Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by Crossref.
Yngve, Agneta
Haapala, Irja
Hodge, Allison
McNeill, Geraldine
and
Tseng, Marilyn
2012.
Interpreting success and failure in food fortification.
Public Health Nutrition,
Vol. 15,
Issue. 10,
p.
1789.
Malpeli, Agustina
Ferrari, María Guillermina
Varea, Ana
Falivene, Mariana
Etchegoyen, Graciela
Vojkovic, María
Carmuega, Estéban
Disalvo, Liliana
Apezteguía, María
Pereyras, Silvia
Tournier, Andrea
Vogliolo, Daniel
and
Gonzalez, Horacio F.
2013.
Short-Term Evaluation of the Impact of a Fortified Food Aid Program on the Micronutrient Nutritional Status of Argentinian Pregnant Women.
Biological Trace Element Research,
Vol. 155,
Issue. 2,
p.
176.
Salam, Rehana A.
Das, Jai K.
and
Bhutta, Zulfiqar A.
2014.
Multiple Micronutrient Supplementation during Pregnancy and Lactation in Low-to-Middle-Income Developing Country Settings: Impact on Pregnancy Outcomes.
Annals of Nutrition and Metabolism,
Vol. 65,
Issue. 1,
p.
4.
Roks, Eveline
2014.
Review of the cost components of introducing industrially fortified rice.
Annals of the New York Academy of Sciences,
Vol. 1324,
Issue. 1,
p.
82.
Serdula, M K
Nichols, E K
Aburto, N J
Masa'd, H
Obaid, B
Wirth, J
Tarawneh, M
Barham, R
Hijawi, B
Sullivan, K M
Haddadin, A
Khatib, I
Al-Sanouri, T M
Galvez, J
Faqih, A
and
Whitehead, R D
2014.
Micronutrient status in Jordan: 2002 and 2010.
European Journal of Clinical Nutrition,
Vol. 68,
Issue. 10,
p.
1124.
Dwyer, Johanna T
Wiemer, Kathryn L
Dary, Omar
Keen, Carl L
King, Janet C
Miller, Kevin B
Philbert, Martin A
Tarasuk, Valerie
Taylor, Christine L
Gaine, P Courtney
Jarvis, Ashley B
and
Bailey, Regan L
2015.
Fortification and Health: Challenges and Opportunities.
Advances in Nutrition,
Vol. 6,
Issue. 1,
p.
124.
Garrett, Greg S.
2018.
Food Fortification in a Globalized World.
p.
53.
Osendarp, Saskia J. M.
Martinez, Homero
Garrett, Greg S.
Neufeld, Lynnette M.
De-Regil, Luz Maria
Vossenaar, Marieke
and
Darnton-Hill, Ian
2018.
Large-Scale Food Fortification and Biofortification in Low- and Middle-Income Countries: A Review of Programs, Trends, Challenges, and Evidence Gaps.
Food and Nutrition Bulletin,
Vol. 39,
Issue. 2,
p.
315.
Garrett, Greg S.
Luthringer, Corey L.
Yetley, Elizabeth A.
and
Neufeld, Lynnette M.
2019.
Encyclopedia of Food Security and Sustainability.
p.
336.
Dasgupta, Susmita
Mustafa, Golam
Paul, Tapas
and
Wheeler, David
2021.
The socioeconomics of fish consumption and child health: An observational cohort study from Bangladesh.
World Development,
Vol. 137,
Issue. ,
p.
105201.
Garrett, Greg S.
2022.
Nutritional Anemia.
p.
351.


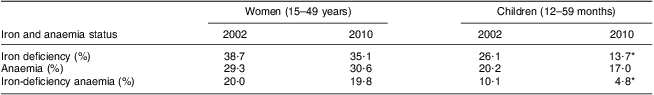

Introduction
Common sense dictates that we should learn from our failures. However, reporting ‘failure’ is more difficult than reporting ‘success’ and it is generally less rewarding, thus there are fewer opportunities to learn from failures than from successes. A study from Brazil in the current issue of this journal( Reference Assunção, Santos and Barros 1 ) reports that iron fortification of flour had no impact on anaemia in children and it is likely that this will be viewed as a ‘failure’. The global community working to reduce undernutrition has agreed that food fortification programmes should be prioritized for sharply increased funding( 2 ). World-renowned economists brought together through the Copenhagen Consensus identified iron fortification of staples as one of the ‘best-buys’ among the thirty interventions considered for addressing the ten great challenges facing global development( 3 ). Therefore the reported ‘failure’ of iron fortification to reduce anaemia in Brazil presents an opportunity to learn and discuss what is indeed needed to improve our practice.
Food fortification is the addition of vitamins and minerals to foods to prevent or correct demonstrated inadequacies in diets( 4 ). It is critically important in the design and evaluation of food fortification programmes to realize that positive health outcomes are directly affected by the quality and amount of nutrients added to the foods rather than just to the consumption of ‘fortified foods’( Reference Dary 5 ).
The fortification formulation is key to success
An appropriate fortification formulation for the food vehicle (wheat flour, in this case) is vitally important in the design of the programme. Ideally the fortification formula should fill the gaps in dietary adequacies of the micronutrients that have been identified by food intake studies in target population groups. In the case of minerals such as iron and zinc, selecting the appropriate form of the fortificant (source of the micronutrient) requires consideration of its bioavailability, its interaction with the food matrix and its cost. Hurrell et al.( Reference Hurrel, Ranum and de Pee 6 ) comprehensively reviewed the efficacy and effectiveness of iron compounds used in fortification programmes. They categorized forms of iron currently being used on the basis of bioavailability and stability and related this to the amount of the vehicle being consumed. These authors presented strong evidence that reduced iron and other forms of iron with low bioavailability are not, and cannot be, efficacious. Hurrell et al.( Reference Hurrel, Ranum and de Pee 6 ) predicted that only nine of seventy-eight national programmes they reviewed ‘could be expected to have positive impact on iron status’; none of these nine used reduced iron. Thus the absence of impact noted in the study from Brazil( Reference Assunção, Santos and Barros 1 ) is consistent with this prediction. This lesson is vitally important for the many existing iron fortification programmes that still allow the use of reduced iron as the source of this mineral.
In addition to bioavailability, food technology and cost must be considered in designing the formula for fortification programmes. When added in the amounts needed to improve iron status in populations, forms of iron with higher bioavailability than reduced iron, e.g. ferrous sulfate, sodium–iron EDTA (NaFeEDTA) and ferrous fumarate, often cause negative interactions with the food matrix at concentrations above – and in some cases even lower than – 30, 30 and 60 mg Fe/kg flour, respectively. Changes in colour, flavour, baking quality or a reduction in shelf-life are often ‘deal-breakers’ for fortification programmes. In many countries, but not Brazil, intakes of wheat flour in target populations are not sufficient to allow these iron fortificants to be added in the amounts that are required to expect desired impacts without risking negative interactions. This characteristic of the fortificants limits somewhat the magnitude of the impact of wheat flour as a vehicle for iron, and measurable changes might only be identified in countries in which wheat flour is consumed in relatively large amounts.
Additionally, forms of iron with greater bioavailability often cost more than those with lesser bioavailability and this creates another constraint to their use. For example, NaFeEDTA has two to three times higher bioavailability than ferrous sulfate or ferrous fumarate in diets rich in iron absorption inhibitors, but it is substantially more expensive than the other two forms of iron. For example, at 30 mg Fe/kg flour, we estimate using the Food Fortification Formulator( Reference Dary and Hainsworth 7 ) and applying 2012 prices for these products that the costs are $US 1·38, $US 0·26 and $US 0·35 per tonne of fortified flour, respectively. Thus, the most promising iron compound from the biological point of view may not necessarily be the most attractive when considering the constraints of operational realities.
In comparison, reduced iron is the cheapest iron fortificant costing less than $US 0·05 per tonne of fortified flour and this is why it is still used in many fortification programmes. The flour fortification standard of Brazil( 8 ) allows the use of several iron fortificants, including reduced iron, and therefore it is not surprising that many of the producers there are using the cheapest form available to comply with the standard.
Linking additional intakes of nutrients to biomarkers of outcome
As with all nutrition interventions, increased investments are needed in evaluating fortification programmes. The WHO 2006 guidelines( 4 ) recommend linking outcomes measured with appropriate biomarkers to changes in the intakes of nutrients measured in the overall diet. Hb is well known to be an insensitive indicator of iron deficiency. It is especially important to recognize this when choosing biochemical parameters to use in measuring the impact of iron fortification programmes that are designed to reduce iron deficiency. As the authors of the Brazil study( Reference Assunção, Santos and Barros 1 ) noted, the absence of data on iron deficiency prior to their 2008 survey meant that the impact of fortification on iron deficiency could not be measured. To understand the causal pathway linking change in nutrient intake to change in biomarker, it is necessary to express identified nutrient gaps in terms of the estimated specific dietary requirements for each nutrient such as the Estimated Average Requirements (EAR) and the Recommended Nutrient Intakes (RNI). The EAR is the nutrient intake that satisfies the needs of half of the population, and this is used as the cut-off point to estimate the adequacy of intakes for most micronutrients in populations( 9 ). The proportion of the population with intakes below the EAR reflects the proportion of the population with inadequate intakes. The RNI is used to estimate the adequacy of micronutrient intakes in individuals, as it represents the nutrient requirement that satisfies the needs of 97·5 % of individuals in the population. Iron differs from many other nutrients in having a large individual variation and therefore, strictly, requires a probabilistic procedure which provides estimates of population inadequacy that are intermediate between those estimated using the EAR and the RNI values.
The selection of indicators for iron status and iron intake plays a vital role in the interpretation of studies evaluating the impact of programmes designed to improve iron status. The Brazil study( Reference Assunção, Santos and Barros 1 ) presented data on total dietary iron intake and a strong statistical analysis of anaemia and iron deficiency in children, but it did not present data on the additional iron provided by the fortified flour. The latter is essential to the interpretation of programme impact because this represents the magnitude of the intervention. The Brazilian standard of wheat flour fortification( 8 ) specifies a total iron content of 4.2 mg Fe/100 g flour but does not specify how much iron must be added by millers to ensure this amount. The intrinsic iron content of unfortified flour is approximately 1.0 mg Fe/100 g and it is highly probable that millers in Brazil who were complying with the standard added about 3.2 mg Fe/100 g flour, just sufficient to reach the iron content specified in the standard. This is a common practice in many countries. With the usual wheat flour intake of Brazilian children aged 2–5 years being 100 g/d, the additional iron intake from flour fortification would be 3.2 g Fe/d. This amount of iron added through fortification corresponds to 62 % and 25 % of the EAR and RNI values, respectively, of this nutrient in a diet with 5 % bioavailability for iron( 10 ). This magnitude of additional iron intake would be expected to cause positive changes in biomarkers associated with iron status( Reference Dary 5 ). The possible explanations for changes not being observed are that either the additional iron was not present in the flour and/or the bioavailability of the iron fortificant was much lower than 5 %.
The 2010 National Micronutrient Survey in Jordan provides a model for the role that appropriate indicators of iron status and iron intake might have in evaluating a national fortification programme( 11 ). The programme began in 2002 and the formulation was designed to include multiple micronutrients with ferrous sulfate added to provide an additional 34 mg Fe/kg flour. In the implementation of the programme, we estimate that the amount of iron actually added was 27 mg Fe/kg, based upon the 79 % compliance with the addition of the premix that was stated in the report. Thus for women of reproductive age consuming 200 g of flour daily in a diet assumed to have 5 % iron bioavailability, fortification added 5.4 mg to iron intake, 20 % of the EAR or 9 % of the RNI. For children aged 36–59 months consuming 100 g of flour daily, the programme added 2.7 mg to iron intake, which corresponded to 52 % of the EAR or 21 % of the RNI. By considering the amount of additional iron consumed because of the programme in relation to the appropriate EAR or RNI, these calculations predict that the programme would have reduced iron deficiency and iron-deficiency anaemia in children, but not in women. The biochemical results from the survey are consistent with this prediction (Table 1).
Table 1 Prevalence of iron deficiency, anaemia and iron-deficiency anaemia, in women and children, before and after iron fortification in Jordan (2002–2010)( 11 )
*Values were significantly different from those in 2010 (P < 0·05).
It is noteworthy that the average consumption of flour and the expected additional intake of iron by children in the Brazil study were similar to those in Jordan. Data on the amount of iron added to flour and longitudinal data describing indicators of iron deficiency allowed the evaluators in Jordan to demonstrate that the national programme there had halved the prevalence of iron deficiency and iron-deficiency anaemia in children but not women, and that the programme had no impact on anaemia in either group. These results lead us to conclude that ferrous sulfate was bioavailable in the Jordanian programme and that the amount of added intake was sufficient to improve iron status in children but not in women. The association between added intake and change in outcomes is more clearly evident when the intake is considered in relation to the proportion of the EAR/RNI that was supplied to each one of these age groups. However, the additional supply of iron in the Jordanian programme was still insufficient, even in children, to cause a statistically significant reduction in the prevalence of anaemia. Switching from ferrous sulfate to NaFeEDTA at the same iron content – if this were possible – would further improve iron status indicators, and perhaps even reduce anaemia in children. However, measurable decreases in anaemia in women may still be improbable.
‘There is nothing so practical as a good theory’ – Kurt Lewin
Evaluations are most useful when based upon a sound programme theory – a causal pathway developed explicitly to identify the critical points through which a programme is predicted to provide the desired impacts. Further, developing a causal pathway provides all stakeholders an opportunity to understand what is needed to make the programme work and to ensure that the necessary information is collected to monitor the process of the intervention and to measure its outcomes. An important limitation of the report from Brazil( Reference Assunção, Santos and Barros 1 ), acknowledged by the authors, was the absence of data showing whether or not the fortification programme was performing as expected – readers do not know whether or not the programme provided sufficient additional iron to justify any expectation of impact. Thus no firm conclusions can be drawn about the impact, or lack of impact, this programme may have had on iron status.
The Program Assessment Guide (PAG) provides one practical option for facilitating the development of programme theories for nutrition programmes( Reference Pelletier, Corsi and Hoey 12 ). More specifically, the PAG is a guide for designing and implementing a participatory workshop that: integrates, and builds capacity to integrate, available evidence, contextual knowledge and experience in the rigorous design, implementation, management and evaluation of programmes; strengthens the shared understanding, commitment and ownership of large-scale interventions within the relevant policy and programme community in the country; and reinforces practices that enhance particular programmes while forging explicit links with broader nutrition, health, food and agricultural interests and agendas. The primary products of the PAG workshop are: an action plan for strengthening the programme; an operations research agenda to address critical knowledge gaps; a list of critical points in the delivery system that should be included in the monitoring and evaluation system; and a strategic plan for overseeing and generating support for the action plan after the workshop.
The public sector is responsible for key roles in effective fortification programmes
A fundamental rationale for the cost efficiency of fortification programmes is that they use products manufactured and distributed by the food industry to increase the supply of vitamins and minerals to target populations. In other words, the delivery mechanism already exists and is without cost to the public sector. Healthy private–public partnerships are essential for effective and sustainable fortification programmes and these depend upon input from both sectors to establish and sustain them. Participation of academic/research institutions, public or private, is crucial in designing the programme; in providing advice to enact the appropriate regulations, including specific reference to the amounts of nutrients added as well as the total expected contents in the fortified food after considering the intrinsic contents in the unfortified vehicles; in evaluating programme impact; and in advocating for sustainability of the programme.
Governments should also continually enforce compliance with the standards at factories and markets. Enforcement is critical for the success of both mandatory and voluntary fortification programmes because it establishes a ‘level playing field’ for fair competition and provides confidence that the product claims being made about the nutrient content are justified. Without this enforcement, companies complying with the standards are disadvantaged in the marketplace by the price of their fortified product in relation to unfortified or ‘semi-fortified’ products being sold as fortified. All companies have a fiduciary responsibility to their shareholders and fortification programmes must accommodate this reality. As reported by Assunção et al.( Reference Assunção, Santos and Barros 1 ), two years after implementation of the national programme in Brazil, almost one in five mills was not adding any iron to its products. In the absence of any government monitoring system to enforce compliance with the mandate, the authors assumed that this situation was no different in 2008.
Academics and researchers are needed to evaluate programmes. This requires measuring changes in intakes of micronutrients and determining if these changes have resulted in desirable outcomes. Collecting, analysing and interpreting data on nutrient consumption and biochemical parameters of nutritional status requires considerable training and expertise in a range of disciplines that are not needed by the private sector. Academics and researchers have also played prominent roles in advocating fortification be implemented. They have been able to convince food industry leaders that their participation is important in improving the national public health and then recognized them for the successes that have been achieved. Experience has demonstrated that changing economic and political contexts often raise barriers to the sustainability of fortification programmes and thus champions are needed to continue advocating for programmes once they have been established( Reference Dary, Martinez and Guamuch 13 ).
Donors and non-government organizations also play key roles in supporting the introduction and scale-up of fortification programmes. Donors such as the Bill and Melinda Gates Foundation, The World Bank, the US Agency for International Development, the Canadian International Development Agency and others provide essential financial and/or technical support required in the initiation of the programmes. Some international non-governmental organizations, e.g. Micronutrient Initiative, Global Alliance for Improved Nutrition, Flour Fortification Initiative and Helen Keller International, are funded by the donors to provide important technical and financial assistance to the programmes. Ideally, this financial and technical assistance is provided in ways that explicitly build the capacity and ownership of the fortification programmes by the governments, regional-level and country-level institutions receiving this assistance. Assistance should be provided not only to the food industries and governmental units involved in the food fortification programmes, but also to academic/research institutions that provide the scientific evidence for assessing the need, the progress and the impact of the programmes.
Conclusion
Hurrell et al.( Reference Hurrel, Ranum and de Pee 6 ) predicted that fortification programmes using reduced iron, such as that reported by Assunção et al.( Reference Assunção, Santos and Barros 1 ) in Brazil, could not be expected to have the desired impacts because of the low bioavailability of this iron fortificant. We agree with the authors of the Brazilian study that the programme of this country requires adjustments and we hope that their study results will help bring about the needed changes. We must learn from our failures. The data on iron deficiency gathered in the 2008 study in Brazil will provide the necessary baseline to assess the impact of any modifications that are made to the programme there. The findings of the study are also highly relevant to managers of fortification in the many other countries that are, or may still be, using reduced iron. Governments and academics play vital roles in increasing awareness of these lessons. We believe it is particularly important for those designing and managing fortification programmes to understand that the impact of their programmes will depend upon the proportion of the nutrient gap that is being filled by the additional bioavailable nutrient supplied through the programmes. It is a mistake to assume that simply consuming ‘fortified foods’, without assessing the additional intake of the nutrients consumed, is sufficient to expect success. Furthermore, changes in biological outcomes should correlate with the additional intake, expressed as a proportion of the EAR/RNI for each age group. The public sector and academia should be provided priority attention for strengthening their roles in food fortification, so that it can achieve its identified potential as one of the most cost-effective approaches we have to improve nutrition globally.
Undernutrition, including deficiencies in vitamins and minerals, has multiple causes and therefore multiple interventions are needed to address it effectively. Iron deficiency and iron-deficiency anaemia are important public health problems that have proved difficult to reduce. Fortifying commonly consumed foods with iron provides one core mechanism for addressing iron deficiency but clearly programmes must be designed and implemented in ways that allow them to work. Other interventions such as supplementation and increasing diet diversity (including improvement of iron bioavailability) will certainly be required to ensure adequate intakes of vitamins and minerals in some target groups.