The metabolic syndrome, a constellation of co-morbidities that includes visceral obesity, hypertension, glucose intolerance and dyslipidaemia, is a highly predisposing condition for CVD(Reference Bricker and Greydanus1). High intake of dietary SFA is associated with increased incidence of CVD(Reference Renaud and de Lorgeril2–Reference Denke5), whereas a high intake of PUFA is known to reduce the incidence of CVD(Reference Dolecek6). Among PUFA, the n-3 PUFA such as EPA and DHA commonly found in fish/fish oil are well known to exert cardioprotective effects. The beneficial effects of n-3 PUFA are attributed to their hypolipidaemic, antithrombotic, antiarrhythmic(Reference Demaison and Moreau7) and insulin-sensitising properties(Reference Vemuri, Kelley and Mackey8). Recently, flax oil derived from flaxseed (Linum usitatissimum) has gained a lot of attention as an important dietary source of n-3 PUFA, especially among the vegetarian populations(Reference Davis and Kris-Etherton9).
Flax oil is a rich source of an essential n-3 PUFA, α-linolenic acid (ALA), which is converted to EPA and DHA by the (Δ6–Δ5) elongase and desaturase enzyme systems in the body. Although there is a debate regarding the efficacy of the conversion of ALA into EPA and DHA in the human body(Reference Davis and Kris-Etherton9), ALA consumption by itself has been reported to exert beneficial effects on the clinical outcomes of renal failure, multiple sclerosis, cancer, hypertension and CVD(Reference Kelley, Branch and Love10, Reference Mantzioris, James and Gibson11). A number of studies have shown that flax oil supplementation can reduce serum TAG and cholesterol concentrations(Reference Cunnane, Gangali and Menard12, Reference Craig13). Moreover, flax oil supplementation has been shown to improve non-alcoholic fatty liver disease (NAFLD) by reducing the lipid content of the liver(Reference Murase, Aoki and Tokimitsu14). Furthermore, it has been proposed that the n-3 PUFA of flaxseed oil have anti-inflammatory properties that are mediated by the production of anti-inflammatory cytokines(Reference Cohen, Moore and Ward15).
Spontaneously hypertensive (SHR)/NDmcr-cp rats, which represent a genetic model of the metabolic syndrome, are derived from a cross between the SHR and the obese Koletsky rat(Reference Junko, Ikeda and Yamori16). SHR/NDmcr-cp rats exhibit hypertension due to the genetic background derived from the SHR, and are severely obese due to the nonsense mutation in the leptin receptor derived from the Koletsky rats. Moreover, obese SHR/NDmcr-cp rats carrying the homozygous mutation in the leptin receptor exhibit most of the abnormalities associated with the metabolic syndrome including hyperglycaemia, hyperinsulinaemia, hyperlipidaemia and fatty liver when compared with their lean counterparts(Reference Yasui, Hiraoka-Yamamoto and Kitamori17). Oxidative stress is also increased in the obese SHR/NDmcr-cp rats, which is similar to the observations in patients of the metabolic syndrome(Reference Yamaguchi, Yamada and Yoshikawa18). Obese SHR/NDmcr-cp rats thus exhibit most of the metabolic derangements observed in patients with the metabolic syndrome, making them one of the most suitable animal models of metabolic syndrome.
In an attempt to identify the beneficial effects of flax oil, diets enriched with either flax oil or lard were fed to both obese and lean SHR/NDmcr-cp rats, and various parameters related to the metabolic syndrome, i.e. hyperlipidaemia, hyperglycaemia, hyperinsulinaemia and oxidative stress, were measured. To gain an insight into the underlying molecular mechanisms, the gene expression of PPAR-α, PPAR-γ and sterol-regulatory element-binding protein (SREBP)-1c was also measured, as PPAR-α is known to regulate the expression of genes involved in β-oxidation of fatty acids and SREBP-1c regulates the expression of genes involved in de novo lipogenesis and fatty acid metabolism, while PPAR-γ expression has been associated with insulin-sensitising effects.
Materials and methods
Animals and diets
All the animals used in the present study were treated in accordance with the Guiding Principles for the Care and Use of Laboratory Animals approved by the Mukogawa Women's University (Nishinomiya, Japan). Male obese and lean SHR/NDmcr-cp rats, aged 7 weeks, were provided by the Disease Model Cooperative Research Association (Kyoto, Japan). Rats were maintained on rodent chow for a period of 1 week before they were fed the experimental diets. After this acclimatisation period, both obese and lean SHR/NDmcr-cp rats were divided into two groups: one group was fed a high-fat diet enriched with flax oil, whereas the other group was fed a high-fat diet enriched with lard. The groups were designated according to their genetic composition and diet as flax oil-fed obese (FO) rats; flax oil-fed lean (FL) rats; lard-fed obese (LO) rats and lard-fed lean (LL) rats. All the groups were maintained on the experimental diets for 4 weeks. Fat-free semi-synthetic sterilised (10 kGy) diet containing (per kg) casein 20 g, dl-methionine 0·3 g, sucrose 30·5 g, maize starch 19 g, fibre 5 g, vitamin mix 1·1 g and mineral mix 4 g was obtained from Funabashi Farms Company Limited (Chiba, Japan). Lard and soyabean oil were obtained from a local supermarket (Japan), whereas lignan-free flax oil was a gift from the Flaxseed Association (Tokyo, Japan). To prepare the experimental diets, 5 % soyabean oil and 10 % of either flax oil or lard were added to the fat-free semi-synthetic diet and stored at − 80°C after flushing with N2 gas. Rats were given fresh diets everyday. Fatty acid composition of the high-fat diets is given in Table 1, which was determined using GLC(Reference Keough and Davis19).
Table 1 Fatty acid composition (percentage of total extracted fatty acids) of the experimental diets*
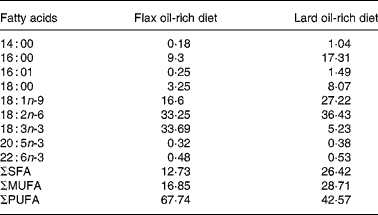
ΣSFA, sum of SFA; ΣMUFA, sum of MUFA; ΣPUFA, sum of PUFA.
* Lipids were extracted from the diets, and fatty acid composition was determined by GC.
The rats were housed in a single room with a 12 h light/12 h dark period cycle. The temperature and humidity were maintained at 21°C and 35 ± 5 %, respectively. Body weights and food consumption of the rats were recorded weekly. At 12 weeks of age, the rats were kept in metabolic cages for 24 h, a day before killing, and their food intake, water intake and urinary excretion were recorded. Rats were fasted for 12 h overnight, and were then killed by anaesthetising with diethyl ether vapour in a closed chamber the next morning. Blood and tissues were collected, weighed and then snap frozen at − 80°C until further analyses.
Serum glucose and NEFA analyses
Blood was collected at the time of killing the rats, and was centrifuged at 3000 g for 15 min to separate the serum. Fasting serum glucose concentration was measured using a commercially available kit, Glucose-C2 no. 439-90901 (Wako Chemicals, Osaka, Japan). Fasting serum NEFA concentrations were measured using kit no. 279-75401 (Wako Chemicals). Fasting serum insulin concentration was measured using kit no. AK RIN-010T (Shibayagi Company Limited, Gunma, Japan).
Serum and hepatic lipid analysis
The cholesterol and TAG content of fasting serum and its various lipoprotein fractions were determined using HPLC by LipoSearch, Skylight Biotech, Inc. (Tokyo, Japan)(Reference Usui, Hara and Hosaki20). Liver lipids were extracted as described previously(Reference Folch, Lees and Sloane Stanley21) and were analysed using enzymatic kit methods for cholesterol (kit no. 439-17501; Wako Chemicals) and TAG (kit no. 432-40201; Wako Chemicals).
Quantitative PCR analysis
Total RNA was isolated from the liver samples using RNeasy Mini kit no. 74104 (Qiagen, Tokyo, Japan), and first-strand complementary DNA was synthesised using SuperScript-III Reverse transcriptase no. 18080-044 (Invitrogen, Tokyo, Japan). Synthesised complementary DNA was mixed with Power SYBR Green PCR Master Mix no. 4367659 (Applied Biosystems, Tokyo, Japan) and gene-specific sense and antisense primers (Table 2). Samples were subjected to real-time PCR quantification using the ABI Prism 7700 Sequence Detection System (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase was used as a housekeeping gene, and no differences were found in the expression of glyceraldehyde-3-phosphate dehydrogenase among various groups. Briefly, standard curves were generated using serial dilution of a control sample for PPAR-α, PPAR-γ, SREBP-1c and glyceraldehyde-3-phosphate dehydrogenase, which were used to calculate the PCR efficiency for each reaction. The expression of each gene per sample was then calculated in relation to the expression of glyceraldehyde-3-phosphate dehydrogenase, thus normalising and correcting the data for the differences in PCR efficiencies for each set of primers. All reactions were performed in duplicate.
Table 2 Sequence of the primers used for the quantitative PCR analysis
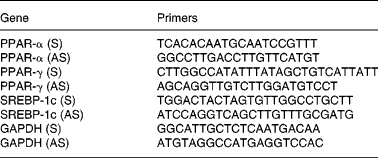
S, sense primer; AS, antisense primer; SREBP, sterol-regulatory element-binding protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Oxidative stress analysis
Concentrations of urinary thiobarbituric acid-reactive substances (TBARS) were used as a marker of oxidative stress. TBARS levels, expressed as malondialdehyde levels, were determined in the urine samples collected for 24 h using commercially available kit no. 10009055 (Cayman Chemicals, Ann Arbor, MI, USA).
Statistical analysis
Data are expressed as means and standard deviations, n 5, in each group. Main effects of genotype, diet and their interaction (genotype × diet) among groups were analysed using two-way ANOVA followed by Tukey's honestly significant difference post hoc analysis (SYSTAT for Windows, version 12.02; SYSTAT Software, Inc., Richmond, CA, USA). Differences having P < 0·05 were considered significant. All assay measurements were made in duplicate.
Results
Body weights, organ weights, food and energetic intake, and serum variables
An obese genotype (P < 0·001, two-way ANOVA) was associated with higher body weight, liver weight and mesenteric-, epididymal- and renal fat-pad weights in both FO and LO rats compared with their lean counterparts after 4 weeks of dietary treatment (FO v. FL, P < 0·001 and LO v. LL, P < 0·001) (Table 3). An obese genotype was also associated with higher food and energetic intake (P < 0·005, two-way ANOVA) in LO rats compared with the LL rats (LO v. LL, P < 0·005); however, no such differences were observed between the FO and FL rats (Table 3).
Table 3 Body weight, organ weights, and food and energetic intake in obese and lean spontaneously hypertensive/NDmcr-cp rats fed high-fat diets rich in flax oil v. lard
(Mean values and standard deviations; n 5)
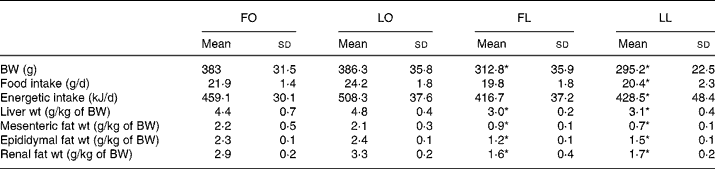
FO, flax oil-fed obese rats; LO, lard-fed obese rats; FL, flax oil-fed lean rats; LL, lard-fed lean rats; BW, body weight.
* Mean values were significantly different from those of the respective obese rats for each diet (P < 0·05).
An obese genotype (P < 0·001, two-way ANOVA) was also associated with higher serum glucose and NEFA concentrations in both FO and LO SHR/NDmcr-cp rats compared with their lean counterparts (FO v. FL, P < 0·001 and LO v. LL, P < 0·01), irrespective of their diet (Table 4). However, an interaction between genotype and diet was observed for the serum insulin concentration (P < 0·001, two-way ANOVA). While both FO and LO rats had higher serum insulin concentration compared with their lean counterparts (FO v. FL, P < 0·001 and LO v. LL, P < 0·001), flax oil feeding was associated with reduced serum insulin concentration in obese rats compared with the LO rats (FO v. LO, P < 0·001) (Table 4).
Table 4 Fasting serum glucose, NEFA and insulin concentrations in obese and lean spontaneously hypertensive/NDmcr-cp rats fed high-fat diets rich in flax oil v. lard
(Mean values and standard deviations; n 5)
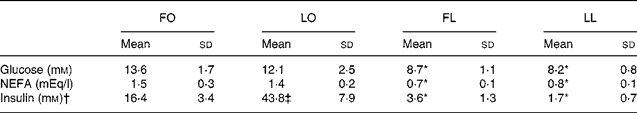
FO, flax oil-fed obese rats; LO, lard-fed obese rats; FL, flax oil-fed lean rats; LL, lard-fed lean rats.
* Mean values were significantly different from those of the respective obese rats for each diet (P < 0·05).
† Two-way ANOVA disclosed an interaction between genotype and diet (P < 0·001) for serum insulin concentrations.
‡ Mean values were significantly different from those of the respective flax oil-fed rats for each genotype (P < 0·05).
Serum and lipoproteins TAG and cholesterol concentrations
An obese genotype (P < 0·001, two-way ANOVA) was associated with higher concentrations of TAG and cholesterol concentrations in serum and VLDL, LDL and HDL fractions in both FO and LO rats compared with their lean counterparts (FO v. FL, P < 0·001 and LO v. LL, P < 0·05), irrespective of their diet (Figs. 1(a)–(d) and 2(a)–(d)). In addition, FL rats had significantly lower LDL-cholesterol concentration compared with the LL rats (FL v. LL, P < 0·05), indicating an effect of diet (P < 0·01, two-way ANOVA) (Fig. 2(c)).

Fig. 1 TAG concentration in (a) whole serum and fractions of (b) VLDL, (c) LDL and (d) HDL in obese and lean spontaneously hypertensive/NDmcr-cp rats fed high-fat diets rich in flax oil v. lard for 4 weeks. Values are expressed as means and standard deviations; n 5. * Mean values were significantly different from those of the respective obese rats for each diet (P < 0·05). FO, flax oil-fed obese rats (□); LO, lard-fed obese rats (▨); FL, flax oil-fed lean rats (![]() ); LL, lard-fed lean rats (
); LL, lard-fed lean rats (![]() ).
).

Fig. 2 Cholesterol concentration in (a) whole serum and fractions of (b) VLDL, (c) LDL and (d) HDL in obese and lean spontaneously hypertensive/NDmcr-cp rats fed high-fat diets rich in flax oil v. lard for 4 weeks. Values are expressed as means and standard deviations; n 5. * Mean values were significantly different from those of the respective obese rats for each diet (P < 0·05). † Mean values were significantly different from those of the respective flax oil-fed rats for each genotype (P < 0·05). FO, flax oil-fed obese rats (□); LO, lard-fed obese rats (▨); FL, flax oil-fed lean rats (![]() ); LL, lard-fed lean rats (
); LL, lard-fed lean rats (![]() ).
).
Hepatic lipid levels
Both genotype (P < 0·005, two-way ANOVA) and diet (P < 0·01, two-way ANOVA) were found to affect the hepatic TAG and cholesterol concentrations in the SHR/NDmcr-cp rats. Flax oil feeding was associated with significantly lower hepatic TAG and cholesterol concentrations in the obese rats compared with the LO rats (FO v. LO, P < 0·05; Fig. 3(a) and (b)), whereas no differences were observed between lean rats fed either a flax oil or lard diet. The reduction in the hepatic TAG and cholesterol concentrations by flax oil feeding was significant enough (67 % reduction) such that no differences were observed between the FO and FL SHR/NDmcr-cp rats, whereas LO rats had significantly higher hepatic TAG and cholesterol concentrations compared with the LL rats (LO v. LL, P < 0·05) (Fig. 3(a) and (b)).

Fig. 3 Concentrations of liver (a) TAG and (b) cholesterol in obese and lean spontaneously hypertensive/NDmcr-cp rats fed high-fat diets rich in flax oil v. lard for 4 weeks. Values are expressed as means and standard deviations; n 5. * Mean values were significantly different from those of the respective obese rats for each diet (P < 0·05). † Mean values were significantly different from those of the respective flax oil-fed rats for each genotype (P < 0·05). FO, flax oil-fed obese rats (□); LO, lard-fed obese rats (▨); FL, flax oil-fed lean rats (![]() ); LL, lard-fed lean rats (
); LL, lard-fed lean rats (![]() ).
).
Hepatic gene expression
A significant interaction between the genotype and diet affected the hepatic mRNA expression of PPAR-α (P < 0·05, two-way ANOVA). An obese genotype was associated with higher hepatic mRNA expression of PPAR-α in the LO rats compared with the LL rats (LO v. LL, P < 0·001; Fig. 4(a)). In addition, flax oil feeding was associated with higher hepatic mRNA expression of PPAR-α in the lean rats compared with the lard-fed rats (FL v. LL, P < 0·05; Fig. 4(a)).

Fig. 4 Hepatic mRNA expression of (a) PPAR-α, (b) PPAR-γ and (c) sterol regulatory element-binding protein (SREBP)-1c in obese and lean spontaneously hypertensive/NDmcr-cp rats fed high-fat diets rich in flax oil v. lard for 4 weeks. Values are expressed as means and standard deviations; n 5. * Mean values were significantly different from those of the respective obese rats for each diet (P < 0·05). † Mean values were significantly different from those of the respective flax oil-fed rats for each genotype (P < 0·05). Two-way ANOVA disclosed an interaction between genotype and diet (P < 0·05) for hepatic mRNA expression of PPAR-α. FO, flax oil-fed obese rats (□); LO, lard-fed obese rats (▨); FL, flax oil-fed lean rats (![]() ); LL, lard-fed lean rats (
); LL, lard-fed lean rats (![]() ).
).
Both genotype (P < 0·001, two-way ANOVA) and diet (P < 0·001, two-way ANOVA) were found to affect the hepatic mRNA expression of PPAR-γ. While an obese genotype was associated with higher hepatic mRNA expression of PPAR-γ in the FO and LO rats compared with their lean counterparts (FO v. FL, P < 0·001 and LO v. LL, P < 0·001), flax oil feeding was associated with higher hepatic mRNA expression of PPAR-γ in both obese and lean rats compared with the lard-fed rats (FO v. LO, P < 0·05 and FL v. LL, P < 0·01) (Fig. 4(b)). No differences were observed for the hepatic mRNA expression of SREBP-1c among various dietary groups of obese and lean rats (Fig. 4(c)).
Since flax oil feeding was specifically associated with an increase in the mRNA expression of PPAR-γ in both obese and lean SHR/NDmcr-cp rats, its correlation analysis with the hepatic TAG and cholesterol concentrations in each dietary group was performed. Interestingly, the hepatic mRNA expression of PPAR-γ correlated negatively with the hepatic TAG concentration (r − 0·98, P < 0·05) in FO SHR/NDmcr-cp rats (Fig. 5(a)). On the other hand, the hepatic mRNA expression of PPAR-γ correlated negatively with the hepatic cholesterol concentration in both FO (r − 0·99, P < 0·001) and FL rats (r − 0·97, P < 0·05) (Fig. 5(b)).

Fig. 5 Correlation analysis of hepatic mRNA expression of PPAR-γ with concentrations of (a) hepatic TAG and (b) cholesterol in the obese and lean spontaneously hypertensive/NDmcr-cp rats fed high-fat diets rich in flax oil v. lard for 4 weeks. (■), Flax oil-fed obese rats (TAG: r − 0·98, P < 0·05; cholesterol: r − 0·99, P < 0·001); (□), flax oil-fed lean rats (TAG: r − 0·87, P>0·05; cholesterol: r − 0·97, P < 0·05); (●), lard-fed obese rats (TAG: r − 0·86, P>0·05; cholesterol: r − 0·86, P>0·05); (○), lard-fed lean rats (TAG: r − 0·72, P>0·05; cholesterol: r − 0·69, P>0·05).
Urinary thiobarbituric acid-reactive substances levels
The urinary TBARS concentration measured as malondialdehyde concentration, a marker for systemic oxidative stress, was affected by a significant interaction between the genotype and diet (P < 0·05, two-way ANOVA). An obese genotype was associated with a higher urinary TBARS concentration in the LO rats compared with the LL rats (LO v. LL, P < 0·01), whereas flax oil feeding was associated with lower urinary TBARS concentration in the obese rats compared with the LO rats (FO v. LO, P < 0·001) (Fig. 6). The reduction in the urinary TBARS concentration by flax oil feeding was significant enough (75 %), such that no differences were observed between the FO and FL SHR/NDmcr-cp rats (Fig. 6).
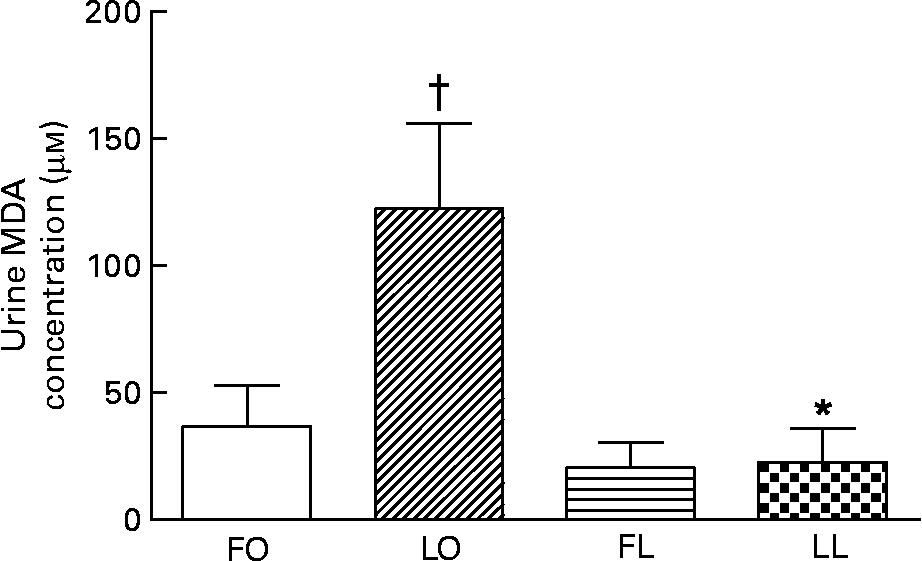
Fig. 6 Concentraion of urinary thiobarbituric acid-reactive substances, measured as malondialdehyde (MDA) concentration, in obese and lean spontaneously hypertensive/NDmcr-cp rats fed high-fat diets rich in flax oil v. lard for 4 weeks. Values are expressed as means and standard deviations; n 5. * Mean values were significantly different from those of the respective obese rats for each diet (P < 0·05). † Mean values were significantly different from those of the respective flax oil-fed rats for each genotype (P < 0·05). Two-way ANOVA disclosed an interaction between genotype and diet (P < 0·05). FO, flax oil-fed obese rats (□); LO, lard-fed obese rats (▨); FL, flax oil-fed lean rats (![]() ); LL, lard-fed lean rats (
); LL, lard-fed lean rats (![]() ).
).
Discussion
The obese SHR/NDmcr-cp rats represent a genetic model of the metabolic syndrome exhibiting obesity, insulin resistance, hepatic steatosis and enhanced oxidative stress. Recent studies have shown that the consumption of flax oil, rich in ALA, an n-3 PUFA, exerts beneficial effects on insulin resistance, dyslipidaemia, hypertension and fatty liver disease(Reference Cunnane, Gangali and Menard12, Reference Murase, Aoki and Tokimitsu14, Reference Chan, Bruce and McDonald22, Reference Ghafoorunissa, Ibrahim and Natarajan23). The present study was designed to investigate the effects of flax oil feeding on various parameters related to the metabolic syndrome such as obesity, hyperlipidaemia, insulin resistance and oxidative stress in obese and lean SHR/NDmcr-cp rats. It was demonstrated that flax oil feeding significantly reduces the hepatic concentrations of TAG and cholesterol, along with a significant reduction in the fasting insulin and 24 h urinary TBARS levels, in the obese SHR/NDmcr-cp rats. Flax oil feeding also induced a significant increase in the hepatic mRNA expression of PPAR-γ in the obese SHR/NDmcr-cp rats, which significantly correlated with a reduction in their hepatic TAG and cholesterol levels.
Liver plays a central role in regulating lipid and cholesterol metabolism in the body. The synthesis, uptake and secretion of various lipids by the liver not only regulate hepatic lipid levels, but also maintain the serum lipid levels. Disturbances in any of these pathways can lead to the accumulation of lipids in the liver, which is characterised as NAFLD, a condition very commonly associated with the metabolic syndrome(Reference Kotronen, Westerbacka and Bergholm24). According to the ‘two-hit hypothesis’ explaining the development of NAFLD(Reference Gan, Adams and Watts25), it is proposed that the ‘first hit’ results from the conditions leading to TAG accumulation in the hepatocytes, such as central obesity and insulin resistance. Other factors, such as increased de novo lipogenesis and increased postprandial TAG delivery, along with reduced mitochondrial as well as peroxisomal oxidation, further enhance the hepatic accumulation of lipids(Reference Chitturi, Abeygunasekera and Farrell26). The ‘second hit’ in the hypothesis involves the emergence and progression of inflammation, which leads to the development of non-alcoholic steatohepatitis. These mechanisms include the pre-inflammatory and pro-apoptotic effects of oxidative stress and other factors including cytokines and endoplasmic stress(Reference Browning and Horton27, Reference Ozcan, Cao and Yilmaz28).
In the present study, FO SHR/NDmcr-cp rats exhibited a significant reduction in the hepatic concentrations of TAG and cholesterol compared with the LO rats, suggesting that flax oil feeding may prove to be an important nutritional tool in the prevention of the ‘first hit’ behind the development of NAFLD. Interestingly, flax oil feeding was also associated with a significant reduction in the urinary TBARS levels in the obese SHR/NDmcr-cp rats, a marker for the systemic oxidative stress. Although the oxidative stress in the liver of these rats was not evaluated, the reduction in the overall oxidative stress levels indicates that flax oil feeding could also prevent the ‘second hit’, i.e. inflammatory development of non-alcoholic steatohepatitis.
A number of previous studies suggesting a preventive role for flax oil supplementation/feeding in the development of NAFLD have attributed its beneficial effects to the much higher content of ALA in flax oil(Reference Morise, Mourot and Riottot29–Reference Vijaimohan, Jainu and Sabitha31). It is reported that ALA-rich flax oil can act as a better substrate for mitochondrial and peroxisomal β-oxidation, thus stimulating increased oxidation of lipids in the liver(Reference Murase, Aoki and Tokimitsu14, Reference Ide, Kobayashi and Ashakumary32). In addition, flax oil is also proposed to suppress fatty acid synthesis, thus inhibiting the accumulation of lipids in the liver(Reference Murase, Aoki and Tokimitsu14). The regulation of hepatic lipid metabolism is mediated by a variety of transcription factors, such as SREBP-1c, PPAR-α and PPAR-γ. The SREBP-1c regulates the expression of genes involved in the synthesis of fatty acids and cholesterol(Reference Osborne33). Flaxseed lignan supplementation, but not flax oil supplementation, has previously been reported to lower the hepatic expression of SREBP-1c, thus inhibiting fatty acid and cholesterol synthesis by the liver and hence reducing the hepatic lipid levels(Reference Fukumitsu, Aida and Ueno34). No differences were, however, observed in the hepatic expression of SREBP-1c levels among flax oil-fed or lard-fed SHR/NDmcr-cp rats, suggesting that flax oil feeding was not involved in the down-regulation of SREBP-1c expression in the obese SHR/NDmcr-cp rats.
PPAR-α is known to regulate the expression of genes involved in the peroxisomal proliferation and β-oxidation of fatty acids(Reference Braissant, Foufelle and Scotto35, Reference Shalev, Siegrist-Kaiser and Yen36). An up-regulation of PPAR-α can lead to increased peroxisomal oxidation of fatty acids which can reduce the accumulation of lipids in the liver, a mechanism that has been previously proposed for the lipid-lowering effects of flax oil supplementation(Reference Vijaimohan, Jainu and Sabitha31). A significant increase in the mRNA expression of liver PPAR-α was observed in the FL rats compared with the LL rats, but not in the FO rats. These findings suggest that the expression of PPAR-α is not associated with reduced hepatic lipid levels in case of the obese SHR/NDmcr-cp rats.
Another member of the PPAR family of transcription factors, PPAR-γ, is predominantly expressed in adipose tissue, while being expressed in very low amounts in the liver(Reference Braissant, Foufelle and Scotto35). Although PPAR-γ is principally expressed in adipose tissue, there is increasing evidence for its up-regulation in the liver, which is associated with obesity and the fatty liver condition(Reference Vidal-Puig, Jimenez-Liñan and Lowell37–Reference Rahimian, MasihKhan and Lo41). However, it remains to be established whether the increased expression of PPAR-γ in the liver causes fatty liver or whether it is the fatty liver condition that up-regulates the expression of PPAR-γ as a restorative mechanism. A significant increase in the hepatic mRNA expression of PPAR-γ was observed in FO SHR/NDmcr-cp rats compared with the LO rats. A similar up-regulation was also observed in the FL SHR/NDmcr-cp rats compared with the LL rats. The reduction in the hepatic TAG and cholesterol levels observed in the FO SHR/NDmcr-cp rats was selectively correlated with an up-regulation of their hepatic mRNA expression of PPAR-γ, thus pointing to an association between the hepatic expression of PPAR-γ and reduction in liver lipids, which may prove beneficial in the prevention of NAFLD.
A recent study reported a significant reduction in the hepatic lipids along with a significant up-regulation in the hepatic mRNA expression of PPAR-γ in ob/ob mice when treated with troglitazone(Reference Memon, Tecott and Nonogaki42). Interestingly, in this study, the hepatic expression of PPAR-γ was much more pronounced than the hepatic expression of PPAR-α, which is similar to our observations. Reduction of liver lipid content in ob/ob mice by troglitazone treatment suggests that PPAR-γ activators may increase the utilisation of lipids in the liver of obese diabetic mice. In addition, other PPAR-γ activators such as candesartan have also been shown to improve insulin resistance by promoting the expression of PPAR-γ in liver and adipose tissue in Wistar rats(Reference Yan, Dou and Pan43). Furthermore, a recent study by Kelley et al. (Reference Kelley, Vemuri and Adkins44) reported that flax oil is associated with the prevention of conjugated linoleic acid-induced insulin resistance and fatty liver in C57Bl/6 mice. Thus, it seems plausible that flax oil mediates its beneficial effects by activating the hepatic expression of PPAR-γ, which perhaps enhances the insulin sensitivity of the liver as well as of the peripheral tissues, similar to the glitazone family of insulin sensitisers. We observed that flax oil feeding was associated with a significant reduction in plasma insulin concentration in the obese SHR/NDmcr-cp rats compared with the LO rats, which appears to support this proposal.
In addition, flax oil feeding was associated with lower LDL-cholesterol concentration in lean SHR/NDmcr-cp rats compared with the LL rats. However, no effects of flax oil feeding were observed for serum and lipoprotein TAG and cholesterol concentrations in the obese rats. These observations are in line with the previous observations, where flax oil did not affect the serum and lipoprotein TAG and cholesterol concentrations in human subjects(Reference Schwab, Callaway and Erkkilä45, Reference Kaul, Kreml and Austria46) and hypercholesterolaemic rabbits(Reference Lee and Prasad47). On the other hand, flaxseed, as opposed to flax oil, supplementation was shown to lower serum TAG and cholesterol concentrations in human subjects(Reference Edralin, Robert and Lisa48). Flaxseed contains both n-3 fatty acids and lignans; flaxseed lignans alone have previously been shown to lower serum lipids in hyperlipidaemic rats(Reference Felmlee, Woo and Simko49). Thus, the presence of lignans could have contributed to the lipid-lowering properties of flaxseed supplementation.
In conclusion, the present study reports that flax oil feeding is associated with a reduction of hepatic lipid accumulation in obese SHR/NDmcr-cp rats, which represent a genetic model of the metabolic syndrome. An increase in the hepatic mRNA expression of PPAR-γ by flax oil feeding showed a negative correlation with hepatic lipid levels in the obese SHR/NDmcr-cp rats. Furthermore, flaxseed oil feeding also lowered serum insulin levels and systemic oxidative stress in obese SHR/NDmcr-cp rats. Thus, it may be proposed that flax oil-mediated activation of PPAR-γ and its insulin-sensitising effects result in the reduction of hepatic lipid accumulation in the obese rats. Future studies are required to prove this proposal.
Acknowledgements
This work was supported by the Open Research Center Project of Mukogawa Women's University, Hyogo, Japan; the Japanese Society for the Promotion of Science (JSPS), Matsumae International Foundation (MIF), Tokyo, Japan; and the Natural Sciences and Engineering Research Council of Canada and Canadian Innovation Fund. S. K. C. is a JSPS fellow and K. C. is a MIF fellow. K. C. was involved in the design of the study, execution of the experiments, data analysis and preparation of the manuscript; N. Y. helped with the animal feeding and other experiments; K. I. and Y. Y. were involved in the conception and execution of the study; and S. K. C. was involved in the conception, design and execution of the study, along with preparation and revisions of the manuscript. The present study represents the original work that has not been published previously, and is not presently being considered by another journal, and that if accepted for the British Journal of Nutrition, will not be published elsewhere in the same form, in English or in any other language, without the written consent of the Nutrition Society. All the procedures used were approved by the Mukogawa Women's University Animal Care Committee. The authors would like to disclose that there are no financial or other contractual agreements that might cause conflicts of interest or be perceived as causing conflicts of interest.












