Nair, Adams-Deutsch and Epstein31–Reference Guy, Choi and Pratt33). These results indicate that the circuitries of reinstatement of food and drug seeking only partially overlap(Reference Nair, Adams-Deutsch and Epstein31).
The aim of the present study was to investigate the effect of the selective dopamine (DA) β-hydroxylase (DBH) inhibitor, nepicastat(Reference Stanley, Li and Bonhaus34), on the reinforcing and motivational properties of a chocolate solution (powdered Nesquik® (Nestlé Italiana) diluted in water) and on relapse to chocolate seeking elicited by chocolate priming and by stimuli previously associated with reward availability and by chocolate priming. Nesquik® (Nestlé Italiana) solution has previously been validated in our laboratory for its highly rewarding, reinforcing and motivational properties(Reference Gessa, Orrù and Lai35–Reference Zaru, Maccioni and Riva38).
In addition, experiments were performed in a separate group of rats to clarify whether the effect of nepicastat was limited to the reinforcing properties of highly palatable foods, such as chocolate, or was extended to the reinforcing properties of regular food elicited by appetite. To this aim, nepicastat was tested in rats subjected to a food restriction regimen producing a magnitude of lever-responding for regular food pellets comparable with that exhibited by fed rats for the chocolate solution.
Nepicastat has recently been found to reproduce the ability of disulfiram, an inhibitor of DBH other than of aldehyde dehydrogenase(Reference Goldstein, Anagnoste and Lauber39, Reference Musacchio, Goldstein and Anagnoste40), to suppress the reinstatement of cocaine-seeking behaviour triggered in rats by a cocaine priming, environmental cues previously paired with cocaine availability and stress(Reference Schroeder, Cooper and Schank41, Reference Schroeder, Epps and Grice42). However, both disulfiram and nepicastat, in doses that suppressed reinstatement to cocaine seeking, have been found to be ineffective in reducing the ongoing cocaine-reinforced operant responding, suggesting that neuronal mechanisms underlying the two phenomena are distinct(Reference Schroeder, Cooper and Schank41). The suppressant effect of nepicastat and disulfiram on the reinstatement of cocaine seeking has been attributed, by Schroeder et al. (Reference Schroeder, Cooper and Schank41, Reference Schroeder, Epps and Grice42), to the inhibition of DBH and the consequent depletion of noradrenaline (NA) in the brain, implying that NA has a critical role in the reinstatement of cocaine seeking, but no role in the maintenance of responding for cocaine.
Nepicastat, originally developed for treating congestive heart failure and hypertension, is presently under advanced clinical evaluation in the treatment of cocaine addiction(43); therefore, to uncover its possible effect on craving for palatable foods and relapse to food seeking would have important clinical and scientific implications. The first aim of the present study was to assess whether treatment with nepicastat was able to affect the reinforcing and motivational properties of a chocolate solution in rats trained to self-administer the chocolate solution under an operant (lever-responding) procedure. To this end, two different experimental procedures were used: (1) fixed-ratio (FR) schedule of reinforcement, in which the response requirement (RR; i.e. the ‘cost’ – in terms of the number of lever responses – of each presentation of the chocolate solution) is predetermined and kept fixed throughout the session (providing measures of the self-administered amount of the chocolate solution and of its reinforcing properties); (2) within-session progressive-ratio (PR) schedule of reinforcement, in which – over the same single session – RR is progressively increased after the delivery of each reinforcer, and the lowest ratio not completed (named breakpoint) is taken as a measure of motivational properties of the chocolate solution(Reference Markou, Weiss and Gold44). The second aim of the present study was to assess whether treatment with nepicastat affected chocolate-primed reinstatement of seeking for the chocolate solution in rats.
Materials and methods
The experimental procedures employed in the present study were in accordance with the Italian Law on the ‘Protection of animals used for experimental and other scientific reasons’.
Animals
Adult, male Wistar rats (Charles River Laboratories), weighing approximately 300 g at the start of the study, were used. Rats used in Expts 1–3 and 5 were housed four per cage; rats used in Expt 4 were housed individually. All cages had wood chip bedding. The animal facility was under an inverted 12 h light–12 h dark cycle (lights on at 21.30 hours), a constant temperature of 22 ± 2°C and a relative humidity of approximately 60 %. A standard rat chow (diet code: 4RF21 (Mucedola) in Expts 1–3 and 5; diet code: 5001 (International Product Supplies Limited) in Expt 4; these two diets were virtually identical in composition) and tap water were always available in the home cage, except as noted below. Rats were extensively habituated to handling and intraperitoneal injection. Each experiment used independent sets of rats.
Self-administration and reinstatement of seeking for chocolate solution
Chocolate solution
The chocolate solution was prepared by diluting powdered Nesquik® (Nestlé Italiana) in tap water. Concentration of the Nesquik® (Nestlé Italiana) chocolate powder was 5 % (w/v) throughout the study. This concentration was selected on the basis of the results of previous experiments in which it had been largely preferred over a wide range of concentrations(Reference Maccioni, Pes and Carai36). The chocolate solution was prepared daily and sipper bottles (see below) were shaken immediately before the start of each session to prevent the development of any deposit. The chocolate solution provided 0·8 kJ/g.
Apparatus
Operant sessions were conducted in modular chambers (Med Associates), located in sound-attenuated cubicles, with fans for ventilation and background white noise. The front panel of each chamber was equipped with (1) one retractable response lever, (2) one green stimulus light mounted above the lever and (3) the retractable spout of a liquid sipper bottle (250 ml capacity) located outside the chamber. A white house light was centred at the top of the back wall of each chamber. Achievement of the RR (see below) resulted in the exposure of the sipper bottle spout (lasting for 5 s in each phase of the experiment) and the illumination of the green light for the period of exposure of the sipper bottle spout.
Experimental procedure
Training and maintenance phase
To facilitate the acquisition of lever-pressing behaviour, rats were water-deprived in their home cage in the 12 h preceding the first two operant sessions. Self-administration sessions were conducted daily, 7 d per week, during the first 4 h of the dark phase of the light–dark cycle. Self-administration sessions lasted 30 min. During the first two sessions, rats were trained to lever-respond on a FR1 schedule of reinforcement for the chocolate solution. FR was progressively increased from FR1 to FR10 over ten sessions. Subsequently, ten additional sessions with FR10 were conducted (maintenance phase), so that the number of lever responses for and the intake of the chocolate solution stabilised in all rats before the start of the test sessions (see below).
Testing under the fixed-ratio schedule (Expt 1)
This experiment used twelve rats. During the test sessions, RR was maintained at the value of FR10. The test sessions lasted 30 min and were conducted on Fridays; six consecutive (Saturday–Thursday) daily baseline sessions elapsed between the test sessions; these baseline sessions were (1) identical to those of the maintenance phase, as no treatment with nepicastat was given, and (2) included in the experimental design to maintain stable levels of self-administration between the test sessions. Nepicastat (Biotie) was dissolved in saline with 0·3 % dimethyl sulphoxide and 0·3 % Tween 80 and administered intraperitoneally (injection volume 2 ml/kg), at the doses of 0, 25, 50 and 100 mg/kg, 3 h before the start of the test sessions. Nepicastat dose range, route of administration and pretreatment time were chosen on the basis of the results of preliminary experiments (this laboratory, data not shown) and literature data suggesting their efficacy in suppressing the reinstatement of cocaine-seeking behaviour in rats(Reference Schroeder, Cooper and Schank41, Reference Schroeder, Epps and Grice42). All doses of nepicastat were tested in each rat under a Latin-square design; specifically, each rat received one of the four tested doses in each of the four different test sessions in order to complete, over 4 weeks, the entire dose–response curve.
Measured variables were (1) the number of lever responses and (2) the amount of self-administered chocolate solution (expressed in ml/kg and determined by weighing the sipper bottle (0·1 g accuracy) before and after the session). Data on the effect of nepicastat on both variables were analysed by separate one-way ANOVA with repeated measures, followed by the Newman–Keuls test for post hoc comparisons.
Testing under the progressive-ratio schedule (Expt 2)
This experiment used nine rats. During the test sessions, RR was increased progressively over the session according to a procedure adapted from that described by Richardson & Roberts(Reference Richardson and Roberts45); namely, RR was increased as follows: 10, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, etc. The test sessions lasted 60 min. The test sessions were conducted on Fridays; six consecutive (Saturday–Thursday) daily regular self-administration sessions (with FR10) elapsed between the test sessions. Nepicastat (dissolved as described above) was administered intraperitoneally (injection volume 2 ml/kg), at the doses of 0, 25, 50 and 100 mg/kg, 3 h before the start of the test sessions. All doses of nepicastat were tested in each rat under a Latin-square design.
Measured variables were (1) the number of lever responses and (2) breakpoint for the chocolate solution, defined as the lowest RR not achieved by the rat (e.g. Maccioni et al. (Reference Maccioni, Pes and Carai36), Rodd et al. (Reference Rodd, Bell and Kuc46), Oster et al. (Reference Oster, Toalston and Kuc47), Madden et al. (Reference Madden, Smethells and Ewan48)). Data on the effect of nepicastat on both variables were analysed by separate one-way ANOVA with repeated measures, followed by the Newman–Keuls test for post hoc comparisons.
Testing under the reinstatement schedule (Expt 3)
This experiment used thirty-two rats, divided into four groups of eight matched for the number of lever responses and the amount of self-administered chocolate solution over the last five sessions of the maintenance phase. At the end of the maintenance phase, rats of all the four groups underwent an extinction phase, whose daily sessions (lasting 60 min) were characterised by the unavailability of the chocolate solution; specifically, the liquid delivery system and stimulus lights were off, and lever-responding was unreinforced. An extinction criterion was set at less than thirty lever responses per session for three consecutive sessions. The day after the achievement of the extinction criterion, each single rat was exposed to a single reinstatement (test) session, during which a stimulus complex associated with the availability of the chocolate solution was presented. This stimulus complex was composed by (1) the click emitted by the introduction, into the chamber, of the sipper spout, (2) the turning on of the stimulus lights and (3) the availability of the chocolate solution for 5 s. This stimulus complex was presented for ten times within 100 s. Immediately after the last presentation of the stimulus complex, the lever was introduced inside the chamber, the house light was switched on and lever responses were recorded. Lever-responding during the test session was unreinforced. Nepicastat (dissolved as described above) was administered acutely and intraperitoneally (injection volume 2 ml/kg), at the doses of 0, 25, 50 and 100 mg/kg, 3 h before the start of the test session.
The measured variable was the number of lever responses during the test session. Data on the effect of nepicastat on this variable were analysed by a two-way (phase (extinction/reinstatement); treatment (nepicastat dose)) ANOVA with repeated measures on the factor phase, followed by the Newman–Keuls test for post hoc comparisons. An additional analysis concerned the number of sessions of the extinction phase needed to achieve the extinction criterion; these data were analysed by a one-way ANOVA.
Self-administration of regular food pellets
Apparatus
Operant sessions were conducted in modular chambers (Med Associates), located in sound-attenuated cubicles, with fans for ventilation and background white noise. The front panel of each chamber was equipped with (1) one retractable response lever, (2) one green stimulus light mounted above the lever and (3) one food trough. A white house light was centred at the top of the back wall of each chamber. Achievement of the RR activated the food dispenser, resulting in the delivery of a 45 mg pellet (grain-based tablet 5TUM; International Product Supplies Limited), the composition of which was identical to that of the chow available in the home cage (see above for details), and illumination of the green light for the period of food delivery.
Experimental procedure (Expt 4)
This experiment used twelve rats. To facilitate acquisition and maintenance of lever-pressing behaviour, rats were kept under a mild food deprivation regimen by feeding a limited amount of food; the latter was calibrated so that baseline levels of lever-responding for food pellets during the self-administration sessions equated those previously recorded in the chocolate self-administration experiment (Expt 1). Self-administration sessions were conducted daily, 7 d per week, during the first 4 h of the dark phase of the light–dark cycle. The self-administration sessions lasted 30 min. During the first two sessions, rats were trained to lever-respond on a FR1 schedule of reinforcement for food pellets. The FR was progressively increased from FR1 to FR10 over ten sessions. Subsequently, ten additional sessions with FR10 were conducted (maintenance phase), so that the number of lever responses for and the intake of food pellets stabilised in all rats before the start of the test sessions.
Test sessions (1) used the FR10 schedule of reinforcement, (2) lasted 30 min and (3) were conducted on Fridays; six consecutive (Saturday–Thursday) daily baseline sessions elapsed between the test sessions; these baseline sessions were (1) identical to those of the maintenance phase, as no treatment with nepicastat was given, and (2) included in the experimental design to maintain stable levels of self-administration between the test sessions. Nepicastat (dissolved as described above) was administered intraperitoneally (injection volume 2 ml/kg) at the doses of 0, 25, 50 and 100 mg/kg, 3 h before the start of the test sessions. All doses of nepicastat were tested in each rat under a Latin-square design.
Measured variables were (1) the number of lever responses and (2) the amount of self-administered food pellets (expressed in g/kg). Data on the effect of nepicastat on both variables were analysed by separate one-way ANOVA with repeated measures, followed by the Newman–Keuls test for post hoc comparisons.
Locomotor activity
Apparatus
Locomotor activity (ambulation) was measured in Plexiglas test cages (480 × 480 × 400 mm) by a computer-operated, photocell-equipped apparatus (Motil; TSE). Photocells were 40 mm spaced. Test cages were located in a sound-proof room, adjacent to the housing room.
Experimental procedure (Expt 5)
Locomotor activity sessions were conducted during the first half of the dark phase of the light–dark cycle. On the test day, rats were divided into four groups of eight to nine animals each and treated acutely with 0, 25, 50 and 100 mg nepicastat/kg. Nepicastat (dissolved as described above) was administered acutely and intraperitoneally (injection volume 2 ml/kg). Then, two locomotor activity sessions, occurring 3 and 24 h after nepicastat administration, respectively, were conducted. Independent groups of rats were used in each session. Rats from each home cage were randomly allocated to the experimental groups. Rats were unfamiliar to the motility cage, in order to provide relatively high baseline levels of spontaneous locomotor activity, i.e. a desirable condition to amplify the possible suppressing effect of nepicastat. Locomotor activity sessions lasted 30 min. At the end of each trial, the motility cage was cleaned thoroughly.
The measured variable was the total number of counts (photocell breaks) recorded automatically by the apparatus in each session. Data on the effect of nepicastat in each session were analysed by separate one-way ANOVA.
Results
Self-administration and reinstatement of seeking for chocolate solution (Expts 1–3)
In all three experiments (FR, PR and reinstatement), all rats easily acquired and steadily maintained self-administration of the chocolate solution. During the 10 d maintenance phase, the number of lever responses and the amount of self-administered chocolate solution over each daily session averaged approximately 1200 and 50 ml/kg, respectively.
Testing under the fixed-ratio schedule (Expt 1)
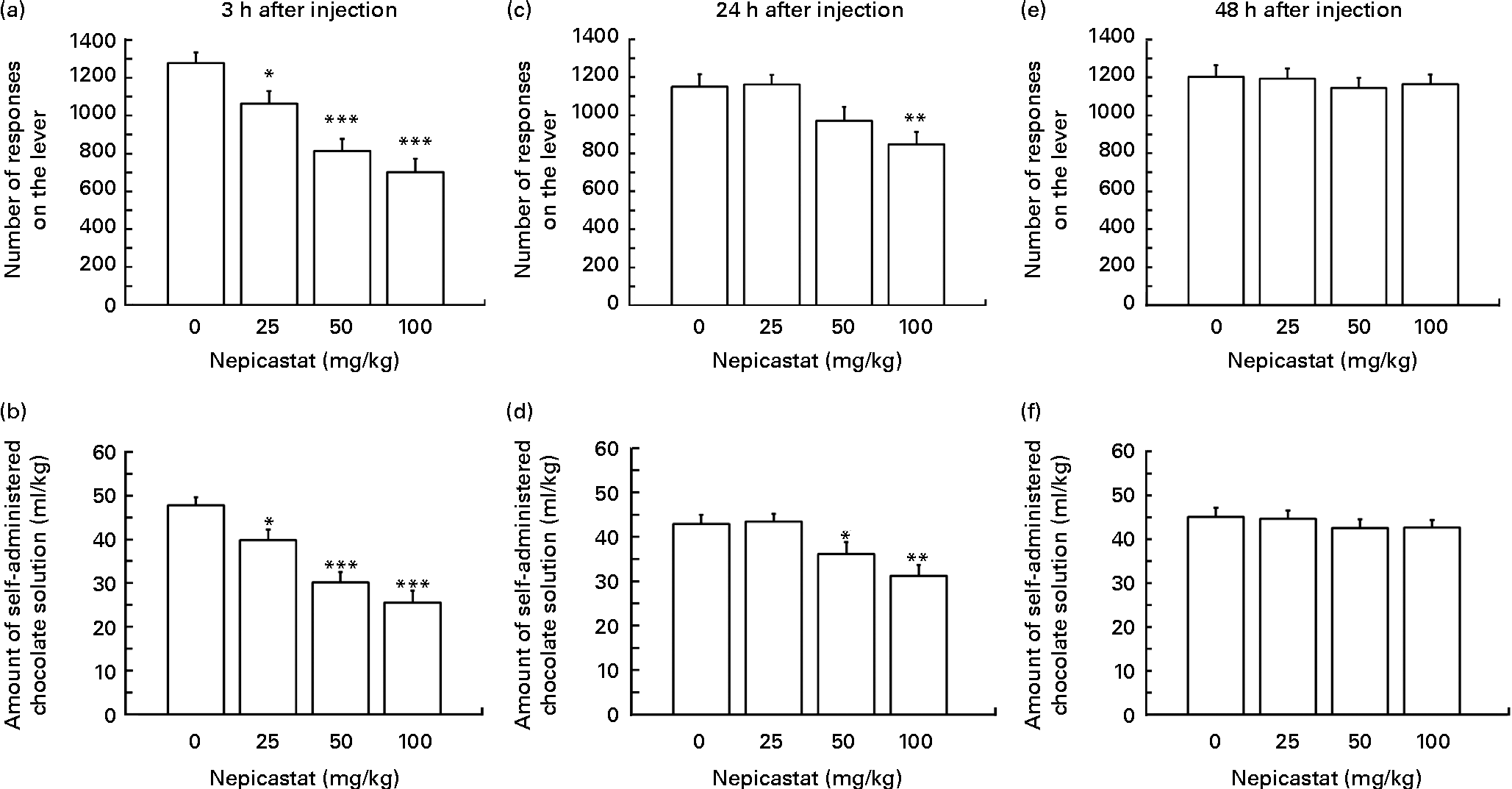
Treatment with nepicastat, given 3 h before the start of the self-administration session, resulted in a dose-dependent reduction in the number of lever responses for the chocolate solution (F(3,33) = 14·85, P= 0·000003; Fig. 1(a)). Specifically, the number of lever responses in rats treated with 25, 50 and 100 mg nepicastat/kg was 17, 36 and 45 % lower, respectively, than that recorded in vehicle-treated rats. The post hoc test revealed that the number of lever responses in the rat groups treated with all three doses of nepicastat was significantly lower than that recorded in vehicle-treated rats. The nepicastat-induced reduction in the number of lever responses was associated with a proportional decrease in the amount of self-administered chocolate solution (F(3,33) = 16·53, P= 0·000001; Fig. 1(b)).

Fig. 1 Effect of treatment with nepicastat on (a, c and e) the number of responses for and (b, d and f) the amount of a chocolate solution in Wistar rats trained to lever-respond (fixed ratio (FR)10) for the chocolate solution (Nesquik® (Nestlé Italiana) chocolate powder was 5 % (w/v)) in daily 30 min sessions; once self-administration behaviour had stabilised, rats were tested with nepicastat under the FR10 schedule of reinforcement. All doses of nepicastat were tested in each rat under a Latin-square design. Values are means of twelve rats, with their standard errors represented by vertical bars. Mean values were significantly different with respect to the vehicle-treated rats (Newman–Keuls test): * P< 0·05; ** P< 0·01; *** P< 0·0005.
Nepicastat was still effective in reducing the number of lever responses for the chocolate solution in the subsequent self-administration session, conducted 24 h after nepicastat administration (F(3,33) = 5·64, P= 0·003099; Fig. 1(c)). The post hoc test revealed, however, that only 100 mg nepicastat/kg significantly reduced the number of lever responses for the chocolate solution (approximately 25 % lower than that recorded in vehicle-treated rats). Nepicastat (given 24 h before the self-administration session) reduced also the amount of self-administered chocolate solution (F(3,33) = 6·98, P= 0·000916; Fig. 1(d)). This effect achieved statistical significance at the post hoc test at the doses of 50 and 100 mg/kg.
Conversely, treatment with nepicastat failed to alter both the number of lever responses for the chocolate solution (F(3,33) = 0·23, P= 0·875379; Fig. 1(e)) and the amount of self-administered chocolate solution (F(3,33) = 0·45, P= 0·718227; Fig. 1(f)) in the self-administration session conducted 48 h after nepicastat administration.
Testing under the progressive-ratio schedule (Expt 2)
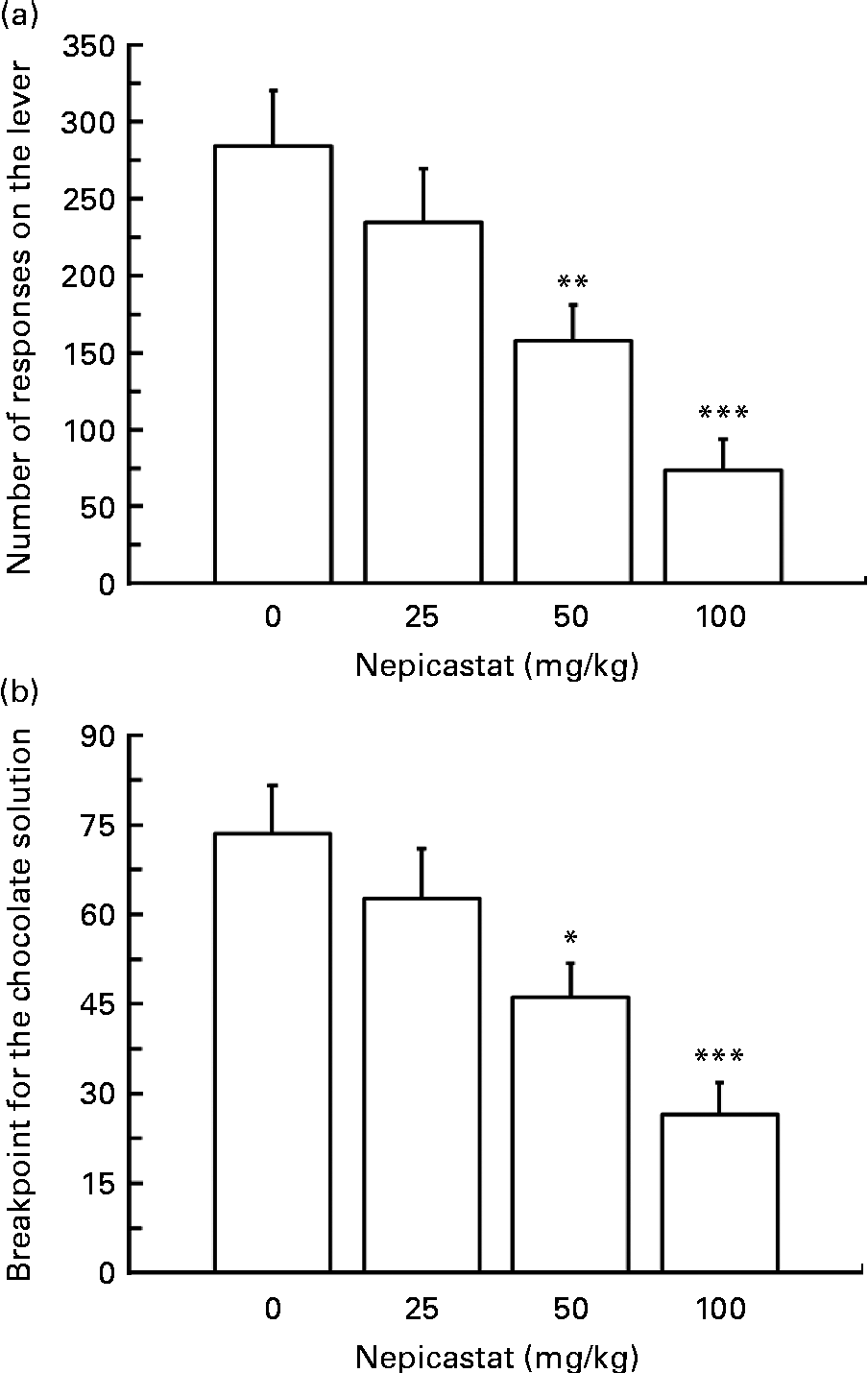
Treatment with nepicastat, given 3 h before the start of the test session, resulted in a dose-dependent reduction in the number of lever responses for the chocolate solution (F(3,24) = 11·07, P= 0·000094; Fig. 2(a)). Specifically, the number of lever responses in rats treated with 25, 50 and 100 mg nepicastat/kg was 17, 45 and 74 % lower, respectively, than that recorded in vehicle-treated rats. The post hoc test revealed that the number of lever responses in the rat groups treated with 50 and 100 mg nepicastat/kg was significantly lower than that recorded in vehicle-treated rats.

Fig. 2 Effect of treatment with nepicastat on (a) the number of lever responses and (b) breakpoint for a chocolate solution in Wistar rats trained to lever-respond (fixed ratio 10) for the chocolate solution (Nesquik® (Nestlé Italiana) chocolate powder was 5 % (w/v)) in daily 30 min sessions; once self-administration behaviour had stabilised, rats were tested with nepicastat under the progressive-ratio schedule of reinforcement in 60 min sessions. All doses of nepicastat were tested in each rat under a Latin-square design. Values are means of nine rats, with their standard errors represented by vertical bars. Mean values were significantly different with respect to the vehicle-treated rats (Newman–Keuls test): * P< 0·05; ** P< 0·01; *** P< 0·0005.
Additionally, treatment with nepicastat reduced, in a dose-dependent fashion, the value of breakpoint for the chocolate solution (F(3,24) = 10·60, P= 0·000125; Fig. 2(b)). Breakpoint averages approximately 75 in vehicle-treated rats; in rats treated with 25, 50 and 100 mg nepicastat/kg, breakpoint resulted to be 15, 37 and 64 % lower, respectively, than that recorded in vehicle-treated rats. The post hoc test revealed that breakpoint in the rat groups treated with 50 and 100 mg nepicastat/kg was significantly lower than that recorded in vehicle-treated rats.
Testing under the reinstatement schedule (Expt 3)
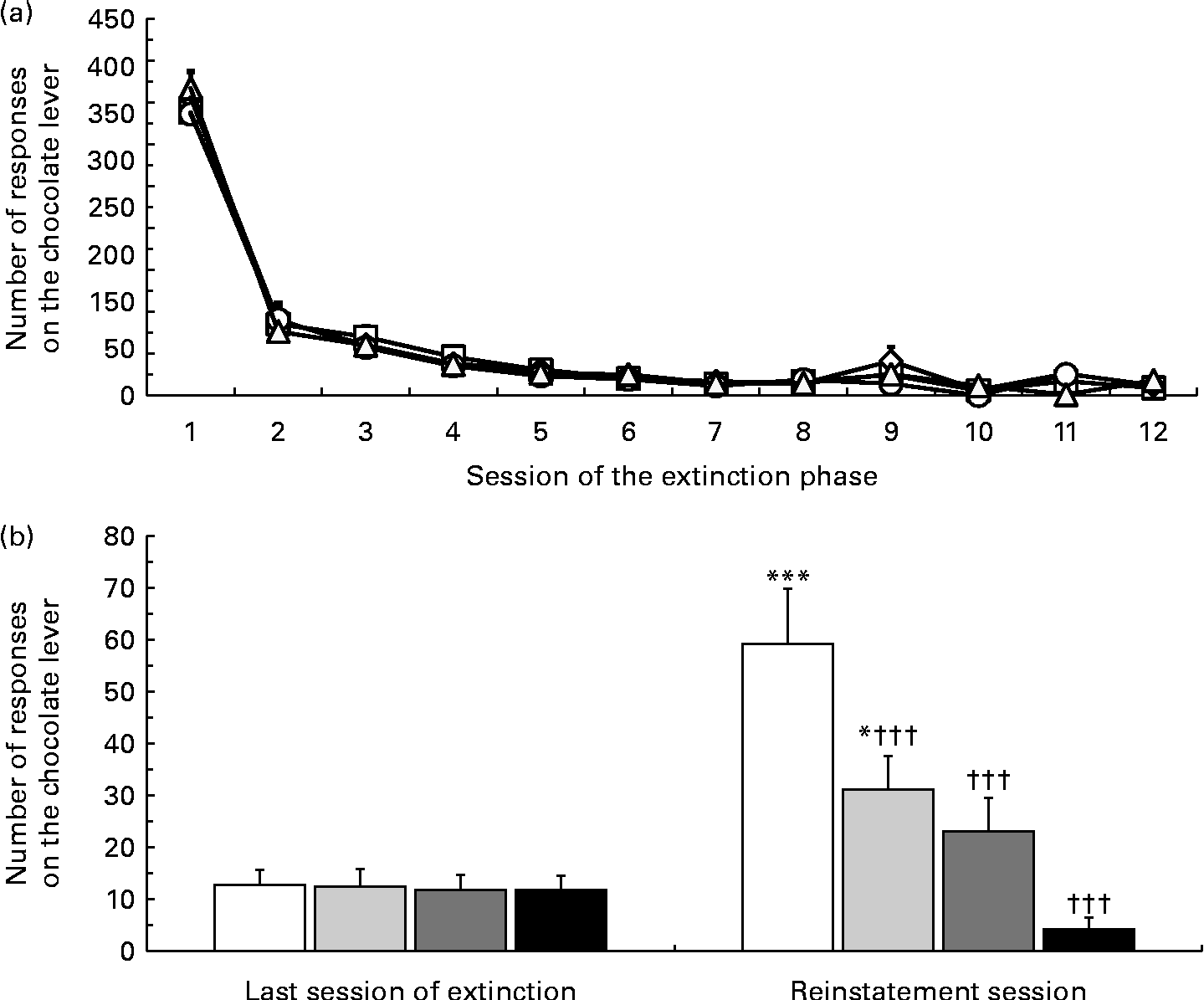
Lever-responding behaviour over the extinction phase was similar between the four rat groups subsequently treated with vehicle or one of the three doses of nepicastat (Fig. 3(a)). The four rat groups did not differ in the number of sessions of the extinction phase needed to achieve the extinction criterion (7·8 (sem 0·8), 8·3 (sem 0·7), 7·6 (sem 0·7) and 7·9 (sem 0·8) in rats subsequently treated with 0, 25, 50 and 100 mg nepicastat/kg, respectively; F(3, 28) = 0·14, P= 0·934496; Fig. 3(a)).

Fig. 3 Effect of treatment with nepicastat on the number of responses on the lever associated with a chocolate solution in Wistar rats initially trained to lever-respond (fixed ratio 10) for the chocolate solution (Nesquik® (Nestlé Italiana) chocolate powder was 5 % (w/v)), then exposed to a period of (a) extinction responding and finally exposed to a 60 min session of (b) reinstatement of chocolate-seeking behaviour. In the test session, lever-responding was reinstated by the repeated presentation of a stimulus complex previously associated with the availability of the chocolate solution. In (a), each point is the mean of sample sizes varying between 1 and 8, depending on the session when each single rat achieved the extinction criterion. In (b), values are means of eight rats, with their standard errors represented by vertical bars. Mean values were significantly different with respect to the same rat group in the last session of the extinction phase (Newman–Keuls test): * P< 0·05; *** P< 0·0005. ††† Mean values were significantly different with respect to the vehicle-treated rat group in the reinstatement session (P< 0·0005; Newman–Keuls test). 0 mg Nepicastat/kg ((a) ![]() , (b) □); 25 mg nepicastat/kg ((a)
, (b) □); 25 mg nepicastat/kg ((a) ![]() , (b)
, (b) ![]() ); 50 mg nepicastat/kg ((a)
); 50 mg nepicastat/kg ((a) ![]() , (b)
, (b) ![]() ); 100 mg nepicastat/kg ((a)
); 100 mg nepicastat/kg ((a) ![]() , (b) ■).
, (b) ■).
ANOVA revealed a significant effect of presentation of the stimulus complex associated with the chocolate solution (F(1, 28) = 28·9, P= 0·000010) and of treatment with nepicastat (F(3, 28) = 7·05, P= 0·001124), as well as a significant interaction between the two factors (F(3, 28) = 12·30, P= 0·000026). The number of lever responses during the last session of the extinction phase was virtually identical in the four rat groups subsequently treated with 0, 25, 50 and 100 mg nepicastat/kg (Fig. 3(b)). Under the vehicle condition, presentation of the stimulus complex associated with the solution robustly reinstated lever-responding: the number of lever responses averaged indeed 59·3 (sem 10·6) and was 4·6 times higher than that recorded in the last extinction session (Fig. 3(b)). Administration of nepicastat resulted in a dose-dependent suppression of lever-responding (Fig. 3(b)); notably, treatment with 100 mg nepicastat/kg resulted in a complete blockade of lever-responding, as indicated by an average number of lever-responding (4·1 (sem 2·3)) that was even largely lower than that recorded in the last extinction session (Fig. 3(b)). In the experimental group treated with 100 mg nepicastat/kg, two rats totally avoided lever-responding.
Self-administration of regular food pellets (Expt 4)
All rats easily acquired and steadily maintained self-administration of regular food pellets. During the 10 d maintenance phase, the number of lever responses and the amount of self-administered food over each daily session averaged approximately 1400 and 14 g/kg, respectively.
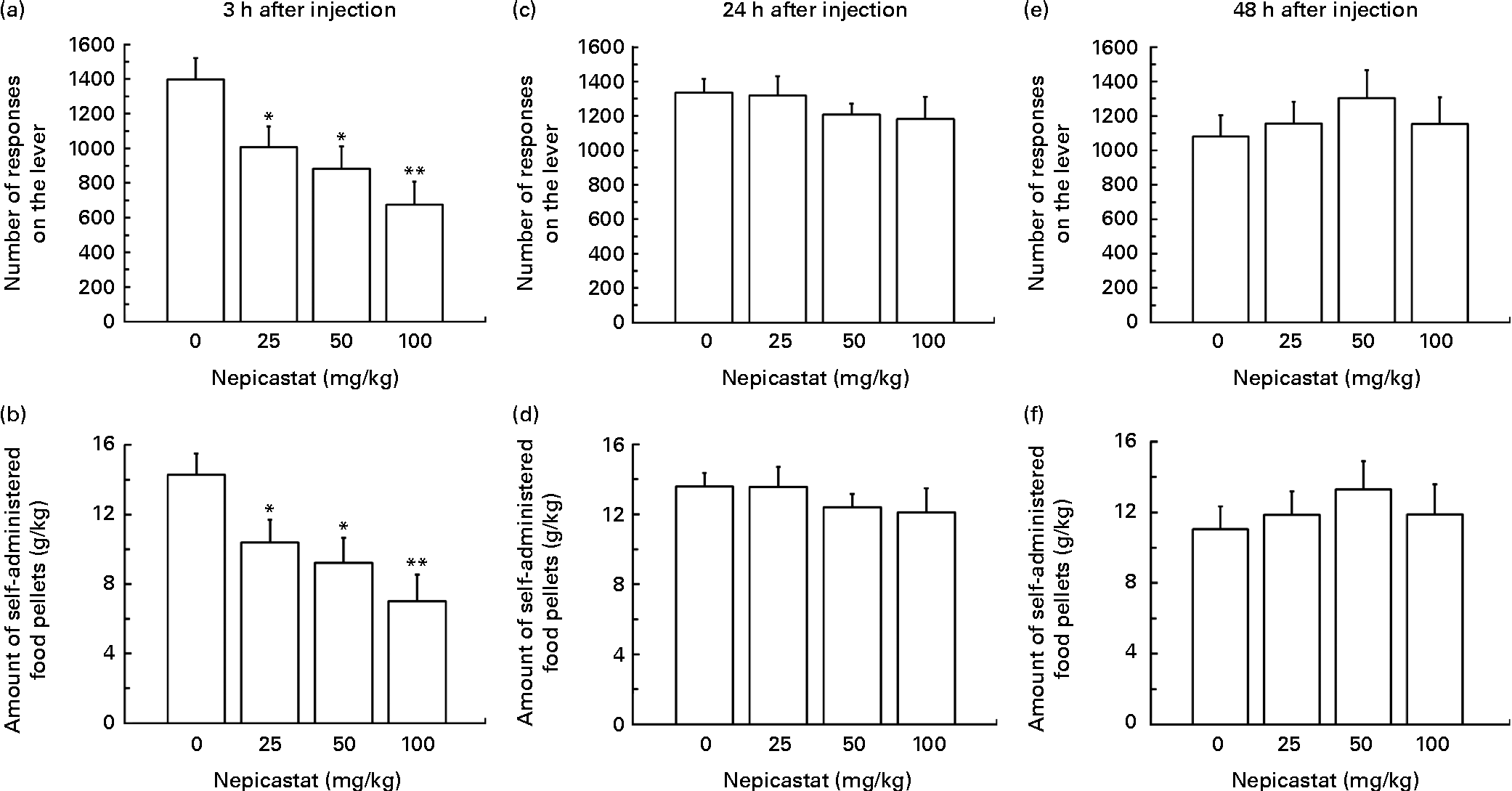
Treatment with nepicastat, given 3 h before the start of the self-administration session, resulted in a dose-dependent reduction in the number of lever responses for regular food pellets (F(3,33) = 5·94, P= 0·002342; Fig. 4(a)). Specifically, the number of lever responses in rats treated with 25, 50 and 100 mg nepicastat/kg was 28, 37 and 52 % lower, respectively, than that recorded in vehicle-treated rats. The post hoc test revealed that the number of lever responses in the rat groups treated with all three doses of nepicastat was significantly lower than that recorded in vehicle-treated rats. The nepicastat-induced reduction in the number of lever responses was associated with a proportional decrease in the amount of self-administered food (F(3,33) = 5·98, P= 0·002264; Fig. 4(b)).

Fig. 4 Effect of treatment with nepicastat on (a, c and e) the number of responses for and (b, d and f) the amount of regular food pellets in Wistar rats trained to lever-respond (fixed ratio (FR)10) for regular food pellets (the composition of which was identical to that of the chow available in the home cage) in daily 30 min sessions; once self-administration behaviour had stabilised, rats were tested with nepicastat under the FR10 schedule of reinforcement. All doses of nepicastat were tested in each rat under a Latin-square design. Values are means of twelve rats, with their standard errors represented by vertical bars. Mean values were significantly different with respect to the vehicle-treated rats (Newman–Keuls test): * P< 0·05; ** P< 0·01.
Nepicastat was ineffective in reducing the number of lever responses for regular food pellets and the amount of self-administered food in the two subsequent self-administration sessions, conducted 24 h (lever responses: F(3,33) = 0·70, P= 0·560495; self-administered food: F(3,33) = 0·55, P= 0·651450; Fig. 4(c) and (d)) and 48 h (lever responses: F(3,33) = 0·60, P= 0·618442; self-administered food: F(3,33) = 0·47, P= 0·702309; Fig. 4(e) and (f)) after nepicastat administration.
Locomotor activity (Expt 5)
Treatment with nepicastat did not affect spontaneous locomotor activity both 3 h (F(3,31) = 1·57, P= 0·215226) and 24 h (F(3,30) = 0·48, P= 0·697289) after drug administration (Table 1).
Table 1 Effect of treatment with nepicastat on spontaneous locomotor activity in Wistar rats exposed to an unfamiliar arena*† (Mean values with their standard errors, n 8–9 rats)
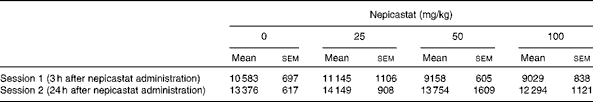
* Locomotor activity sessions lasted 30 min.
† The measured variable was the total number of counts (photocell breaks) recorded automatically by the apparatus in each session.
Discussion
Confirming previous observations(Reference Maccioni, Pes and Carai36–Reference Zaru, Maccioni and Riva38), we found that the chocolate solution exhibited intense reinforcing and motivational properties, as indicated by the rapid acquisition and steady maintenance of operant self-administration behaviour, remarkably high numbers of lever responses in each self-administration session, large amounts of self-administered solution and high values of breakpoint under the PR schedule of reinforcement.
In the reinstatement experiment, extinction of the chocolate-seeking behaviour was reached in all rats after an extinction phase of approximately 8 d, during which lever-responding was not reinforced. Reinstatement of chocolate seeking was produced by a non-contingent exposure to the chocolate solution combined with environmental cues (light plus tone) previously associated with the availability of the reward. The reinstatement procedure intended to reproduce relapse in chocolate ‘dieters’, usually triggered by stimuli previously associated with chocolate availability and by a lapse back to chocolate tasting after a period of abstinence(Reference Grilo, Shiffman and Wing10, Reference Van Gucht, Vansteenwegen and Beckers49).
The operant responding maintained by the chocolate solution was probably driven by its orosensory attributes (sweetness, chocolate flavour and aroma) rather than by appetite and its energy content, with the animals being fed ad libitum in their home cage and the energy content of the chocolate solution being 1/17 that of the regular food pellets.
The results of the present study show that nepicastat reduced the reinforcing and motivational properties of the chocolate solution and totally suppressed the reinstatement of chocolate seeking. Thus, nepicastat reduced in a dose-related manner both lever-responding under the FR schedule of reinforcement (a measure of the reinforcing properties of the chocolate solution) and breakpoint under the PR schedule of reinforcement (a measure of the motivational properties of the chocolate solution).
The suppressant effect of nepicastat on self-administration of the chocolate solution was long-lasting, with the highest dose still being significantly inhibitory 24 h after dosing, and not secondary to any sedative effect, since no dose of nepicastat caused any impairment of spontaneous locomotor activity. However, the suppressant effect of nepicastat was not specific for the operant responding driven by the hedonic qualities of food; in fact, the compound was equally effective in inhibiting operant responding maintained by appetite in rats subjected to a food restriction regimen calibrated to generate lever-responding for regular food pellets equal to that produced by the chocolate solution in fed rats. These results suggest that nepicastat may be effective in reducing the reinforcing properties of food, when sustained by either palatability or appetite; these results also suggest that a common neural substrate controls both conditions.
The finding that nepicastat reduced lever-responding for regular food pellets is in contrast with the observation by Schroeder et al. (Reference Schroeder, Cooper and Schank41, Reference Schroeder, Epps and Grice42), who found that nepicastat and the other DBH inhibitor, disulfiram, had no effect on lever-responding for regular food pellets in Sprague–Dawley rats. A possible explanation for the discrepancy between the present data and those by Schroeder et al. (Reference Schroeder, Cooper and Schank41, Reference Schroeder, Epps and Grice42) might be the highly different baseline levels of lever-responding in the two studies (approximately 100 lever responses/session under a FR1 schedule of reinforcement, with a maximum number of sixty reinforcers available in the study by Schroeder et al. (Reference Schroeder, Cooper and Schank41, Reference Schroeder, Epps and Grice42); approximately 1400 lever responses/session under a FR10 schedule of reinforcement and no limit in the number of reinforcers available in the present study). Additionally, the studies by Schroeder et al. (Reference Schroeder, Cooper and Schank41, Reference Schroeder, Epps and Grice42) tested a single dose of nepicastat (50 mg/kg, intraperitoneal), missing possible effects of other doses.
Additionally, Schroeder et al. (Reference Schroeder, Epps and Grice42) found that 50 mg nepicastat/kg (intraperitoneal) were totally ineffective on self-administration of sucrose pellets in Sprague–Dawley rats exposed to the PR schedule of reinforcement. These data are in some contrast with the present data demonstrating the capability of different doses of nepicastat (including 50 mg/kg, intraperitoneal) to reduce lever-responding for the chocolate solution in Wistar rats exposed to the PR schedule of reinforcement. Several methodological differences – including rat strain, self-administration procedure, different baseline levels of lever-responding and palatability of the reinforcer – may, however, account for these discrepancies. Future studies should clarify this issue.
The suppressant effect of nepicastat on the reinstatement of chocolate seeking reproduced the reported inhibitory effect of nepicastat and disulfiram on the reinstatement of cocaine seeking in rats(Reference Schroeder, Cooper and Schank41, Reference Schroeder, Epps and Grice42), suggesting that a common mechanism and neuronal circuitry mediate the ability of different stimuli (e.g. cocaine, chocolate, cues and stress) to precipitate relapse to the sought reward. In apparent contrast with these data and interpretation, nepicastat and disulfiram failed to prevent food-primed reinstatement of seeking for regular food pellets in rats subjected to a food restriction regimen(Reference Schroeder, Cooper and Schank41); however, in that study(Reference Schroeder, Cooper and Schank41), food pellets were delivered at regular intervals during the reinstatement session, so that the availability of a given food pellet could be interpreted by the rat as contingent to its lever-responding, probably having a ‘reinforcing’ impact on the subsequent lever-responding behaviour.
On the other hand, neuronal mechanisms controlling the reinforcing properties of food seem to be different from those underlying cocaine self-administration. Indeed, nepicastat inhibited the self-administration of the chocolate solution and regular food pellets under a FR10 schedule of reinforcement, which is in contrast to previous observations that disulfiram and nepicastat failed to modify operant responding for cocaine under a FR1 schedule of reinforcement(Reference Schroeder, Cooper and Schank41) and to the notion that pharmacological manipulation of noradrenergic transmission does not affect self-administration of cocaine or other psychostimulants in rats or non-human primates(Reference Gaval-Cruz and Weinshenker50).
The ability of nepicastat and disulfiram to suppress the reinstatement of cocaine seeking has been attributed(Reference Schroeder, Cooper and Schank41, Reference Schroeder, Epps and Grice42, Reference Gaval-Cruz and Weinshenker50) to the reduced NA production and the consequent loss of an α1-adrenoceptor-mediated stimulatory tonus on mesolimbic dopaminergic neurons, which is needed so that environmental stimuli are able to trigger DA release in the nucleus accumbens (the key phenomenon mediating relapse)(Reference Leri, Flores and Rodaros51–Reference Platt, Rowlett and Spealman54). This mechanism might also explain the ability of nepicastat to suppress the reinstatement of chocolate seeking observed in the present study. This hypothesis implies that NA plays a positive role in the ability of various stimuli to trigger the reinstatement of both drug and palatable food seeking. However, NA seems to be involved in palatable food self-administration but not in that of cocaine.
It should be noted that recent results from our laboratory suggest an alternative hypothesis to explain nepicastat effect on the reinstatement of cocaine and, possibly, food seeking. We found that nepicastat and disulfiram not only reduced – as expected by DBH inhibition – NA release in different brain areas, but also caused a marked increase in DA release in the prefrontal cortex(Reference Devoto, Flore and Saba55, Reference Devoto, Flore and Saba56). To explain this effect, we postulated that the two DBH inhibitors, by removing NA from α2-adrenoceptors, would relieve noradrenergic and dopaminergic terminals in the prefrontal cortex from the inhibitory control exerted by NA, thereby causing an unrestrained DA release from these terminals. Since cortical DA is thought to exert an inhibitory control on glutamatergic excitatory projections from the prefrontal cortex to the nucleus accumbens, which play a critical role in relapse to drug and food seeking(Reference McFarland, Lapish and Kalivas25, Reference Peters and Kalivas57, Reference LaLumiere and Kalivas58), we suggest that DA accumulation in the prefrontal cortex may contribute to the suppressant effect of nepicastat on the reinstatement of cocaine and food seeking. Notably, alterations in DA function in mesolimbic ‘reward’ pathways may have an enormous impact on several behaviours related to drugs of abuse and palatable food, including – as an example – increased vulnerability to drug addiction and overeating of palatable food in those individuals with gene-based hyporesponsitivity of the DA ‘reward’ circuitry (e.g. Volkow et al. (Reference Volkow, Wang and Telang59), Kenny(Reference Kenny60), Blum et al. (Reference Blum, Oscar-Berman and Giordano61), Stice et al. (Reference Stice, Yokum and Burger62–Reference Stice, Figlewicz and Gosnell64)).
In conclusion, further research is needed to clarify the exact mechanism of nepicastat suppressant effect on food self-administration and reinstatement of food seeking. These studies should clarify the role of NA on the reinforcing effect of food and in the control of how environmental triggers promote relapse to food seeking.
Acknowledgements
The authors are grateful to Mrs Carla Acciaro for technical support. All authors conceived the study and designed the experiments. The authors' contributions are as follows: G. C. and G. L. G. managed the literature searches and summaries of previous related work; A. Z. and P. M. conducted the experiments and analysed the data; G. L. G. drafted the manuscript and supervised the study. All authors contributed to and approved the final draft of the manuscript. G. L. G. takes responsibility for the paper as a whole. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The authors declare that there is no conflict of interest.