Cancer is one of the leading causes of mortality in the world and treatment and management of cancer cases is placing a substantial burden on health-care systems globally(Reference Fitzmaurice, Allen and Barber1). While cancer-specific mortality is generally declining in high-income countries due to improved early detection, reduction in active and passive smoking, and access to improved and targeted treatments(Reference Siegel, Miller and Jemal2,Reference Arnold, Sierra and Laversanne3) , the number of deaths from cancers is increasing in low- and middle-income countries(Reference Fitzmaurice, Allen and Barber1,Reference Sankaranarayanan, Swaminathan and Brenner4) . Africa has among the lowest cancer survival rates in the world. According to the International Agency for Research on Cancer’s Global Cancer Observatory (GCO) databases issued in 2018, over one million cases of cancer, excluding skin cancer, were diagnosed in Africa, among which over 690 000 individuals died from the disease(Reference Bray, Ferlay and Soerjomataram5). It is estimated that based solely on current trends in incidence and an aging population, the annual number of deaths from cancer in Africa will be close to a million by 2030(Reference Sylla and Wild6,Reference Bray, Jemal and Grey7) .
Primary prevention of cancer is a major public health priority alongside other strategies such as lowering cancer treatment costs, and increasing and improving routine screening facilities, to lessen cancer incidence and mortality in the coming years. At least one-third of the cancer cases in Africa are potentially preventable by lifestyle changes including higher engagement in protected sex and vaccination against specific oncogenic infections, avoidance of tobacco use, weight control, increased physical activity, reduction of alcohol consumption and the promotion of a healthy, balanced diet(Reference Stein and Colditz8). Dietary recommendations for reducing cancer risk, based on studies from high-income countries, generally include increased intake of fruits, vegetables and wholegrain foods, and lower intake of red and processed meats, salt-preserved foods, high-sugar and high-fat foods, sweetened and alcoholic beverages(Reference Norat, Scoccianti and Boutron-Ruault9). Based on the current nutrition transition in Africa, with the progressive replacement of traditional plant-based diets by a Westernised diet rich in fats and added sugars and low in dietary fibres(Reference Hawkes10–Reference Kosulwat14), cancer incidence and mortality are likely to increase in the coming years as observed earlier in other countries(Reference Zhang, Dhakal and Zhao15). Despite these projections, there is a critical lack of epidemiological data on the relationship between diet, overall lifestyle and cancer development and survival in the African population to target cancer research and prevention among particularly vulnerable populations.
In the absence of specific epidemiological studies requiring collection of individual-level data on diet and other cancer risk factors as well as cancer end points, ecological analyses can be useful in identifying how the prevalence of specific dietary risk factors correlates with cancer incidence at the population level. For example, early ecological studies(Reference Armstrong and Doll16) revealed that consumption of meat products and animal fats was correlated with certain cancer types, thus providing important preliminary data that led to a substantial body of experimental and epidemiological studies to later confirm the hypothesis. The expansion of cancer registries worldwide and the improvement of diagnostic tools have rendered ecological studies an attractive approach to reveal and monitor specific associations of environmental and lifestyle parameters with cancer trends.
The current analysis aimed to evaluate changes in population-level dietary factors and their association with cancer incidence across eighteen sub-Saharan African countries in which both cancer registry and dietary data are available.
Methods
Cancer incidence data
Cancer sites selected for the present study were those for which diet has been suggested as an important aetiological factor and include cancers of the colon and rectum, thyroid, oesophagus, pancreas, stomach, breast and prostate(17). Cancer types with a strong link to infections (e.g. cervical) or those with a specific environmental exposure risk factor (e.g. skin cancer) or those with very low incidence rates (e.g. male breast cancer) were not considered in the analysis. Cancer incidence data were obtained from population-based cancer registries affiliated to the African Cancer Registries Network. The African Cancer Registries Network provides the facilities and features of a regional hub for sub-Saharan Africa, as part of the Global Initiative for Cancer Registration (GICR) coordinated by the International Agency for Research on Cancer. Cancer data were presented as age-standardised incidence rates (ASR) per 100 000 person-years. The method of calculation of the ASR was identical to that applied for GLOBOCAN databases and the Cancer in Sub-Saharan Africa report(18,Reference Parkin, Ferlay and Jemal19) . Briefly, an ASR is calculated using reported cancer cases in the population covered by a registry considering the world standard population(Reference Doll, Payne and Waterhouse20). ASR are utilised because they allow the comparison of incidence across populations without regard to age, a strong determinant for several cancers. In the case that multiple population-based cancer registries are established in a country, a national ASR was estimated as the weighted average of the recorded ASR obtained from the multiple cancer registries(Reference Bray, Ferlay and Soerjomataram5). Cancer registries and country-specific data available from the eighteen African countries (see the online supplementary material, Supplemental Fig. S1) considered are summarised in Table 1. The years of collection of the cancer data are country-specific and span from 2001 to 2015.
Table 1 Characteristics of the cancer registries for the eighteen African countries included in the analysis
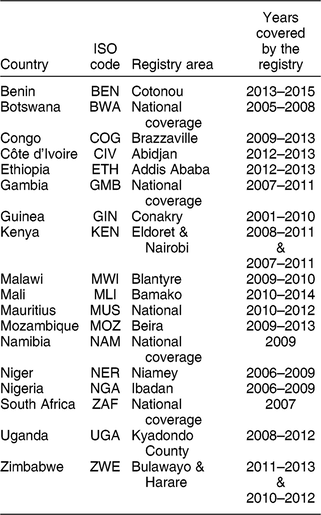
ISO, International Organization for Standardization.
Dietary data
Major food group supply data were obtained from the food balance sheets of the FAO (http://www.fao.org/faostat). Food balance sheets are prepared from an established calculation method using individual country’s domestic production, importation and exportation data, with adjustment for agricultural seeds, animal feeds and potential losses during storage(21). Food balance sheets data are available for all the African countries and are updated yearly since 1961. Data include per country and per capita estimations for major foods and food groups available for human consumption, as well as energy, proteins, fats and carbohydrates values drawn from these food groups. Food items considered for our analysis were: animal products (meat, red meat, animal fats), plant products (cereals, starchy roots, vegetables, fruits, sugar, vegetable oils) and alcoholic beverages. Data on energy availability from animal sources and plant products, as well as total energy (plant plus animal sources of energy) and the ratio of animal energy to plant energy, were additionally considered for the analysis. The description of the food commodities is presented in the online supplementary material, Supplemental Table S1. Data available in the food balance sheets were presented either as kilograms per capita per year or converted to kilocalories per capita per day to recover the energy contribution of the food considered. As the time between ‘exposure’ to any diet as a risk or protective factor and tumour development can vary from 5 to over 20 years(Reference Grant22), we considered five specific latency time points: 0 (years covered by the cancer registry, as a ‘reference’) and 5, 10, 15 and 20 preceding years (T 0, T –5, T –10, T –15 and T –20, respectively). We hypothesised that the strength of the correlations between food availability and cancer incidence might follow a particular trend starting from the ‘reference’ towards the time points (respectively, T –5, T –10, T –15, T –20). In the cases where the cancer data spanned several years, we used averaged food groups data for the analysis.
Covariates
Country-specific smoking data were collated from the WHO’s Global Health Observatory (GHO; http://www.who.int/gho/tobacco/use/en/). The national human development index for each country was sourced from the United Nations Development Programme’s database (http://hdr.undp.org/en/content/human-development-index-hdi). Mean BMI data were obtained from the Non-Communicable Diseases Risk Factor Collaboration consortium (http://ncdrisc.org/). Smoking was considered an important covariate because it is a major risk factor for the development of several of the cancers of interest in the present analysis(Reference Sasco, Secretan and Straif23). National human development indices were considered a proxy for quality of life, associated with life expectancy at birth, adult literacy rate and national gross income per capita. Likewise, BMI was considered as a covariate because it is a risk factor for thirteen cancers(Reference Lauby-Secretan, Scoccianti and Loomis24) and is associated with diet and lifestyle.
Statistical analysis
Food group availability data and cancer ASR were log-transformed to achieve normality. The relationship between food availability and cancer ASR was determined by calculating Pearson partial correlation coefficients in men and women separately, at each time point, using the PCORRMAT command in Stata(Reference Buis25). The correlation coefficients were adjusted for smoking rate, human development index and mean BMI. Comparison between food data across years was performed using a paired t test. In a subsequent analysis, we replaced mean BMI by the proportion of the population with BMI above 30·0 kg/m2 and the smoking rate by the average number of cigarettes per capita. We additionally applied the Benjamini–Hochberg procedure(Reference Benjamini and Hochberg26) to correct for multiple comparisons. We considered P values below 0·05 to be statistically significant. All analyses were performed using the statistical software package Stata version 14.
Results
The ASR (per 100 000 person-years) for the cancer sites considered per country and by gender are presented in Table 2. Overall, prostate and breast cancers were the most diagnosed cancers in men and women, respectively. Mauritius showed the highest incidence for colorectal cancer in both men (ASR = 14·9/100 000 for 2010–2012) and women (ASR = 11·1/100 000 for 2010–2012), and for breast cancer (ASR = 51·7/100 000). The incidence rates for oesophageal cancer were higher in Eastern–Southern African countries (Kenya, Malawi, Uganda and Zimbabwe) compared with other regions. The incidence of thyroid, pancreatic and stomach cancers was low in all countries expect in Mali where stomach cancer incidence was higher (in men, ASR = 19·1/100 000 for 2010–2014; in women, ASR = 15·3/100 000).
Table 2 Age-standardised rates (/100 000 person-years) of the cancers in the eighteen African countries considered in the analysis
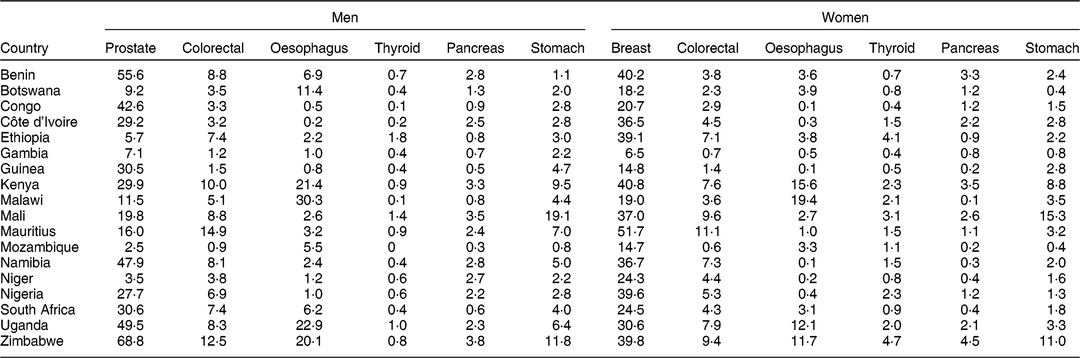
Table 3 summarises mean values for food group data for the countries considered in the current analysis. Mean energy availability for the preceding 20 years (T –20) was 9142 kJ/capita per d (2185 kcal/capita per d), and increased by 883 kJ/capita per d (211 kcal/capita per d) between T –20 and T 0. While this increase in energy availability was due to greater supply of energy from both plant and animal sources (P comparing T –20 and T 0 for both animal and plant energy <0·0001), the sharp increase in the average ratio of animal to plant energy in 20 years (T –20 = 0·073; T 0 = 0·087; P for difference <0·001) suggests that the proportion of animal products consumed, compared with plant products, increased significantly in Africa over the past two decades.
Table 3 Average food and energy availability over time for the eighteen African countries included in the analysis
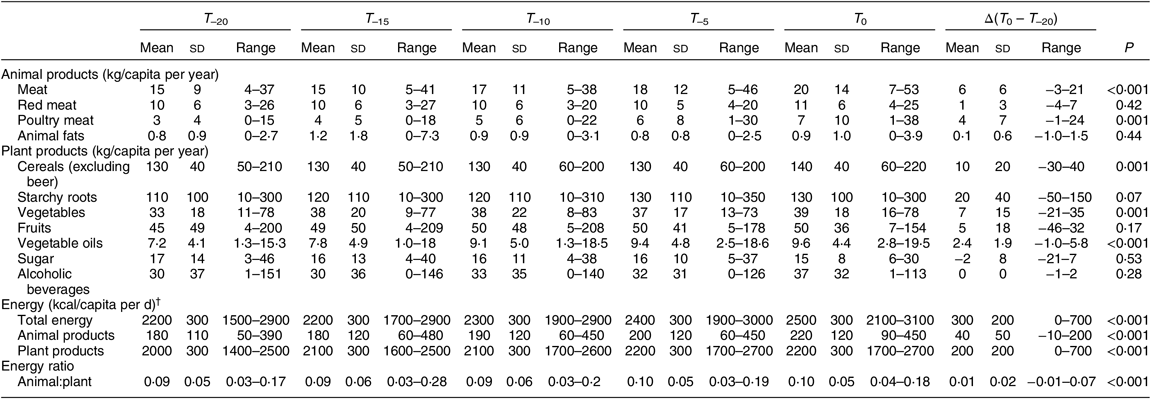
T 0, time of collecting cancer age-standardised cancer rates; T –5, T –10, T –15 and T –20, 5, 10, 15 and 20 preceding years, respectively.
† To convert to kJ, multiply kcal values by 4·184.
Table 4 presents the results of the correlations of food groups and energy availability with the ASR of cancer. Red meat was significantly positively correlated with pancreatic cancer in men (for T –20: r –20 = 0·61, P < 0·05), stomach cancer in women (for T 0: r 0 = 0·58, P < 0·05) and colorectal cancer (Fig. 1) in both men (for T –20: r –20 = 0·53, P < 0·05) and women (for T 0, T –5, T –20: respectively r 0 = 0·63, r –5 = 0·58, r –20 = 0·58, all P < 0·05), whereas poultry meat did not show any significant associations. Meat availability was significantly positively correlated with colorectal cancer in men (for T 0, T –5, T –20: respectively r 0 = 0·72, P < 0·01; r –5 = 0·60, P < 0·05; r –20 = 0·64, P < 0·01) and women (for T –5, T –20: respectively r –5 = 0·54, r –20 = 0·54, all P < 0·05). Moreover, meat availability was also positively correlated in men to pancreatic cancer (for T 0, T –5, T –15 T –20: respectively r 0 = 0·59, r –5 = 0·61, r –15 = 0·58, all P < 0·05; r –20 = 0·75, P < 0·01) and stomach cancer (for T 0: r 0 = 0·55, P < 0·05), and in women to breast cancer (for T –5: r –5 = 0·54, P < 0·05). Overall, availability of animal products including meat, animal fats and energy from animal sources tended to be positively correlated with colorectal, pancreas, stomach, thyroid and breast cancer.
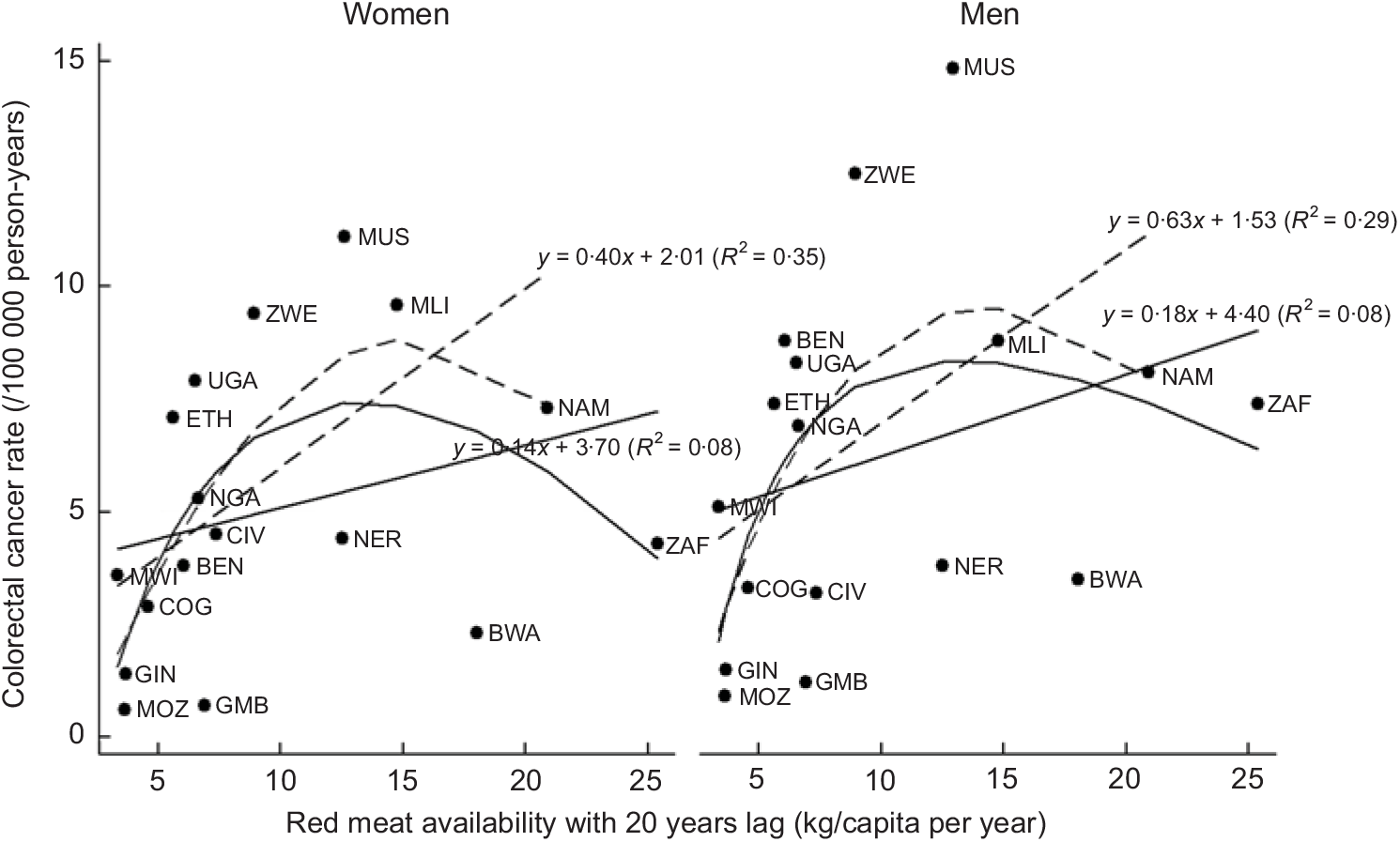
Fig. 1 Red meat and colorectal cancer in eighteen African countries. Solid lines represent data for all eighteen countries, whereas the dashed lines represent the data with outlier countries excluded (Mauritius, South Africa and Botswana). The relationships are presented as a linear or as a polynomial fit. Countries are presented with their ISO (International Organization for Standardization) codes (see Table 1 for explanation)
Table 4 Ecological partial correlation (r) between age-standardised rates of cancers and availability of food and energy, adjusted for human development index, smoking and obesity rates, for the eighteen African countries included in the analysis
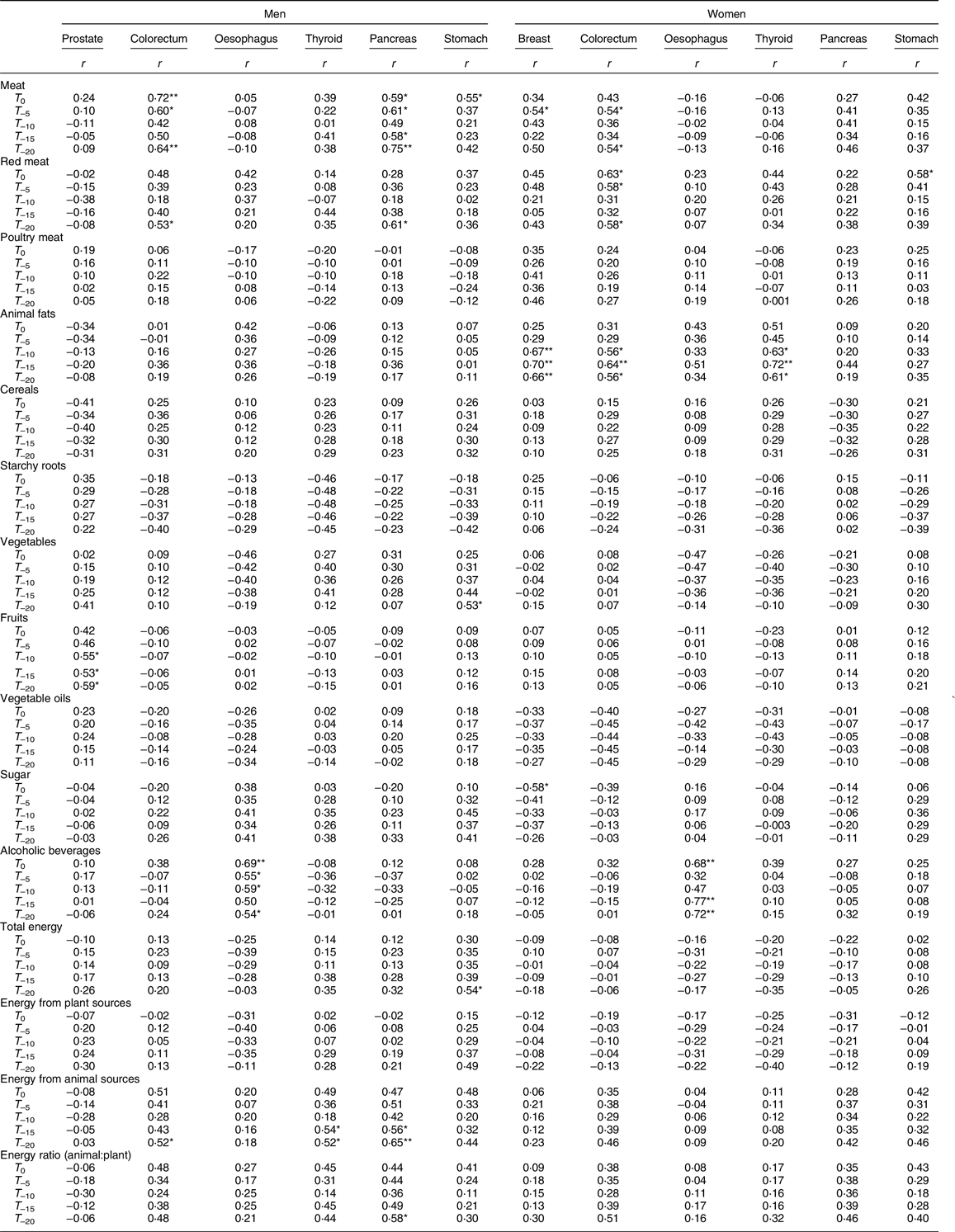
T 0, time of collecting cancer age-standardised cancer rates; T –5, T –10, T –15 and T –20, 5, 10, 15 and 20 preceding years, respectively.
* P < 0·05, **P < 0·01.
Although not statistically significant, starchy roots showed a negative correlation with ASR for colorectal and thyroid cancer in both men and women. In men, energy from animal sources was significantly positively correlated to thyroid, colorectal and pancreatic cancer rates; and higher energy supply from animal sources over plant-based sources was positively correlated with pancreatic cancer (for T –20: r –20 = 0·58, P < 0·05). In both men and women, alcoholic beverages were positively correlated to oesophageal cancer incidence. The correlation coefficients between alcoholic beverages and oesophageal cancer were: in men, for T 0 (r 0 = 0·69, P < 0·01), T –5 (r –5 = 0·55, P < 0·05), T –10 (r –10 = 0·59, P < 0·05) and T –20 (r –20 = 0·54, P < 0·05); in women, for T 0 (r 0 = 0·68, P < 0·01), T –15 (r –15 = 0·77, P < 0·01) and T –20 (r –20 = 0·72, P < 0·01).
In a stepwise approach, we verified whether the inclusion or exclusion of countries with extreme food availability or cancer data might modify the trend of the correlations and found no apparent differences (results not shown). When we applied the conservative Benjamini–Hochberg procedure to correct for false discovery, none of the correlations remained significant (results not shown).
Discussion
Availability of all major food groups and total energy supply have increased over the preceding 20 years in Africa. In our analysis, we found that the amount of red meat available at the national level was positively correlated with colorectal cancer incidence. We also found that the availability of meat, as well as that of animal fats and energy from animal sources in general, tended to be positively correlated with colorectal, breast, pancreatic, thyroid and stomach cancer. In contrast, starchy roots tended to be inversely associated with colorectal cancer as well as thyroid cancer incidence. These ecological data support the hypothesis that an increase in the consumption of animal-derived products and a concomitant reduction in the traditional plant-based diet are potentially driving the rising incidence of colorectal mainly and other cancers in many sub-Saharan African countries. In addition, the availability of alcoholic beverages showed a positive correlation with the incidence of oesophageal cancer.
Foods positively associated with cancer
The role of red meat and animal products in cancer development, particularly colorectal cancer, has been extensively investigated in both epidemiological studies and experimental models(Reference Aune, De Stefani and Ronco27,Reference Genkinger and Koushik28) . Indeed, the World Cancer Research Fund International and the European Code against Cancer have concluded there is convincing evidence that the consumption of red meat increases the risk for colorectal cancer(Reference Norat, Scoccianti and Boutron-Ruault9,17) . Likewise, the International Agency for Research on Cancer’s monograph on red and processed meat concluded that processed meat is carcinogenic and red meat is probably carcinogenic to men(Reference Bouvard, Loomis and Guyton29). Given the background of experimental and observational evidence linking red and processed meat consumption and colorectal cancer, it is noteworthy that our ecological analysis showed a positive correlation between availability of red meat and animal fats and colorectal cancer incidence. Colorectal cancer rates have been rising in Africa in recent years(Reference Graham, Adeloye and Grant30,Reference Irabor31) ; it is possible that increases in the consumption of red meat and animal fats are, in part, driving this trend. Most of the African countries are experiencing the nutrition transition at a faster pace, and therefore are shifting rapidly from traditional foods towards predominantly animal-sourced foods and processed and highly processed diets, which results in a rise in the prevalence in obesity and diet-related non-communicable diseases, including cancers(Reference Popkin and Du12–Reference Kosulwat14). Red meat and meat products intake may also be associated with other cancers including breast, pancreatic, stomach or thyroid cancer, but the associations require additional evidence(Reference Wie, Cho and Kang32–Reference Zheng, Guinter and Merchant34). The absence of significant correlations between poultry meat and risk of cancers reinforces the importance of red meats as the major animal source most likely to influence the risk for cancers. Further research into the extent to which the shift from traditional diets towards animal-based diets is related to cancer development and particularly colorectal cancer in Africa is warranted.
We found that increased energy availability from animal sources over vegetal sources was positively correlated to pancreatic cancer in men. Similarly, previous studies have also reported a significant association between animal fat availability and pancreatic cancer incidence in thirty-five upper-middle-income and high-income countries(Reference Zhang, Zhao and Berkel35), or mortality in twenty-nine countries(Reference Ghadirian, Thouez and PetitClerc36). However, unlike for colorectal cancer, epidemiological studies have reported discrepant results regarding the association between the intake of animal products and pancreatic cancer. In one of the largest studies conducted to date, the National Institutes of Health–AARP Diet and Health Study reported a positive relationship between animal fat consumption and pancreatic cancer risk(Reference Thiebaut, Jiao and Silverman37). In contrast, a more recent meta-analysis including nineteen studies (thirteen case–control and six cohort studies) reported no clear association between animal fat consumption and pancreatic cancer(Reference Shen and Yao38). Further, the mechanisms linking animal fat, meat and pancreatic cancer are less well defined than for colorectal cancer. Overall, more specific research is needed to further investigate the role of animal fats, meat and this malignancy in Africa.
In Africa, there exists an ‘oesophageal cancer corridor’, stretching from Ethiopia to South Africa and including the Rift Valley, which is characterised by an exceptionally high prevalence of this malignancy. The reasons underlying the high prevalence of oesophageal cancer in this region are not fully elucidated but may include micronutrient deficiencies(Reference Schaafsma, Wakefield and Hanisch39) and consumption of very hot beverages in addition to smoking and infection by human papilloma virus(Reference Patel, Wakhisi and Mining40). High levels of alcohol consumption have been linked also to oesophageal cancer, particularly squamous cell carcinoma, and a recent study in western Kenya estimated that half of the oesophageal cancer burden can be attributed to alcohol intake, particularly the routine consumption of locally brewed beer-like alcoholic drinks and distilled spirits(Reference Menya, Kigen and Oduor41). Our results corroborate these findings and suggest that increased availability of alcoholic beverages might partially explain the higher incidence of oesophageal cancer in Africa.
Foods inversely associated with cancer
Traditional African diets are plant-based, rich in fibres from cereals (e.g. maize, millet, sorghum, teff) and starchy roots (e.g. cassava, yam, sweet potato, taro). Kane-Diallo et al.(Reference Kane-Diallo, Srour and Sellem42) showed that a predominantly pro-plant-based diet was inversely associated with overall cancer risk, which corroborates our findings. In our analysis we also found that the availability of starchy roots tended to be inversely associated with colorectal as well as thyroid cancer incidence. A role for dietary fibre in the development of colorectal cancer was first proposed by Burkitt(Reference Burkitt43) in his investigations comparing stool weight and colorectal cancer incidence in European and rural African populations. Since the publication of Burkitt’s work, the incidence of colorectal cancer has risen substantially in many regions of Africa and, for example, has more than doubled in Kampala in Uganda over the past four decades. The World Cancer Research Fund International has classified high consumption of foods containing dietary fibre to provide a convincing decreased risk for colorectal cancer(17). Mechanisms underlying the link between dietary fibre intake and colorectal cancer include the reduction of intestinal transit time and an increase in the consistency of faeces(Reference Aune, Chan and Greenwood44). In addition, a diet rich in fibre may be protective against colorectal cancer because of alterations in bile acid metabolism, improvements in insulin sensitivity or beneficial modifications to the gut microbiota(Reference Simpson and Campbell45). Interestingly, a recent ‘diet-swap’ intervention study conducted in rural Africans and urban African-Americans demonstrated that uptake of a traditional African diet high in dietary fibre and lower in meat and animal fat reduced colon cell proliferation and inflammation while promoting changes in the gut microbiota that would hypothetically lower colorectal cancer risk(Reference O’Keefe, Li and Lahti46).
Strengths and limitations
The present study has a number of limitations. First, the approach used is ecological and is subject to ‘ecological fallacy’, suggesting that the findings may not necessarily be extrapolated to the individual level. Furthermore, due to the large number of correlation tests, some associations may have occurred by chance. We sought to address this by using a conservative method to control for multiple tests and while none of the associations survived such a correction, we note that many of the correlations were consistent with epidemiological evidence from studies conducted in other regions of the world, suggesting that they may be valid. An additional limitation was the use of FAO food balance sheets as a proxy for individual-level food consumption. Data from food balance sheets do not necessarily reflect individual food consumption, but rather an approximation of food consumption, and should therefore be interpreted with caution. Apart from animal fats and energy from animal sources that showed specific patterns in the strength of the correlation coefficients across time periods, with the strongest correlations found over longer latency periods, the overall picture was not clear for other food groups, which may be due to the quality of cancer, food or covariate data. The quality of the data may vary from one food group to another, alcoholic beverages being one group often poorly reported. Further, the FAO data do not include foods and beverages produced domestically. In addition, it should be noted that multi-layered disparities in food consumption between rural v. urban dwellers, low v. high educated individuals and men v. women and children, among others, are particularly pronounced in Africa. In general, rural dwellers and often lower educated individuals consume a more traditional diet and have physically active working conditions, and men tend to eat more meat and animal products than women(Reference Ntandou, Delisle and Agueh47–Reference White, Shoemaker and Park49). Nevertheless, these disparities cannot be captured through food balance sheets that provide information only at a country level.
On the other hand, cancer incidence data derived from local registries in Africa can be of variable quality, are not always nationally representative, and the quality of the data is not necessarily uniform across cancer types. For example, in our case, the incidence of prostate cancer is 2·5 and 68·8 in Mozambique and Zimbabwe, respectively, two neighbouring countries. Across Africa, there is a high disparity in cancer incidence between countries which may be a result of data quality as well as reflecting true variation. Improvement in cancer registration to parallel the establishment of robust epidemiological studies in African countries is now required. To address the potential impact of data quality on our results, we tested, for each cancer site included in the study, the influence of extreme national ASR on the results by removing the data from each country in a stepwise fashion and observed similar trends – albeit the significance of some of the results was lost as a result of smaller sample size. Another potential limitation is the lack of data on confounding factors such as physical activity, sedentary behaviour and other lifestyle and environmental factors that may also have changed over the years evaluated in the current analysis. Despite the fact that most cancer incidence is higher in HIV patients(Reference Jensen, Oette and Haes50), we did not consider HIV infection as a confounder in our analysis because it has been shown previously that African countries with higher HIV infection rates likely have an increased burden of non-communicable diseases, in a situation of coexistence(Reference Angkurawaranon, Nitsch and Larke51).
Epidemiological studies that collect data at the individual level are urgently needed to investigate the relationship between the nutrition transition, diet and cancer in Africa. A number of epidemiological studies are gradually being established in Africa to investigate nutritional and lifestyle factors and cancer. For example, the South Africa Breast Cancer project (SABC; http://sabc.iarc.fr/)(Reference Jacobs, Taljaard-Krugell and Ricci52) and the Study of Determinants of Breast Cancer in Morocco (EDSMAR; http://edsmar.iarc.fr/) are among the first studies that aim to investigate the influence of diet, obesity and metabolic health on cancer incidence in Africa.
Conclusion
In conclusion, the present ecological study is the first to report on the association between food availability and changes in cancer incidence in Africa. More research and high-quality epidemiological studies are needed to better understand the effects of the food environment, lifestyle, and other social and cultural aspects of the diet in Africa and their role in cancer development. Given the lack of economic resources in many regions of Africa, generating more evidence to support cancer prevention strategies within the African context should be considered a priority.
Acknowledgements
Acknowledgements: The authors would like to thank Mr Eric Masuyer and Mr Jacques Ferlay, from the Cancer Surveillance Section at the International Agency for Research on Cancer, for their support with the cancer database and the calculations of the age-standardised cancer rates. Financial support: This study was undertaken during the tenure of the postdoctoral fellowship of E.K.A., from the International Agency for Research on Cancer, partially supported by the European Commission FP7 Marie Curie Actions–People–Cofounding of Regional, National and International Programmes (COFUND). No funding was received for this study. Conflict of interest: None. Authorship: M.J.G. proposed the study concept and supervised the whole study process. F.B. provided the cancer data through the African Cancer Registry Network. M.J.G., F.B., P.F., N.S. and F.Z. proposed the design of the study. E.K.A. analysed the data under the supervision of P.F. and drafted the manuscript. V.C. and I.H. provided expertise comments on the quality and the interpretation of the dietary data, and specific aspects of the findings. All the authors have read, contributed to and approved the final manuscript. Ethics of human subject participation: Not applicable. Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980019000831
Author ORCID
Elom K Aglago, 0000-0002-0442-3284.
Appendix
Members of the African Cancer Registry Network
Dr Max Parkin, Coordinator of the African Cancer Registry Network; Professor Dismand Houinato, Programme National de Lutte contre les Maladies Non Transmissibles (Bénin); Dr Malebogo Kebabonye-Pusoentsi, Botswana National Cancer Registry (Botswana); Professor Charles Gombe, Registre des Cancers de Brazzaville (Congo); Dr Guy N’da, Registre des Cancers d’Abidjan (Côte d’Ivoire); Dr Mathewos Assefa, Addis Ababa Cancer Registry (Ethiopia); Dr Lamin Giana, Gambia Cancer Registry (Gambia); Dr Baffour Awuah, Kumasi Cancer Registry (Ghana); Professor Moussa Koulibaly, Registre de Cancer de Guinée (Guinea); Dr Nathan Buziba, Eldoret Cancer Registry (Kenya); Dr Anne Korir, Nairobi Cancer Registry (Kenya); Dr Charles Dzamalala, Malawi Cancer Registry (Malawi); Dr Shyam Manraj, Mauritius Cancer Registry (Mauritius); Dr Josefo Ferro, Registro de Cancro de Beira (Mozambique); Dr Reinette Koegelenberg, Namibian National Cancer Registry (Namibia); Professor Hassan Nouhou, Registre des Cancers du Niger (Niger); Dr Festus Egbinoba, Abuja Cancer Registry (Nigeria); Professor Ima-Obong Ekanem, Calabar Cancer Registry (Nigeria); Professor Olufemi Ogunbiyi, Ibadan Cancer Registry (Nigeria); Professor Clement Adebamowo, Nigerian National System of Cancer Registries (Nigeria); Ms Anne Finesse, Seychelles National Cancer Registry (Seychelles); Ms Nontuthuzelo Somdyala, Eastern Cape Province Cancer Registry (South Africa); Professor Cristina Stefan, South African Children’s Cancer Group (SACCSG) Tumour Registry (South Africa); Dr Elvira Singh, South African National Cancer Registry (South Africa); Professor Henry Wabinga, Kampala Cancer Registry (Uganda); Mr Eric Chokunonga, Zimbabwe National Cancer Registry (Zimbabwe).







