Introduction
Alveolar echinococcosis (AE) and cystic echinococcosis (CE) are major zoonoses in central Asia. AE is caused by the parasite Echinococcus multilocularis whilst CE is caused by Echinococcus granulosus s.l. Both diseases cause extensive human morbidity, and AE is usually fatal if left untreated (Torgerson et al., Reference Torgerson, Schweiger, Deplazes, Pohar, Reichen, Ammann, Tarr, Halkik and Müllhaupt2008). In Kyrgyzstan, there is now evidence for a major epidemic of AE (Usubalieva et al., Reference Usubalieva, Minbaeva, Ziadinov, Deplazes and Torgerson2013; Raimkylov et al., Reference Raimkylov, Kuttubaev and Toigombaeva2015; Counotte et al., Reference Counotte, Minbaeva, Usubalieva, Abdykerimov and Torgerson2016; Paternoster et al., Reference Paternoster, Boo, Wang, Minbaeva, Usubalieva, Raimkulov, Zhoroev, Abdykerimov, Kronenberg, Müllhaupt, Furrer, Deplazes and Torgerson2020). The annual burden of echinococcosis in Kyrgyzstan for 2013 was estimated to be 11 915 [95% uncertainty interval (UI): 4705–27 114] disability-adjusted life years (DALYs) for AE and 3052 [95% UI: 1508–5205] DALYs for CE (Counotte et al., Reference Counotte, Minbaeva, Usubalieva, Abdykerimov and Torgerson2016).
Foxes (Vulpes vulpes) are one of the usual definitive hosts of E. multilocularis. Studies on foxes in Naryn district in Kyrgyzstan have demonstrated a prevalence of E. multilocularis infection of 64% (Ziadinov et al., Reference Ziadinov, Deplazes, Mathis, Mutunova, Abdykerimov, Nurgaziev and Torgerson2010). Dogs have been shown to be suitable definitive hosts for E. multilocularis with similar egg reproduction to foxes (Kapel et al., Reference Kapel, Torgerson, Thompson and Deplazes2006). In dogs in Naryn district, the prevalence of E. granulosus s.l. has previously been reported to be ~18%, whilst that of E. multilocularis is ~19% (Ziadinov et al., Reference Ziadinov, Mathis, Trachsel, Rysmukhambetova, Abdyjaparov, Kuttubaev, Deplazes and Torgerson2008). Echinococcus spp. have complex life cycles where carnivore hosts play an important role in the transmission in Kyrgyzstan. It is hypothesized that in Kyrgyzstan dogs play an important role in the zoonotic risk; however, the wild animal cycle including foxes functions as a reservoir for E. multilocularis (Ziadinov et al., Reference Ziadinov, Mathis, Trachsel, Rysmukhambetova, Abdyjaparov, Kuttubaev, Deplazes and Torgerson2008, Reference Ziadinov, Deplazes, Mathis, Mutunova, Abdykerimov, Nurgaziev and Torgerson2010; Mastin et al., Reference Mastin, Van Kesteren, Torgerson, Ziadinov, Mytynova, Rogan, Tursunov and Craig2015; Van Kesteren et al., Reference Van Kesteren, Mastin, Torgerson, Mytynova and Craig2017).
It has been hypothesized that an increase in human CE and AE in central Asia may be related to the dissolution of the Soviet Union in 1991 with fundamental sociocultural, economic and structural changes (Torgerson, Reference Torgerson2013). Furthermore, a decline in the activities of veterinary services is reported, including a decrease or absence of veterinary control over meat production. The process of domestic slaughter has increased, as well as the privatization of large livestock farms. A further consequence of the crisis has been increasing human poverty (Torgerson, Reference Torgerson2013) along with an increase in the number of dogs in both rural and urban areas (Torgerson et al., Reference Torgerson, Oguljahan, Muminov, Karaeva, Kuttubaev, Aminjanov and Shaikenov2006).
The first case of human AE in Kyrgyzstan was diagnosed in 1996, but until 2003 only small numbers of cases were registered annually. The number of reported cases has increased significantly since 2004 (Usubalieva et al., Reference Usubalieva, Minbaeva, Ziadinov, Deplazes and Torgerson2013). Human AE has a prolonged latent period. The epidemic of human AE started ~15 years after the dissolution of the Soviet Union. Therefore, it is hypothesized that the socioeconomic changes that lead to the increase in incidence of CE also created the conditions that allowed the colonization of dogs by E. multilocularis. This has resulted in an onward transmission to humans due to the close contact between dogs and humans and hence an epidemic of human AE (Torgerson, Reference Torgerson2013).
In Kyrgyzstan, the national annual incidence of reported human cases of CE 25 years ago was ~4 cases per 100 000. In recent years it is ~15 per 100 000. Of serious concern is that the much more pathogenic form of AE has emerged as a major public health problem. During the last 20 years AE has increased to ~200 reported cases per year (Usubalieva et al., Reference Usubalieva, Minbaeva, Ziadinov, Deplazes and Torgerson2013; Raimkylov et al., Reference Raimkylov, Kuttubaev and Toigombaeva2015; Counotte et al., Reference Counotte, Minbaeva, Usubalieva, Abdykerimov and Torgerson2016; Paternoster et al., Reference Paternoster, Boo, Wang, Minbaeva, Usubalieva, Raimkulov, Zhoroev, Abdykerimov, Kronenberg, Müllhaupt, Furrer, Deplazes and Torgerson2020). In certain districts, very high prevalences of human AE have been detected by ultrasound surveillance. For example, an ultrasound study in the south of Kyrgyzstan (Bebezov et al., Reference Bebezov, Mamashev, Umetaliev, Ziadinov, Craig, Joekel, Deplazes, Grimm and Torgerson2018) documented a prevalence of AE of 4.2%.
The aim of this study is to investigate the environmental contamination of taeniid eggs in high and lower incidence areas for AE. We also wished to investigate seasonal associations which may give indications of transmission and if control could be targeted. We also hypothesized if there may be a link between the contamination with eggs of E. multilocularis in public areas and the local incidence of human AE and if dogs are the main source of infection for the human population.
Materials and methods
Study area and sample collection
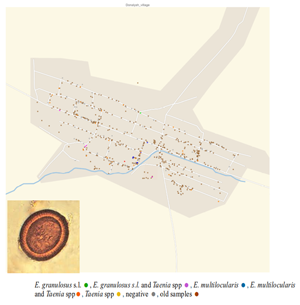
For the study, villages were selected based on human incidence data (Paternoster et al., Reference Paternoster, Boo, Wang, Minbaeva, Usubalieva, Raimkulov, Zhoroev, Abdykerimov, Kronenberg, Müllhaupt, Furrer, Deplazes and Torgerson2020). Five villages were selected in the Alay district where the reported incidence of AE between 2014 and 2016 was amongst the highest in Kyrgyzstan. For comparison 5 villages were selected in the Kochkor district which has a substantially lower reported incidence of human AE. Terek, Sopu-Korgon, Sogondu, Kun-Elek and Chii-Talaa villages were explored in Alay district; Mantysh, Komsomol, Don-Alysh, Chekildek and Ak-Talaa villages in Kochkor district (Fig. 1). For each village, samples of all fresh dog feces were collected and assigned a specific identification number. Fresh feces were intact feces that had the appearance of being recently deposited. For older fecal samples, which were dried out with some evidence of degradation, the number and position were recorded. Dog feces were identified by morphology, size and content. The GPS coordinates of all observed fecal samples (fresh and old) were recorded. It was only possible to access public parts of the villages for sample collection and not, for example, enclosed private gardens. The area of the parts of the village that was accessible, and samples taken was estimated using the Google Maps Area Calculator Tool (Frančula, Reference Frančula2020).

Figure 1. Location of the expedition points in Kyrgyzstan and separately on top of the Kochkor (low AE incidence) district and on the left side of the Alay (high AE incidence) district.
All samples were frozen for 5 days at minus 80°C prior to analysis for safety reasons.
Mapping of surgical cases
The national surveillance system for surgical cases of human echinococcosis compiled by the State Sanitary and Epidemiological Service in Bishkek, Kyrgyzstan was used. These data have been previously reported by Paternoster et al. (Reference Paternoster, Boo, Wang, Minbaeva, Usubalieva, Raimkulov, Zhoroev, Abdykerimov, Kronenberg, Müllhaupt, Furrer, Deplazes and Torgerson2020). For this study the community level incidence of AE was used to explore if there was any association with the level of Echinococcus spp. contamination in dog feces and density of environmental contamination and with human incidence was used.
Egg isolation
The eggs were isolated according to a previously described study (Mathis et al., Reference Mathis, Deplazes and Eckert1996) with some modifications. In brief, the samples were thawed; then 2 g of feces were weighed into a 15 mL Falcon tube. Subsequently, a 1:4 solution of phosphate-buffered saline–Tween–sodium azide was added, shaken vigorously for 1 min and centrifuged for 10 min at 500g. After removing the supernatant, the pellet was re-suspended in 8 mL of zinc chloride solution (density 1.45), then the samples were shaken again and centrifuged for 30 min at 400g. Afterwards, the supernatant was passed through nylon meshes with a mesh size of 40 and 21 μm (Lanz-Anliker, Rohrbach, Switzerland). A 21 μm nylon mesh was used to collect the taeniid eggs. The sediment was placed in a flat-walled Nunc tube and examined for the presence of taeniid eggs using an inverted microscope. Positive samples were collected and centrifuged at 500g for 5 min.
DNA isolation from taeniid eggs
An isolation of the DNA was achieved by using a method adapted from that previously described (Trachsel et al., Reference Trachsel, Deplazes and Mathis2007). In brief, the pellet was transferred into a 1.5 mL Eppendorf tube and centrifuged for 1 min at 8000g. The supernatant was removed, and the pelleted eggs were re-suspended in 200 μL distilled water. Then 25 μL of 1 m potassium hydroxide and 7 μL dithiothreitol were added to the sample and vortexed, followed by an incubation at 65°C for 15 min.
Furthermore, 60 μL 2 m Tris-hydrochloric acid (HCl) (pH 8.4) and 2 μL concentrated HCl (12.4 N/>37%) were added. After the addition of 200 μL of buffer AL (QIAamp DNA Mini Kit, Qiagen) and 20 μL proteinase K, the sample was incubated at 56°C for 10 min. A 50 μL of Chelex (50%) was added and the mix was put on a rotor at room temperature between 30 min and 3 h. The tubes were then centrifuged at 16 000g for 1 min and the supernatant was transferred to a fresh tube together with 200 μL pure ethanol. The whole mix was vortexed and spun down.
The samples were applied to Qiagen spin columns and centrifuged for 1 min at 8000g. The columns were washed with 300 μL buffer AW1 and centrifuged for 1 min at 8000g. This washing step was repeated once again. Then the columns were washed with buffer AW2 and centrifuged for 1 min at 8000g. After the repetition of the washing step with AW2, the columns were centrifuged empty at full speed for 3 min. Next, the columns were placed in a 1.5 mL Eppendorf tube, 100 μL AE elution buffer was added and incubated for 1 min at room temperature, to elute the DNA from the membrane. As a last step, the columns were centrifuged for 1 min at 8000g and the flow-through was collected.
Polymerase chain reaction (PCR)
For the PCR a multiplex PCR adapted from a previous protocol (Trachsel et al., Reference Trachsel, Deplazes and Mathis2007) with a commercial kit (Qiagen Multiplex PCR Kit, Qiagen, Hilden, Germany) was used. First, a mastermix containing 25 μL Qiagen Mastermix, 18 μL distilled water, both from the multiplex PCR Kit, as well as 5 μL primermix were prepared in PCR tubes. The primermix is composed of 80 μL Cest5 primers and 10 μL from Cest1, 2, 3, 4 primers as well as 380 μL distilled water. Primers Cest1 (5′-TGC TGA TTT GTT AAA GTT AGT GAT C-3′) and Cest2 (5′-CAT AAA TCA ATG GAA ACA ACA ACA AG-3′) amplified a 395 bp stretch of the nad1 gene of E. multilocularis; primers Cest3 (5′-YGA YTC TTT TTA GGG GAA GGT GTG-3′) and Cest5 (5′-GCG GTG TGT ACM TGA GCT AAA C-3′) amplified a 267 bp stretch of the rrnS of Taenia spp. and primers Cest4 (5′-GTT TTT GTG TGT TAC ATT AAT AAG GGT G-3′) and Cest5 amplified a 117 bp stretch of the rrnS of E. granulosus (s.l.). Finally, 2 μL of DNA solution was added. To confirm the validity of the PCR, positive as well as negative controls were implemented.
The cycling conditions were 15 min at 95°C, 30 s at 94°C, 90 s at 58°C with a repetition of 40 cycles and another 10 min at 72°C.
Sequencing
For a more accurate determination of the taeniid species, all sequencing was performed at Microsynth Switzerland. Therefore, the DNA was purified by using a commercial kit (MinElute PCR Purification Kit, Qiagen) and sent for sequencing.
Density of contamination
Estimates of the fecal egg counts were performed using the McMaster technique (Deplazes and Eckert, Reference Deplazes and Eckert1988a). For estimations of the mean fecal egg count only samples with mono-infections with taeniids, with E. granulosus s.l., E. multilocularis or Taenia spp. (species not determined), respectively, were used. These infections were diagnosed by PCR as described above.
To calculate the average mass of a canine fecal sample, 50 samples were collected, randomly weighed and the mean weight was calculated. Total numbers of eggs were the eggs per g multiplied by the average fecal weight. Density of contamination was estimated according to:
where epg = mean eggs per g of E. granulosus s.l., E. multilocularis or Taenia spp. eggs; weight = mean weight of a single fecal sample; pv = proportion of fresh fecal samples containing Echinococcus spp. or Taenia spp. (including mixed infections); total = total number of fecal samples found (fresh and old) and area = sample area from which feces were searched and collected.
Statistical analysis
For the statistical evaluation the statistic program R (R Core Team, 2022) was used. Poisson regression was used to examine if there was any association between the prevalence of Echinococcus spp. in the canine fecal samples or the level of contamination with parasite eggs and the incidence of echinococcosis from the national surveillance reports.
For egg contamination per m2, the fecal egg counts were analysed using the eggCounts package in R (Torgerson et al., Reference Torgerson, Paul and Furrer2014). Uncertainty in the total egg contamination was estimated by using equation (1). Here, the uncertainty in the proportion of fecal samples containing parasite eggs was estimated by using 2000 beta random variables from Beta (a + 1, b + 1) where a is the number of fresh fecal samples positive for Echinococcus spp. or Taenia spp. and b is the number of fresh fecal samples that were negative. Uncertainty in the fecal egg counts was calculated by using 2000 draws from the posterior distribution from the single sample egg count function in eggCounts. Each of these random draws was analysed in equation (1). The 2.5, 50 and 97.5% quantiles of the distribution of the 2000 resulting samples were estimates of the median and 95% confidence interval (CI) limits of the fecal egg contamination in terms of eggs per m2. All data and R code are provided as Supplementary files 1 and 2.
Results
Taeniid egg contamination in fecal samples of dogs
A total of 2013 fresh samples of dog feces was examined. In 53 samples E. multilocularis was detected (of which 10 were mixed infections with Taenia spp.), 54 samples with E. granulosus s.l. (of which 27 were mixed infections with Taenia spp.) and 285 samples positive for Taenia spp. (of which 37 were mixed infections with Echinococcus spp.). Results of all samples are summarized in Table 1.
Table 1. Overview of the number of positive taeniid samples in dog feces (2017–2018)

A total of 7065 old samples of dog feces were detected and recorded. The minimum number of old samples of dog feces found was in February in the Alay district, which can be explained by the large amount of snow that fell during that period. Also, the minimum number of old samples of dog feces found was in the Alay district in June, since at that time more dogs were with farmers on summer pastures in the mountains. The maximum number of old samples of dog feces in the Alay district was found in September and November.
In the Kochkor district, fewer old dog fecal samples were found in June than in other months (September, November and February), as at that moment more dogs were on summer pastures in the mountains with farmers.
After PCR testing of dog fecal samples, we selected 298 samples for the quantitative egg counts by the McMaster method, 26 samples with E. granulosus s.l., 42 with E. multilocularis and 230 with Taenia spp. The mean eggs per g were 1607 (CI 1188–2144) for E. granulosus s.l.; 1287 (CI 1009–1623) for E. multilocularis and 1199 (CI 1092–1314) for Taenia spp. There was little variation in the proportion of samples in which Taenia spp. and Echinococcus spp. eggs were found across the 4 sampling periods. Only between September 2017 and February 2018 an apparent decrease in the proportion of samples containing E. granulosus s.l. eggs (P < 0.01, Fig. 2) was observed.

Figure 2. Proportion of fecal samples (±95% confidence intervals) containing Echinococcus granulosus s.l., Echinococcus multilocularis and Taenia spp. eggs across the 4 sampling periods.
There was some heterogeneity in the level of contamination of eggs of E. multilocularis, which ranged from 17 eggs per m2 (CI 8.6–31) for the village of Chekildek in Kochkor district, to several villages having no E. multilocularis detected. There were no significant differences between the levels of contamination in villages in Kochkor district compared to villages in Alay district. There was some evidence of decreased contamination of the environment in the winter months in the Alay district, whereas in the Kochkor district there was some evidence of increased levels of contamination (Fig. 3).

Figure 3. Estimated contamination of E. multilocularis eggs in canine feces: Alay district (left) and Kochkor district (right).
For E. granulosus s.l. there was also considerable heterogeneity with the level of contamination ranging from 21 eggs per m2 (CI 9.9–40.5) for the village of Chekildek in the Kochkor district to 2.9 eggs per m2 (CI 0.9–7.2) in Chii-Talaa in the Alay district (Fig. 4). There was some evidence of lower contamination rates in both districts during the winter (Fig. 4).

Figure 4. Estimated contamination of E. granulosus s.l. eggs in canine feces: Alay district (left) and Kochkor district (right).
Contamination with Taenia spp. eggs decreased from the autumn throughout the winter before increasing again the following autumn in the Alay district (Fig. 5). In Kochkor district, there was a continuous decrease during the period of this study.

Figure 5. Estimated contamination of Taenia spp. eggs in canine feces: Alay district (left) and Kochkor district (right).
There was no association with the level of environmental contamination in public areas, and the reported village level incidence reported in the national surveillance system. There was no evidence for a difference in the proportion of dog feces containing E. multilocularis eggs in the high incidence district of Alay in comparison to the lower incidence district of Kochkor. The same is true in respect of the level of contamination. There was also no evidence of an association between the proportions of dog fecal samples containing E. granulosus s.l. eggs and the incidence of CE in the human population (P > 0.05) and the same is true in respect of the level of contamination. Summary data together with the proportion of dog fecal samples containing Echinococcus spp. parasite eggs isolated from dog feces and the level of contamination are given in Table 2.
Table 2. Proportion of fecal samples containing Echinococcus spp. eggs and the level of contamination in public areas of the high and low incidence districts

a Annual incidence per 100 000 population.
b Proportion of canine fecal samples (%) (95% CI).
c Estimated total eggs per m2 from September 2017 to November 2018 (95% CI).
Sequencing of randomly selected positive Taenia spp. fecal samples of dogs
For the Taenia species to be identified, 15 samples from each year were randomly selected and sequenced (Trachsel et al., Reference Trachsel, Deplazes and Mathis2007). Of these 30 samples, 26 were Taenia hydatigena, 3 were Taenia crassiceps and 1 was Taenia ovis.
Discussion
Widespread contamination of village public spaces with dog feces containing eggs of E. multilocularis, E. granulosus and Taenia spp. was demonstrated. This represents a source of infection for the human population for Echinococcus spp. and T. crassiceps. However, it was not possible to detect any association between the intensity of environmental contamination and the officially reported incidence of AE or CE. The incidence of AE in the Alay district was nearly 4 times higher compared to the Kochkor district (Table 2). Yet there was a very similar proportion of dog feces containing E. multilocularis eggs and similar levels of contamination. Likewise, no differences could be detected in the proportion of fecal samples with eggs of E. granulosus s.l. or the level of contamination. This would support the hypothesis that a direct environmental contamination of public areas is not the main driver of this epidemic of human echinococcosis in Kyrgyzstan. However, a weakness of our approach was our method for estimating the level of contamination in the villages. We were not able to access considerable parts of the villages, such as private gardens, to collect samples and check for fecal contamination. It is possible that these areas had a different intensity of contamination compared to the areas we were able to access, and this could generate bias. Arguably, there might also be greater contact between dog feces and humans in areas such as private gardens which might be a greater driver of transmission. However, it is worth noting that there appears to be quite considerable accumulations of dog feces in places where local people frequent, often such as around the village school, mosque or shops, where we were able to obtain samples (see figures in Supplementary file 3). It was also assumed that the feces observed and/or collected were dog feces based on the morphology. It is possible that some may have been feces of foxes or other wild carnivores. Ideally DNA analysis of the samples would confirm the species origin of the samples. In Tibetan plateau village communities, which have a similarly high incidence of human AE as the villages in the present study, 13 of 155 carnivore fecal samples (8.3%) were shown to be fox feces by PCR analysis. The remainder were dog feces (Vaniscotte et al., Reference Vaniscotte, Raoul, Poulle, Romig, Dinkel, Takahashi, Guislain, Moss, Tiaoying, Wang and Qiu2011). However, regardless of this the estimated contamination levels would be valid.
There is also a long latent period between infection and the development of the disease. Therefore, cases reported in the national surveillance system will represent infection events of some time, possibly of years ago. Thus, the levels of contamination may have changed between when the infection event occurred and the detection of human cases through surveillance. It is also assumed that the reporting of cases to the national surveillance system is accurate and has no under-reporting in either of the 2 districts. Nearly all cases of AE and CE are treated in either Bishkek or Osh. Suitable medical facilities are not available in the rural areas where this study was undertaken. But the patient origin is reported from the hospital records, and this would suggest there is minimal bias in reporting, as nearly all reports originate from these main medical centres. In the Alay district an ultrasound surveillance study was previously undertaken (Bebezov et al., Reference Bebezov, Mamashev, Umetaliev, Ziadinov, Craig, Joekel, Deplazes, Grimm and Torgerson2018) and reported an AE prevalence of 4.2% in that district, which is consistent with the very high reported incidence of treated cases.
Nevertheless, our data might also point to other sources of infection. The water supply has been shown to be a risk factor for human echinococcosis and a systematic review and a meta-analysis has suggested it may represent a considerable attributable fraction for human echinococcosis (Torgerson et al., Reference Torgerson, Robertson, Enemark, Foehr, van der Giessen, Kapel, Klun and Trevisan2020). Investigations into potential contamination of the water supplies of these villages are ongoing. It is noteworthy that much of the population of these villages does not have access to a safe and sanitary water supply.
There was some, albeit inconsistent evidence for seasonal variations in the contamination of village spaces. Thus, in the Alay district, the contamination levels for both Echinococcus species and for Taenia spp. declined in the winter before recovering by the following autumn. It can be hypothesized that the large number of eggs of the parasites in the autumn period of the year is due to the fact that dogs descend with farmers from summer pastures to villages before the onset of winter. Farmers on summer pastures in the mountains slaughter sheep themselves; they feed the affected organs with echinococcosis cysts (lungs and liver) to dogs. Dogs may also prey on small mammals which are a natural reservoir of E. multilocularis and T. crassiceps. Data of the results from a previous study (Abdyjaparov and Kuttubaev, Reference Abdyjaparov, Kuttubaev, Torgerson and Shaikenov2004) indicate the presence of AE invasion in 10 species of rodents in alpine districts in Kyrgyzstan. These included the area around Sari Tash (altitude 3170 m), where E. multilocularis infection of 0.8% of rodents was documented. This included 4% of red marmots (Marmota caudata). In Sary Mogal (altitude 2980 m), which is a neighbouring settlement to Sari Tash, the study by Bebezov et al. (Reference Bebezov, Mamashev, Umetaliev, Ziadinov, Craig, Joekel, Deplazes, Grimm and Torgerson2018) documented a high prevalence of human AE. The result shows that the majority of Taenia infections were T. hydatigena, a species maintained in a domestic cycle mainly with dogs and sheep and goats, i.e. the same as E. granulosus; on the other hand T. crassiceps has a cycle comparable with E. multilocularis. Three of 30 Taenia spp. samples sequenced were shown to be T. crassiceps which is further evidence that domestic dogs are preying on small mammal species. However, there were insufficient numbers of T. crassiceps samples to infer any differences in predation behaviour of dogs in different villages. Taenia crassiceps cysticercosis has occasionally caused severe disease in humans (Deplazes et al., Reference Deplazes, Eichenberger and Grimm2019).
An interesting observation was that in fecal samples only eggs of E. granulosus s.l. had significantly higher egg count than samples containing Taenia spp. Gemmell (Reference Gemmell1990) demonstrated that T. hydatigena had a substantially higher biotic potential than E. granulosus s.l. Dogs infected with T. hydatigena producing ~38 000 eggs per day were compared to dogs infected with E. granulosus with an estimated 8470 eggs per day. The majority of the Taenia spp. samples that were sequenced were shown to be T. hydatigena. Thus it could be expected that the intensity of egg contamination of feces of dogs infected with Taenia spp. would be far higher than those infected with E. granulosus s.l. This anomaly is likely explained by the fact that most Taenia spp. eggs remain in the proglottids following expulsion from an infected dog, and the proglottids are either voided separately from the feces or migrate from the feces following defecation by the dog. Deplazes and Eckert (Reference Deplazes and Eckert1988b) observed that only 36% of the expelled proglottids from dogs experimentally infected with T. hydatigena were associated with defecation. Further analyses revealed that only 16% of the total estimated number of eggs were recovered from the feces; the remainder were excreted independently of defecation with the proglottids. Assuming this is the case in the present study, the total number of eggs voided by an infected dog would be over 6 times the numbers detected in fecal samples. This then reconciles the results of the present study to earlier studies demonstrating the much higher biotic potential of T. hydatigena compared to E. granulosus s.l.
Dogs are the main definitive hosts for E. granulosus s.l. Data suggest that, for E. multilocularis, they may be an important source of infection for human AE (Torgerson et al., Reference Torgerson, Robertson, Enemark, Foehr, van der Giessen, Kapel, Klun and Trevisan2020). Consequently, regular treatment of dogs with praziquantel is an important intervention to avoid human disease (World Health Organization, Reference World Health Organization2011). The efficiency of an intervention programme in which dogs are medicated depends on the number of registered dogs. Furthermore, the acceptance of the community as well as the access to households are decisive points for the efficiency of such an intervention programme. An aggravating factor in the control of these zoonotic diseases is the traditional movement of livestock. The farmers move their animals between the valley and the pastures for 6–8 months per year in order to find rich grazing land. With the livestock the dogs also move each season which increases the possibility of a reinfection (World Bank, 2011).
Although this study found that there is no obvious correlation between the increase in AE (and CE) cases in humans and the contamination with E. multilocularis (or E. granulosus s.l.) in dog feces in public areas, the role of the dog cannot be excluded given the increase in human AE. To clarify this question conclusively, further parameters need to be examined. The involvement of wild definitive hosts contaminating the environment, changes in the infection cycle and in the pathogenicity of the parasite, the susceptibility of the population as well as changes in environmental factors are only a few parameters that need to be investigated in more detail.
It has been argued that humans are relatively resistant to AE. This might result in the low incidence of human AE in Switzerland despite widespread contamination of the environment with fox feces and hence potential exposure of the population (Gottstein et al., Reference Gottstein, Wang, Boubaker, Marinova, Spiliotis, Müller and Hemphill2015). However, there are subtle suggestions in our results that may provide evidence against this hypothesis. The contamination of the public areas with E. multilocularis eggs is similar in the 2 districts; yet there are marked differences in the reported human incidence of disease, although different levels of contamination in private gardens cannot be discounted. Both populations are ethnic Kyrgyz and it would seem unlikely that 1 population would be markedly more resistant to the parasite than the other. Furthermore, the level of contamination with E. granulosus s.l. seems to be somewhat higher that of E. multilocularis in the Alay districts. Yet in Alay there is a considerably higher incidence of AE than CE, which may point to a greater susceptibility to AE.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003118202300118X.
Data availability statement
All data is available in the manuscript or supporting material.
Acknowledgements
The authors acknowledge the Swiss School of Public Health, European Union's Horizon 2020 research and innovation programme under grant agreement no. 801076.
Author's contribution
Conceptualization: K. K. A. and P. R. T. Investigation: K. K. A., P. A. K., M. I., G. P., P. D. and P. R. T. Methodology: K. K. A. and P. R. T. Software: P. R. T. Laboratory analyses: K. K. A. and P. A. K. Formal analysis and funding acquisition: P. R. T. and P. D. Resources: K. K. A., P. D. and P. R. T. Writing – original draft preparation: K. K. A. and P. R. T. Writing – review and editing: K. K. A., P. A. K., G. P., P. D. and P. R. T. All authors have read and agreed to the submitted version of the article.
Financial support
This research was funded by the Swiss National Science Foundation, grant number: 173131 – ‘Transmission modelling of emergent echinococcosis in Kyrgyzstan’.
Competing interests
None.