The Baby-Friendly Hospital Initiative (BFHI) was put into practice in the early 1990s by the WHO and UNICEF. ‘Ten Steps to Successful Breastfeeding’ has been a key component of the BFHI(1). Among the ten steps, steps 4 and 6 suggest that ‘Facilitate immediate and uninterrupted skin-to-skin contact and support mothers to initiate breastfeeding as soon as possible after birth’ and ‘Do not provide breastfed newborns any food or fluids other than breast milk, unless medically indicated’(1). Prelacteal feeding (PLF) can be defined as to be given any foods and drinks (mostly sugary water, honey, tea, animal milk, baby food or plain water) to a newborn before the lactation and breast-feeding are established within the first 3 d after delivery(2,3) . Avoidance of PLF during the first 3 d of life also promotes breast-feeding practices including duration of exclusive breast-feeding and any breast-feeding(Reference Pérez-Escamilla, Segura-Millán and Canahuati4–Reference Pérez-Escamilla, Maulén-Radovan and Dewey6). In spite of much efforts spent for call attention to breast-feeding, 43 % of newborns are given liquids or foods other than breast milk in the first 3 d of life(2). Global estimation studies showed that approximately 800 000 under-five deaths are associated with suboptimal breast-feeding practices(Reference Rollins, Bhandari and Hajeebhoy7,Reference Victora, Bahl and Barros8) .
In Turkey, BFHI was started in 1991 with the collaboration of the Ministry of Health and UNICEF immediately following the world breast-feeding developments, and ‘Ten Steps to Successful Breastfeeding’ and ‘Global Strategy for Infant and Young Child Feeding’ are still in force(Reference Çaylan, Kılıç and Tetik9). Data on breast-feeding prevalence for monitoring the programme are obtained from the Turkey Demographic and Health Surveys (TDHS), which are conducted every 5 years regularly by the Hacettepe University Institute of Population Studies(10–13).
PLF ratio is an important indicator to monitor baby-friendly practices since it demonstrates the problems associated with early breast-feeding support. Investigating the factors affecting PLF will contribute to identify intervention strategies and improvement of these practices. The aim of the current study is to analyse the trends, determinants of PLF and its relations with the mode of delivery among infants younger than 24 months over the years 2003–2018.
Methods
Study setting
The current study used the data from the TDHS collected in 2003, 2008, 2013 and 2018(10–13). The data collection method was established as interviewing a Turkish population determined via a weighted, multi-stage, stratified cluster sampling method via internationally validated instruments. The analysis for the current study restricted to mother–infant dyads who met the following inclusion criteria: (i) ever breastfed infants born in the past 24 months preceding the survey (when the mother had two children under 24 months, the youngest was included), (ii) singleton birth, (iii) being alive, (iv) living with mother and (v) infants with known PLF status. Individual questionnaire sets for women of reproductive age were used to collect the TDHS data.
Outcome variables
The outcome variable in the study was PLF, based on reports of the mothers who were interviewed in the surveys. In the TDHS woman’s questionnaire, mothers were asked ‘During the first three days after delivery, was [child name] given any fluid other than breast milk?’ If the answer is ‘yes’, what was [child name] given? (Options include infant formula, sugar/glucose water, salt/sugar solution, plain water, milk other than breast milk, honey, tea/infusions, fruit juice and others).
Independent variables
The independent variables included were those previously identified as being associated with the risk of PLF that were available in the pooled data set(Reference Pérez-Escamilla, Segura-Millán and Canahuati4,Reference Giugliani, do Espirito Santo and de Oliveira5,Reference Khanal, Adhikari and Sauer14–Reference El-Gilany and Abdel-Hady16) . These variables included maternal age and education, paternal age and education, region, residence, wealth index, mother tongue, number of living child, gender of infant, preceding birth interval (first birth, <24 months and ≥24 months), number of antenatal care (ANC) visits (<4, 4–7 and ≥8), place of delivery (home, public hospital and private hospital), delivery type, perceived size of child at birth (smaller than average, average and larger than average), birth weight, initiation time of breast-feeding (within 1 h, 1 to <2 h, 2–23 h and ≥24 h) and birth season. The wealth index is a measure that has been tested in a number of countries in relation to inequities in household income, use of health services and health outcomes, and the variable is categorised into lowest (poorest), second (poorer), middle, fourth (richer) and highest (richest) wealth quintiles(Reference Rutstein and Johnson17).
Ethics
Necessary permissions and survey data were obtained from Hacettepe University, Institute of Population Studies.
Data analyses
Data were analysed using IBM SPSS version 22.0 statistical software package. Initially, weighted case numbers and frequencies were taken as descriptive statistics of the general characteristics. Then, distributions of PLF according to individual characteristics were calculated as frequencies and CI with complex sample analysis, and absolute changes between survey years were measured. Next, maternal and infant factors for giving PLF were analysed by using complex sample multiple logistic regression analysis in four survey data, separately and merged database. Finally, the factors associated with PLF were analysed in cases having caesarean section and vaginal delivery separately.
Results
Data on mother–infant dyads having inclusion criteria (n 4942 (n 1439 in 2003, n 1336 in 2008, n 1252 in 2013 and n 915 in 2018)) enrolled for the study. Slightly more than half of the children were male (50·5 %). A higher proportion of mothers at the time of birth were within the ages of 25–29 years (32·4 %) and 20–24 years (26·7 %), and 52·4 % of mothers had 5- to 7-year education. While in 2003, 18·4 % of deliveries took place at home, in 2018, home delivery percentage decreased to 0·6 %. In overall, 92·5 % of deliveries took place at health facilities. Percentage of eight or more ANC visits increased from 26·1 % in 2003 to 72·6 % in 2018. The rate of caesarean section was 24·5 % in 2003 and gradually increased to 53·2 % in 2018. The overall prevalence of caesarean section was 40·2 %. While the lowest rate of initiation of breast-feeding within 1 h after delivery was detected as 51·0 % in 2008, the highest rate was found to be 73·6 % in 2018. The prevalence of initiation of breast-feeding within 1 h after delivery was 60·1 % in the overall analysis. General characteristics of the mothers and index infants are presented in Table 1.
The overall prevalence of PLF was 35·5 %; the prevalence fluctuated between 29·3 % in 2008 and 41·4 % in 2018. The rate of PLF was 42·0 % in mothers who delivered by caesarean section and 31·1 % in those who delivered vaginally (Table 1). In the overall analysis, the most common types of prelacteal feeds were infant formula (61·1 %) followed by sugar/glucose water (24·9 %), plain water (9·3 %) and milk other than breast milk (5·9 %). The proportion of infant formula among given prelacteal feeds increased from 32·0 % in 2003 to 92·1 % in 2018 (2·9-fold increase). The proportion of sugar/glucose water decreased from 51·5 % in 2003 to 3·5 % in 2018 (14·7-fold decrease). The distribution and variations of prelacteal food types by years are shown in Table 2.
Table 2 Distribution and variations of prelacteal food types by years (2003–2018)*
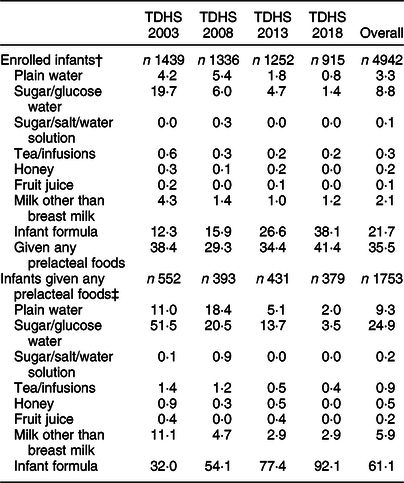
TDHS, Turkey Demographic and Health Surveys; PLF, prelacteal feeding.
* Weighted percentages were given.
† Percentage distribution of given PLF in survey groups.
‡ Percentage distribution of type of food in infants given PLF.
Variations in frequency distributions of prelacteal feeding by key factors
Estimated rates and 95 % CI and absolute changes of PLF over the years 2003–2018 were calculated according to mother–infant pair characteristics and are given in Table 3. Estimated rates of PLF according to mother–infant pair characteristics in vaginally delivered and caesarean section delivered mothers and in overall data are documented in Table 4.
Table 3 Estimated prevalence (rates), 95 % CI and absolute changes of prelacteal feeding according to mother–infant pair characteristics (Turkey Demographic and Health Surveys (TDHS) 2003–2018)
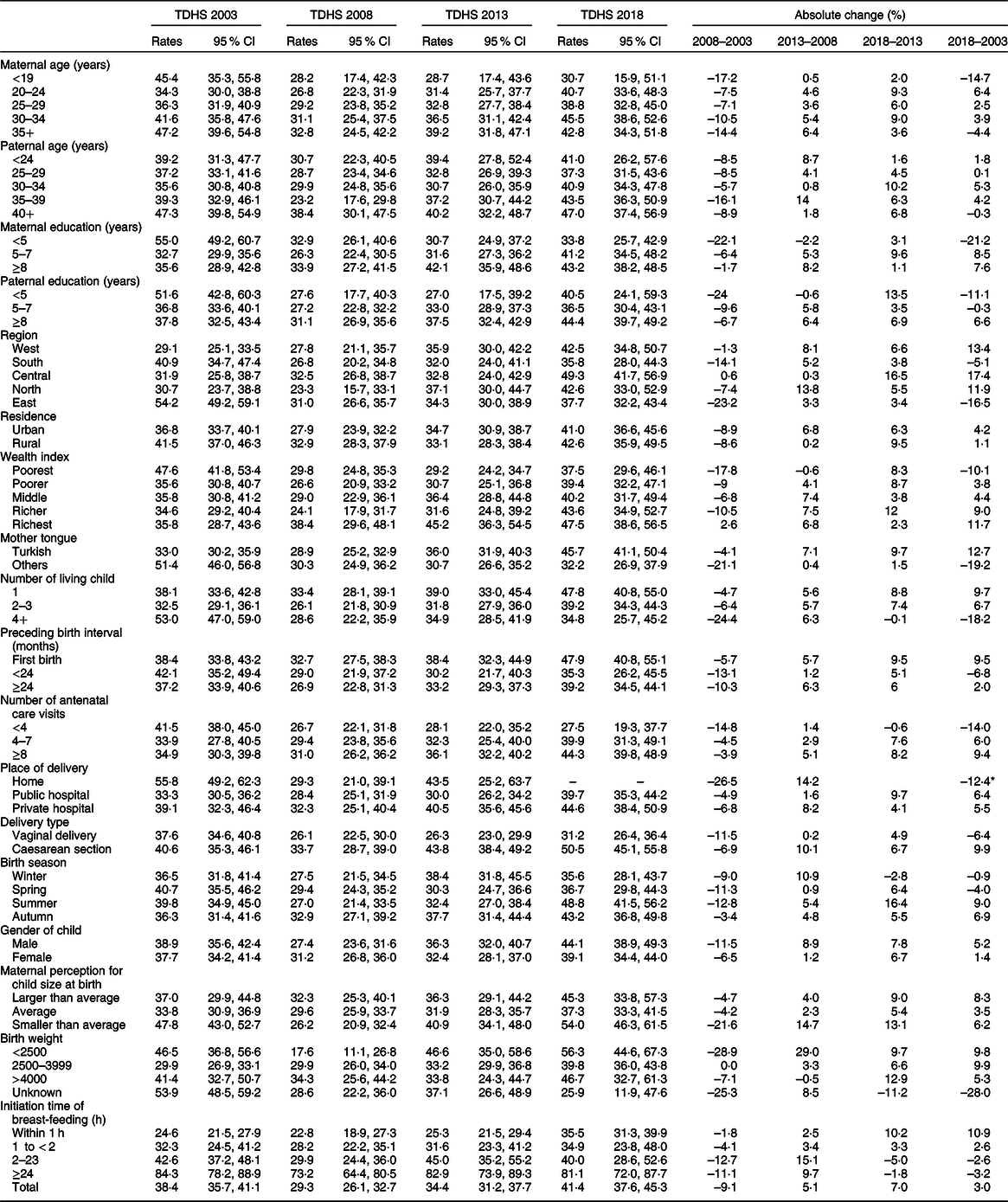
* Absolute change percentage of home delivery represents 2013–2003.
Table 4 Estimated prevalence (rates) of prelacteal feeding and its associations according to delivery type
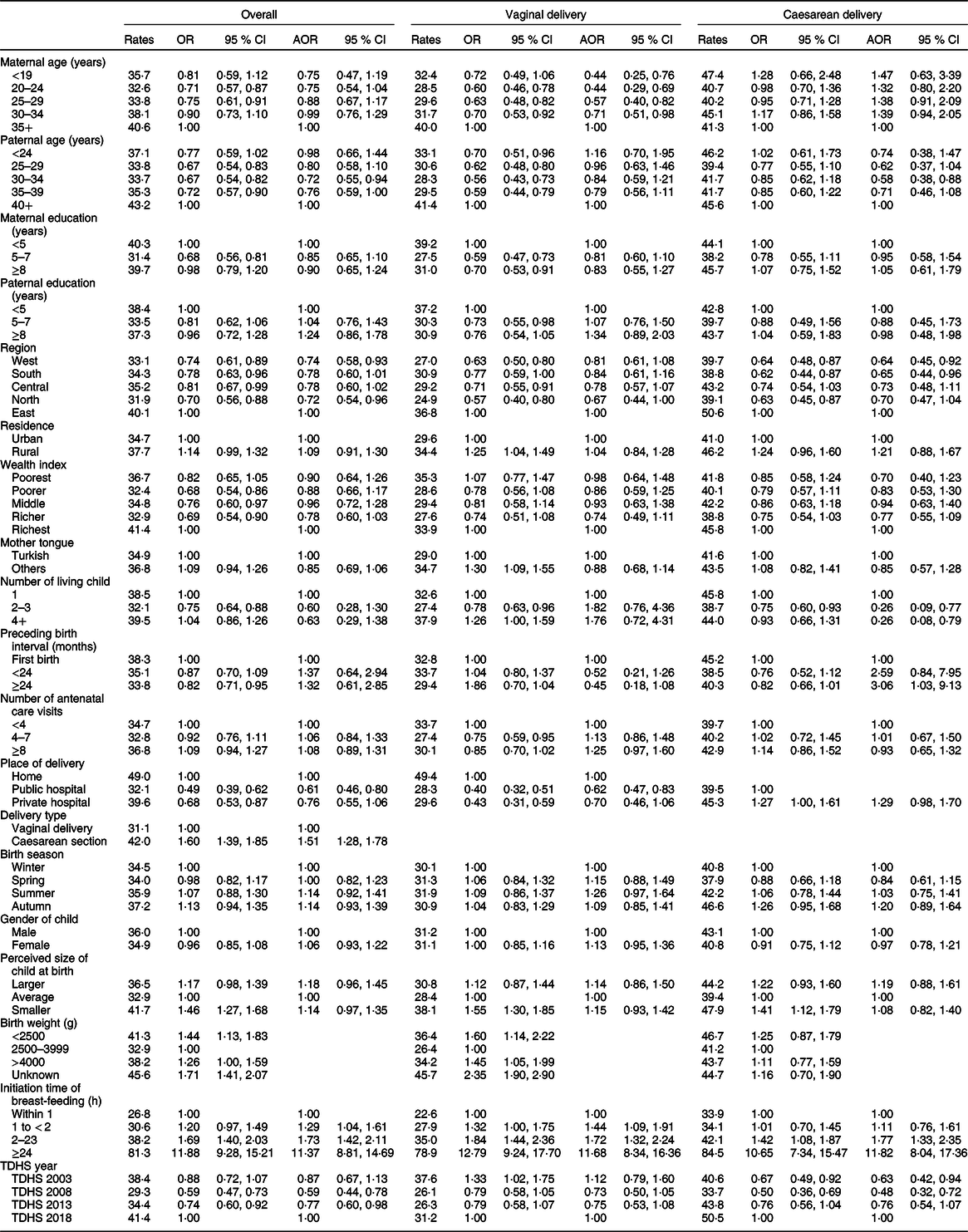
AOR, adjusted OR; TDHS, Turkey Demographic and Health Surveys.
* Factors used in the computation are fixed at the following values: maternal age ≥ 35 years; paternal age ≥ 40 years; maternal education < 5 years; paternal education < 5 years; region = East; residence = urban; wealth index = richest; mother tongue = Turkish; number of living child = 1; gender of child = male; preceding birth interval = first pregnancy; number of antenatal visits < 4; place of delivery = home; delivery type = vaginal delivery; perceived size of child at birth = average; initiation time of breast-feeding: within 1 h; birth season = winter; TDHS year = 2018.
† All factors included except delivery type.
Highest PLF rates were observed in older parents from 2003 to 2018 (Table 3). Lowest PLF was seen in mothers aged 20–29 years and fathers aged 25–39 years compared with old parents in vaginal delivery cases and overall cases. Similar changes were not present in caesarean delivery cases (Table 4).
In 2003, PLF practices were significantly more frequent in parents with low education. However, this relationship disappeared over the years after 2003 (Table 3). There were more than 20 % decrease in PLF of cases with low paternal education between 2003 and 2008. Overall, maternal education showed ‘u’ band change in PLF, lowest at the education level of 5–7 years (Table 3).
In 2003, while the highest PLF rate was observed in the Eastern region (54·2 %, 95 % CI 49·2, 59·1), the lowest PLF rates were detected in the Western region (29·1 %, 95 % CI 25·1, 33·5). There was no regional difference after 2003 (Table 3). When the trend in the frequency of PLF in regions by years is examined, the most significant absolute change was observed in the Central (17·4 % increase) and Eastern regions (16·5 % decrease, Table 3). In the overall analysis, while the highest PLF rate was observed in the Eastern region (40·1 %), the lowest PLF rates were detected in the Northern and Western regions (North 31·9 %; West 33·1 %). Furthermore, the Eastern region had the highest PLF rates in both caesarean section (50·6 %, 95 % CI 45·4–55·8) and vaginal delivery (36·8 %, 95 % CI 33·7–39·9). Merged data analysis revealed that all regions had lower odds for PLF than the Eastern region (Table 4).
Based on 2003 and 2018 TDHS results, there was an association between mother tongue and PLF (Table 3). Although in 2003, PLF practices were significantly more frequent in mothers speaking other mother tongue (51·4 %, 95 % CI 46·0, 56·8) compared with those speaking Turkish (33·0 %, 95 % CI 30·2, 35·9); in 2018, PLF rate was higher in mothers speaking Turkish (45·7 %, 95 % CI 41·1, 50·4) compared with those speaking other mother tongue (32·2 %, 95 % CI 26·9, 37·9). When the absolute change of PLF rate between 2018 and 2003 was examined, it was observed that while the PLF rate increased in mothers speaking Turkish (12·7 %), the rate decreased in those speaking other mother tongue (–19·2 %) (Table 3). In the overall analysis, vaginally delivered mothers speaking other mother tongue had the highest PLF rates compared with vaginally delivered mothers speaking Turkish (34·7 v. 29·0 %; OR 1·30, 95 % CI 1·09, 1·55).
According to 2018 TDHS results, PLF rates were higher in mothers having ≥8 ANC visits (44·3 %, 95 % CI 39·8, 48·9) than those having <4 ANC (27·5 %, 95 % CI 19·3, 37·7) (Table 3). Overall analysis revealed no significant change in PLF rates by ANC (Table 4).
The TDHS in 2003 showed that newborns who were delivered at home were more likely to receive PLF (55·8 %, 95 % CI 49·2, 62·3) compared with those were delivered in a health facility (public hospital: 33·3 %, 95 % CI 30·5, 36·2; private hospital: 39·1 %, 95 % CI 32·3, 46·4). In 2018, home deliveries were not included in the analysis due to the very low numbers (Table 3). In the overall analysis, the babies born at home had the higher PLF rates (49·0 %, 95 % CI 43·6, 54·5) compared with those born in public hospitals (32·1 %, 95 % CI 30·4, 33·9) and in private hospitals (39·6 %, 95 % CI 36·3, 43·0). In addition, the private hospital’s PLF rate was statistically significantly higher than those of public hospital’s PLF rate (OR 1·27, 95 % CI 1·00, 1·61) in caesarean delivery group (Table 4).
The current study showed that statistically significant increase in PLF rates was seen in newborns who were delivered by caesarean section compared with those delivered by vaginal delivery in 2013 and 2018 TDHS (Fig. 1). When the absolute change of PLF rate between 2018 and 2003 was examined, it was found that while the PLF rate increased in caesarean deliveries (9·9 %), the rate decreased in vaginal deliveries (–6·4 %) (Table 3). In the overall analysis, newborns who were delivered by caesarean section were more likely to receive PLF compared with those who were delivered by vaginal delivery (42·0 v. 31·1 %; OR 1·60, 95 % Cl 1·39, 1·85; Table 4).
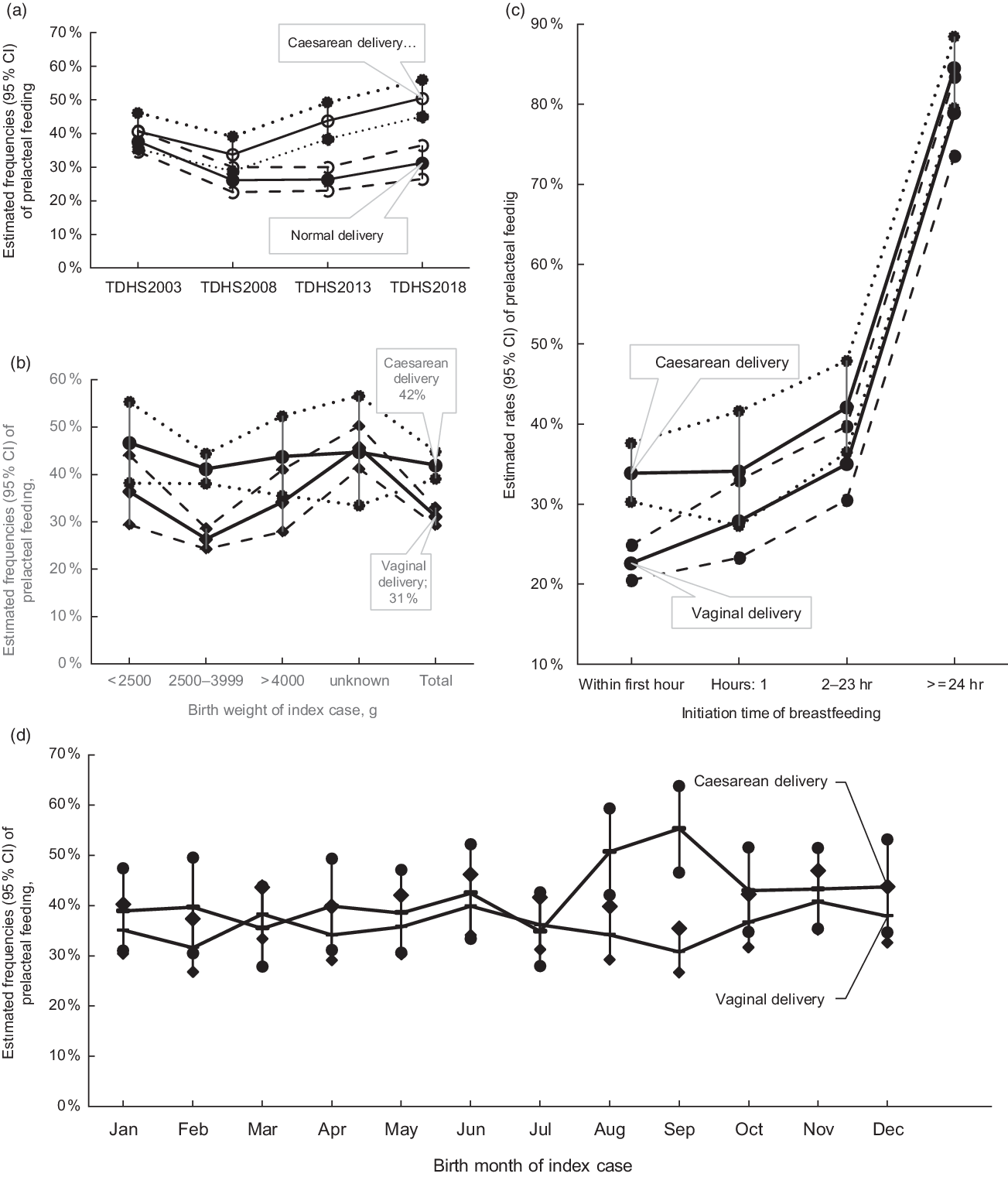
Fig. 1 Estimated rates and 95 % CI of prelacteal feeding according to delivery type with survey time from Turkey Demographic and Health Survey (TDHS) 2003 to TDHS 2018 (a), with birth weight (b), with initiation time of breast-feeding (c) and with birth month (d)
Delayed initiation of breast-feeding was associated with an increased PLF frequency in all surveys and overall analysis (Tables 3 and 4, see online supplementary material, Supplemental Table 1). When the trend in the percentage of PLF according to the initiation time of breast-feeding by years was examined, the most significant absolute change was observed in within 1 h (10·9 % increase) (Table 3). We also examined the effect of mode of delivery on the initiation time of breast-feeding and PLF relationship. While the PLF rate in cases with early initiation was 22·6 % (95 % CI 20·5, 24·9) for vaginal delivery, this rate was 33·9 % (95 % CI 30·3, 37·6) for caesarean delivery (Table 4 and Fig. 1). The univariate analysis of overall data showed that delayed initiation of breast-feeding after delivery was associated with significantly higher odds of introduction of PLF (2–23 h: OR 1·69, 95 % CI 1·40, 2·03; ≥24 h: OR 11·88, 95 % CI: 9·28, 15·21) compared with those within 1 h after delivery. Similar relationships were determined in both birth types when vaginal and caesarean births were analysed separately (Table 4).
Birth weight had an impact on the prevalence of PLF in merged data and vaginal delivery cases; however, no influence of birth weight in PLF was detected in caesarean delivery cases (Fig. 1 and Table 4).
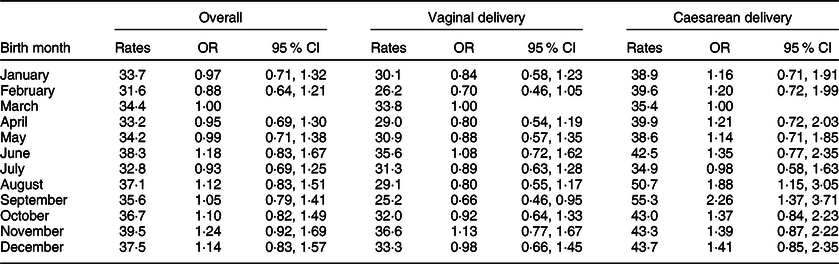
Trends in PLF status by birth month and delivery type were also examined (Fig. 1). The results of the study showed that the rate of PLF in newborns born by caesarean section was statistically significantly higher in September (55·3 %, 95 % CI 46·5, 63·8) compared with newborns born in March and July (March: 35·4 %, 95 % CI 27·9, 43·8; July: 34·9 %, 95 % CI 27·9, 42·6). In the univariate analysis, while the PLF rate in vaginal deliveries did not related to birth months, PLF risk was 1·88-fold higher in caesarean deliveries in August (95 % CI 1·15, 3·05) and 2·26-fold higher in September (95 % CI 1·37, 3·71) compared with March (Table 5 and Fig. 1).
Table 5 Estimated monthly prevalence (rates) of prelacteal feeding and its associations according to delivery type
Multivariate analysis
Overall multivariate analysis of the four TDHS data showed that living in the Northern and Western regions decreased the PLF risk by 28 % (adjusted OR (AOR) 0·72, 95 % CI 0·54, 0·96) and 26 % (AOR 0·74, 95 % CI 0·58, 0·93), respectively, compared with the Eastern region. Caesarean deliveries in the Western (AOR 0·64, 95 % CI 0·45, 0·92) and Southern regions (AOR 0·65, 95 % CI 0·44, 0·96) decreased the PLF risk compared with the Eastern region (Table 4).
In the multivariate analysis, public hospital deliveries decreased PLF risk by 39 % compared with home deliveries (AOR 0·61, 95 % CI 0·46, 0·80). Among vaginal births, public hospital deliveries decreased the PLF risk by 38 % compared with home deliveries (AOR 0·62, 95 % CI 0·47, 0·83). In caesarean deliveries, there was no statistically significant difference among public and private hospitals in terms of PLF (Table 4). PLF rate was 1·51 times higher (95 % CI 1·28, 1·78) in mothers delivered by caesarean section as compared with those delivered by vaginal route (Table 4).
The multivariate analysis of overall data showed that delayed initiation of breast-feeding after delivery was associated with significantly higher odds of introduction of PLF compared with the first hour, and also a dose–response association was found (1 ≤ 2 h: AOR 1·29, 95 % CI 1·04, 1·61; 2–23 h: AOR 1·73, 95 % CI 1·42, 2·11; ≥24 h: AOR 11·37, 95 % CI 8·81, 14·69). A similar initiation time and PLF relationship were observed in mothers both vaginally delivered and caesarean section delivered (Table 4).
Other factors significantly associated with PLF were maternal age <35 years in vaginal deliveries, 30–34 years paternal age, having ≥2 live children in caesarean section deliveries, and birth interval >24 months in caesarean section deliveries (Table 4).
We also compared the change in PLF risk over the surveys. When compared with 2018, PLF risk was 41 and 23 % lower in 2008 (AOR 0·59, 95 % CI 0·44, 0·78) and 2013 (AOR 0·77, 95 % CI 0·60, 0·98), respectively (Table 4).
In the multivariate analysis, maternal and paternal education, residence, wealth index, mother tongue, gender of the child, number of antenatal visits, perceived birth size of child and season of birth were not associated with PLF (Table 4).
Discussion
The main objective of the current paper was to provide an overall view of the factors and trends associated with PLF among <24-month-old infants over the years covering 1998–2018 by four consecutive TDHS in Turkey. In the current study, although the onset of breast-feeding was relatively high within the first hour after delivery, more than one-third of infants received prelacteal feeds other than breast milk during the first 3 d of life. There are several studies investigating the frequency of PLF and the associated factors in the literature(Reference Khanal, Adhikari and Sauer14,Reference Patel, Banerjee and Kaletwad15,Reference Boccolini, Perez-Escamilla and Giugliani18–Reference Berde, Yalcin and Ozcebe23) . In the study including seven Latin American and Caribbean countries, the overall prevalence of PLF was reported to be 32·8 %(Reference Boccolini, Perez-Escamilla and Giugliani18). In that study, PLF frequency reportedly ranged from 18·0 % in Guiana to 55·2 % in Dominican Republic(Reference Boccolini, Perez-Escamilla and Giugliani18). In another study involving twenty-two sub-Saharan African countries, the overall prevalence of PLF was reported as 32·2 %(Reference Berde and Ozcebe19); the frequency of PLF was reported to be 2·5 % in Malawi with the lowest frequency and 67·0 % in Cote d’Ivoire with the highest frequency(Reference Berde and Ozcebe19). Studies done in the Asian countries reported a PLF prevalence of 26·5 % in Nepal and 16·9 % in India(Reference Khanal, Adhikari and Sauer14,Reference Patel, Banerjee and Kaletwad15) . In a recent study using data from the most recent Demographic and Health Surveys (2000–2013) from fifty-seven countries, overall ‘avoidance of PLF’ frequency was reported as 49·2 %. At the regional level, while the highest prevalence for avoidance was in Latin America (65·2 %), the lowest prevalence was reported in South/Southeast Asia (41·0 %)(Reference Oakley, Benova and Macleod20).
PLF and breast-feeding practices vary across countries and regions, and also with different racial and ethnic within the same country(Reference Oakley, Benova and Macleod20,Reference Alghamdi, Horodynski and Stommel24–Reference Quah, Cheng and Cheung26) . In our study, regional variations were observed in the 2003 TDHS; however, in the subsequent years, regional differences were disappeared. In 2003 and in the overall analysis, the Eastern region was found to be the most disadvantaged region in terms of PLF risk. All regions except Central region showed a decline in PLF between 2003 and 2008. During survey periods, the Central region showed no improvement in PLF and reached the highest regional rates in TDHS 2018. At the end, in all regions except the Southern and Eastern regions, there was an upward trend in PLF in TDHS 2018 compared with TDHS 2003. In the current study, based on the 2003 and 2018 TDHS results, there was also an association between mother tongue and PLF. Although in 2003, PLF practices were significantly more frequent in mothers speaking other mother tongue compared with those speaking Turkish; in 2018, PLF rate was higher in mothers speaking Turkish compared with those speaking other mother tongue. Turkey is a country that hosts a large number of cultural diversity. The other mother tongue category consists of largely Kurdish and Arabic languages, and these mother tongues are especially common in the Southern and Eastern regions. Among other factors, it is possible that there will be some additional cultural and regional changes after the Syrian migration that started in 2011. A recent study documented lower rates of exclusive breast-feeding in the Syrian refugee mothers than Turkish mothers(Reference Bayram Deger, Ertem and Cifci27). However, it is not possible to identify regional and mother tongue relations and to distinguish the differences with the current study. The current study has revealed the necessity of conducting qualitative and semi-qualitative further field researches on breast-feeding and PLF practices.
The changes in culture, local beliefs and access to food and drinks have an impact on the type of prelacteal feeds(Reference Khanal, Adhikari and Sauer14,Reference Rogers, Abdi and Moore28,Reference Saka, Ertem and Musayeva29) . While in West and Central Africa, plain water is the most common fluid given to infants in the first 3 d after birth, in some Latin American and Caribbean countries, the most common type of PLF is infant formula(Reference Boccolini, Perez-Escamilla and Giugliani18,Reference Berde and Ozcebe19) . Our study showed that although in 2003, the most common given PLF was plain water, in subsequent years, the most common PLF was varied as infant formula. Furthermore, in 2018 THDS, the ratio of infant formula given as prelacteal feeds increased to 92·1 %. Based on the 2019 UNICEF report, the rise in use of breast milk substitutes was reported as an area of growing concern globally. Sales of milk-based formula grew by 72 % in upper middle-income countries such as Brazil, China and Turkey from 2008 to 2013(2). Turkey has enacted legislation or other legal measures encompassing a few provisions of ‘International Code of Marketing of Breast-milk Substitutes’, and advertising of only infant formula products used for the first 6 months was banned. Thus, intensive cross-promotion of infant formula indirectly via the promotion of follow-up formula and foods for infants and young children is among the most important threatenings of BFHI. Also, easy access to infant formula, inadequate and inappropriate counselling on breast-feeding during prenatal follow-up visits to pregnant women and inability to support early postnatal breast-feeding are considered as possible additional reasons of this outcome(Reference Erkul, Yalcin and Kilic30).
The incidence of caesarean section delivery is increasing globally(Reference Betran, Merialdi and Lauer31). Currently, Turkey is among the countries having the highest caesarean delivery rates in the world and has the highest caesarean delivery rate in OECD (Organisation for Economic Co-operation and Development) countries(Reference Berde, Yalcin and Ozcebe23,32,33) . There is evidence in the literature documenting a positive association between caesarean section (especially an elective pre-labour caesarean section) and suboptimal breast-feeding practices(Reference Prior, Santhakumaran and Gale34,Reference Orun, Yalcin and Madendag35) . In agreement with previous studies conducted in different world regions, we found that caesarean section delivery substantially increased the risk of introduction of PLF(Reference Patel, Banerjee and Kaletwad15,Reference Boccolini, Perez-Escamilla and Giugliani18,Reference Nguyen, Withers and Hajeebhoy21,Reference Prior, Santhakumaran and Gale34,Reference Raheem, Binns and Chih36) . However, this association was statistically significant since 2013, compatible with the increasing trend in caesarean rates. The usage of anaesthesia, delayed recovery of mothers due to surgical procedures, post-operative care routines that interrupt bonding and mother–infant interaction, delay in initiation of breast-feeding after delivery and delayed onset of lactation are the possible reasons to explain this relationship(Reference Prior, Santhakumaran and Gale34,Reference Chang and Heaman37–Reference Chapman and Perez-Escamilla39) . Providing additional breast-feeding support for caesarean delivered mothers may be beneficial to prevent the introduction of PLF(Reference Prior, Santhakumaran and Gale34–Reference Chang and Heaman37,Reference Dewey40) .
In our study, consistent with the results of previous studies, mothers who delivered in public hospitals had lower odds of giving prelacteal feeds compared with mothers who delivered at home(Reference Berde and Ozcebe19,Reference Oakley, Benova and Macleod20,Reference Kumar, Agarwal and Swami41) . In a recent study describing early breast-feeding practices in fifty-seven countries, home deliveries with a skilled birth attendant and deliveries in public sector were associated with a higher prevalence of positive breast-feeding practices(Reference Oakley, Benova and Macleod20). In Turkey, caesarean section percentages are markedly lower in public hospitals compared with private hospitals(33). In addition, breast-feeding is likely to be more supported and promoted by health professionals in public hospitals(Reference Berde, Yalcin and Ozcebe23).
Timely initiation of breast-feeding has a positive impact to decrease PLF practices(Reference Patel, Banerjee and Kaletwad15,Reference Champeny, Pries and Hou42,Reference Temesgen, Negesse and Woyraw43) . Our results showed that the risk of PLF increased gradually as the initiation time of breast-feeding increased. However, as an important result of the study, approximately one-quarter of newborns who initiate breast-feeding timely received PLF. It is noteworthy that this ratio increased to 35·5 % in 2018. Although this seems to be a contradiction, increased frequency of PLF within 1 h after delivery partly appears to be associated with caesarean deliveries (Fig. 1). This result also supports the negative effect of caesarean delivery on early mother–infant interaction and timely initiation of breast-feeding. However, it is necessary to make sure that the concept of ‘within 1 h after birth’ is understood correctly by especially caesarean delivered mothers. Further, the longitudinal clinical studies are likely to be more descriptive in this issue.
The current study showed that PLF risk was higher in caesarean births in August and September which are the hot months. There are some controversial studies about environmental heat and insufficient breast milk(Reference Zia, Golombek and Lemon44–Reference Lavagno, Camozzi and Renzi47). Past studies documented a non-significant seasonal variation in weight loss(Reference Bhat, Lewis and David45,Reference Uras, Karadag and Dogan46) . Previous studies reported excessive weight loss and early neonatal hypernatraemia during the initiation of breast-feeding in hot months(Reference Lavagno, Camozzi and Renzi47,Reference Davanzo, Cannioto and Ronfani48) . Davanzo et al. (Reference Davanzo, Cannioto and Ronfani48) reported that neonatal weight loss ≥8 % was associated with caesarean section, hot season, any formula feeding and jaundice not requiring phototherapy in healthy term infants. However, a recent study documented no seasonal association with weight loss(Reference Zia, Golombek and Lemon44). We postulated that the health professionals’ concerns about the exacerbation of the effects of lactation failure due to environmental heat in the hot season months in cases having caesarean delivery which may have increased the tendency to offer PLF especially. However, the TDHS data set does not contain questions to explain this relationship. Therefore, further prospective studies are needed to explain the rationale of seasonal variations.
Another important result of the study is that in the multivariate analysis, the number of ANC visits was not associated with PLF. This result means that ANC visits could not be used effectively regarding prenatal breast-feeding counselling. Among Ten Steps to Successful breast-feeding, step 3 suggesting ‘antenatal counseling about the benefits and management of breast-feeding for pregnant women and their families’ is among the most important key clinical practices(1,Reference Erkul, Yalcin and Kilic30) . Especially from the third trimester, every follow-up visit with pregnant women and their families should be considered as an opportunity for breast-feeding education, and special attention should be paid to this issue by the healthcare professionals.
Strengths and limitations
Our study has some limitations. First, due to the cross-sectional nature of the study design, attention should be paid that identified determinants of PLF do not indicate causality. In the current study, we included in analysis for potential independent variables which subject to recall bias. There may also be other variables that are unknown and not included in the study. Additionally, some known factors such as some cultural practices and beliefs, healthcare professionals’ advices, mothers’ own intention could not be included in the analysis. TDHS does not involve any question about breast-feeding support offered in the maternity wards. The current study does not include the issues such as counselling on breast-feeding during antenatal visits, and effect of BFHI. As another limitation, international standard questionnaires are used in TDHS and unfortunately, the current study does not contain a qualitative methodology. Additional studies with the qualitative component would be more effective to explain PLF relationships.
On the other hand, the study has the strength of being a nationally representative study with a high response rate. In addition, complex sample analysis was performed to account for the sampling strategy and sample weight, and thus, the findings are generalisable to the entire country.
Conclusion
To eliminate suboptimal breast-feeding practices in Turkey, interventions targeting to decrease PLF rates and to extend the duration of breast-feeding are among vital importance issues. Counselling on breast-feeding, delivery type during antenatal visits, postnatal lactation management support and social support should be provided to all mothers and families. In mothers delivered by caesarean section, special attention should be given to early breast-feeding support of mothers who had repeated caesarean births and >2 years between births.
Many of the health facilities in Turkey have adopted BFHI, and these health facilities implement ‘Ten Steps to Successful Breastfeeding’ policy. However, more than a quarter century after the start of the programme, sustainability of the gained standards became increasingly important both at the facility and at the country-level implementation(Reference Çaylan, Kılıç and Tetik9). Therefore, periodic self-monitoring and external evaluation of certified hospitals are vital importance. In addition, to decrease PLF rates, the adoption and implementation of step 4 and step 6 in all maternity hospitals should also be carefully monitored. In Turkey, revitalising and strengthening of the baby-friendly practices which enable the improvement of breast-feeding indicators over the past 20 years should be among priorities.
Acknowledgements
Acknowledgements: None. Financial support: The current research received no specific grant from any funding agency, commercial or not-for-profit sectors. No other entity besides the authors had a role in the design, analysis or writing of the current article. However, the financial support of the 2008, 2013 and 2018 Turkey Demographic and Healths Surveys has been provided by the Scientific and Technological Research Council of Turkey (TÜBİTAK) within the scope of the 1007 Support Programme for Research and Development Projects of Public Institutions. Conflicts of interest: There are no conflicts of interest. Authorship: S.S.Y. was responsible for formulating the research question, S.S.Y., S.Y. and M.A.E. for analysing the data and S.S.Y. and N.C. for writing the article. All authors reviewed and edited the final version of manuscript. Ethics of human subject participation: The current research did not involve human subjects but relied on the secondary data analysis of a deidentified data set. Ethical standards were upheld in the research process. Necessary permissions and survey data were obtained from Hacettepe University Institute of Population Studies. The study protocol of TDHS had been approved by Hacettepe University Ethics Committee.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020002037.










