Introduction
Weed management is consistently ranked among the top priorities of the United States sweetpotato industry. Amaranth species can drastically reduce yields (up to 85%) in sweetpotato (Basinger et al. Reference Basinger, Jennings, Monks, Jordan, Everman, Hestir, Waldschmidt, Smith and Brownie2019; Meyers et al. Reference Meyers, Jennings, Schultheis and Monks2010; Semidey at al. 1987; Smith et al Reference Smith, Jennings, Monks, Chaudhari, Schultheis and Reberg-Horton2020). Yellow and purple nutsedge (Cyperus esculentus L. and C. rotundus L., respectively) negatively affect sweetpotato yield and quality, and losses from 18% to 96% have been reported (Meyers and Shankle Reference Meyers and Shankle2015). Large crabgrass [Digitaria sanguinalis (L.) Scop.] at densities of 1 to 16 plants m−1 of row reduced yields from 35% to 76% in sweetpotato (Basinger et al. Reference Basinger, Jennings, Monks, Jordan, Everman, Hestir, Waldschmidt, Smith and Brownie2019). Grieg and Al-Tikriti (Reference Grieg and Al-Tikriti1966) and Glaze et al. (Reference Glaze, Harmon and Phatak1981) found that yields of sweetpotato plots were reduced by more than 90% in weedy treatments compared with treatment plots receiving herbicides, hand-weeding, and cultivation. Delaying the onset of weeding sweetpotato beyond 2 wk after planting resulted in substantial yield reduction (Levett Reference Levett1992; Seem et al. Reference Seem, Creamer and Monks2003). Semidey et al. (Reference Semidey, Liu and Ortiz1987) examined the effect of various populations of Amaranthus spp. on ‘Miguela’ sweetpotato and found that season-long density of four spleen amaranth (Amaranthus dubius Mart. Ex Thell.) plants per square meter reduced sweetpotato yield.
Conventional sweetpotato growers use herbicides, between-row cultivation, mowing, and hand-weeding. Herbicides commonly used for weed management in sweetpotato include flumioxazin, S-metolachlor [registered for use in some states under §24(c) of the Federal Insecticide, Fungicide, and Rodenticide Act], clomazone, and two graminicides (sethoxydim and clethodim). Although napropamide and DCPA are registered for use with sweetpotato crops, they provide inconsistent and often inadequate weed control (Weir Reference Weir2001). Each of the registered herbicides have drawbacks. Flumioxazin, S-metolachlor, and clomazone all require a rainfall or irrigation for activation, but few producers have the infrastructure for overhead irrigation. If rainfall is not timely, weeds emerge prior to activation and are not controlled. Flumioxazin must be applied before transplanting and requires that planting ridges be formed and the top of the ridge leveled. If not done properly, the herbicide is removed from the center of the planted row during transplanting, thereby providing little weed control in the row. Weeds that escape control in the row cannot be controlled with cultivation and compete the most with the developing crop.
Mechanical weed control is a common practice among sweetpotato producers, who use multiple tillage times during field preparation and two to three cultivations during the growing season. Although producers use wick/wiper applicators and mowing as weed management tactics, neither is successful, because to treat escaped weeds, they must first grow above the sweetpotato canopy where they compete with the crop for light (Coleman Reference Coleman2014). Escaped weeds are removed by hand. Many sweetpotato fields in the southeastern United States are hand-weeded at an estimated expense of $510 per acre (Tregeagle and Washburn Reference Tregeagle and Washburn2020). The lack of adequate weed control is the most important obstacle to the adoption of organic production or sustainable cultural practices (i.e., no-tillage or minimum tillage).
The leading U.S. sweetpotato cultivars (‘Beauregard’ and ‘Covington’) are highly susceptible to weed interference to the extent that total crop failure has been reported under high weed pressure. Seem et al. (Reference Seem, Creamer and Monks2003) reported that yields for late planted Beauregard sweetpotato (June 20 and 28) were reduced less by weed interference than those planted earlier (May 31 and June 6), which was attributed to lower weed density and a more rapid rate of ground cover by sweetpotato vines at the later planting date. They concluded that the critical weed-free period for Beauregard, the amount of time required to maintain weed-free fields to prevent a decrease in yield, was between 2 and 6 wk after planting. Meyers et al. (Reference Meyers, Jennings, Schultheis and Monks2010) found that Palmer amaranth (A. palmeri S. Watson) populations of 6 plants m−2 reduced Beauregard and ‘Covington’ marketable root yield by more than 80%. The critical weed-free period was 2 wk for the interaction between Covington and Palmer amaranth (Knezevic et al. Reference Knezevic, Evans, Blankenship, Van Acker and Lindquist2002; Smith et al. Reference Smith, Jennings, Monks, Chaudhari, Schultheis and Reberg-Horton2020). These results imply that Palmer amaranth can be detrimental to yield even if the weed is allowed to grow for a short time after emergence prior to removal. Additionally, weeds can occupy different spatial niches and growth habits in an agroecosystem, thus additional weeds growing alongside Palmer amaranth will further decrease crop competitiveness (Cutulle et al. Reference Cutulle, Derr, McCall, Horvath and Nichols2013).
Cultivars that are tolerant of weed interference can be important components in integrated weed management in conventional and organic production. Research on weed interference has been reported for sweetpotato, and the data suggest that some cultivars may be more tolerant to weeds than others. LaBonte et al. (Reference LaBonte, Harrison and Motsenbocker1999) examined the effect of sweetpotato vine morphology on weed interference, and reported that yields of one clone, W-241 (subsequently named ‘Carolina Bunch’), were reduced by less than 20% by weed interference in comparison to weed-free plots, whereas all other clones in the study were reduced by between 50% and 70%. Carolina Bunch possesses a semi-erect vine growth habit (i.e., maximum main vine length between 75 and 150 cm) as described by the vine growth descriptors developed by the International Potato Center (Huamán Reference Huamán1991). Sweetpotato plants with erect growth have shorter internodes, which produces a denser canopy with greater height in the early growth stages, compared to the spreading vine growth. The superior weed suppression observed with this plant habit may result from the more effective shading provided by the canopy as it expands. Although genotypes with spreading vines grow rapidly in terms of spreading outward, much of the soil between vines is left bare during early growth, and weeds can emerge through the open canopy. Harrison and Jackson (Reference Harrison and Jackson2011) compared to the weed-free intervals of Carolina Bunch (semi-erect habit) and Beauregard (spreading habit) and reported a difference between cultivars in yield reduction caused by weed interference. This evidence suggests that the two clones may vary substantially in the weed-free interval required to produce maximum yields. The difference between clones is also evident in terms of the reduction of sweetpotato shoot biomass caused by weed interference and the suppression of weed growth by sweetpotato.
Many insect pests damage sweetpotato roots in the United States (Chalfant et al. Reference Chalfant, Jansson, Seal and Schalk1990; Cuthbert Reference Cuthbert1967; Cuthbert and Davis Reference Cuthbert and Davis1970). Injury by white grubs (primarily Phyllophaga spp.) and sweetpotato flea beetle (Chaetocnema confinis Crotch) can be variable. The most widespread across the United States is the WirewormDiabroticaSystena (WDS) complex, which consists of several species of wireworms (e.g., Melanotus communis Gyllenhal and Conoderus sp.), banded and spotted cucumber beetles (Diabrotica balteata J. L. LeConte and D. undecimpunctata howardi Barber), and Systena flea beetles. The sweetpotato weevil, Cylas formicarius elegantulus Summers, is the most important pest of sweetpotato worldwide including the coastal areas of the southern United States (Jansson et al. Reference Jansson, Hunsberger, Lecrone and O’Hair1990).
To provide additional weed and insect management strategies for sweetpotato, we initiated development of insect-resistant germplasm that also has competitive weed tolerance potential by breeding and selecting for sweetpotato clones that are fast growing and have semi-erect to erect canopy architecture. In 2015, the Sweetpotato Breeding and Genetics Program within the U.S. Vegetable Laboratory (USVL; a division of the U.S. Department of Agriculture [USDA] Agricultural Research Service) initiated a recurrent selection approach to generate sweetpotato clones with vigorous growth, compact plant habit, high yields, resistance to ground-dwelling insect pests, and that are competitive with weeds. In this study we compared the performance of six advanced sweetpotato clones to three control cultivars (Beauregard, Covington, and Monaco) over two seasons under various weed-free intervals. The results highlight the potential for the development of germplasm with erect plant architecture to mitigate weed interference and have resistance to insect pests.
Materials and Methods
Field studies were conducted in 2018 and 2019 at the USVL (32.80127°N, 80.06566°W) on a Yonges loamy fine sand (Aeric Paleaquults; <1% organic matter) and a soil pH 6.0 to 6.4. The experiment was designed as a randomized complete block with a split-plot arrangement where main plots were weed-free period and subplots were sweetpotato clone. A total of nine sweetpotato clones were planted each year (Table 1). Three sweetpotato cultivars were used as controls, two insect-susceptible with a spreading habit (Beauregard and Covington) and an insect-resistant with a semi-erect habit (Monaco). The remaining six clones were advanced selections from the USVL sweetpotato breeding program and have erect to semi-erect plant habit (maximum main vine length <75 cm or 75 to 150 cm, respectively). Plots were hand-weeded twice a week to maintain intervals free of naturally occurring weed species for 2, 3, or 4 wk after planting, and a cultivation-only treatment served as a control. The cultivation treatment was conducted 2 wk after planting on all plots. Treatments were replicated four times, and subplots measured four rows wide and 54.9 m long. Corn (Zea mays L.) had been planted preceding the trials, and the residue was mowed and disced into the soil prior to bed formation. Sweetpotato slips (∼30 cm long) were planted into narrow beds (∼38 cm wide by 30 cm tall) that were prepared by forming soil into rows ∼1 m apart. Fertilizer (1,121 kg ha−1 of 4N-3.5P-10K, Nutrien; Saskatoon, SK, Canada) was incorporated into the soil before bedding. Slips were planted at a spacing of 30 cm on June 20, 2018, and June 19, 2019. When weekly rainfall was not adequate (<2.54 cm) during the growing season, supplemental overhead irrigation was applied until all plots had received a total of 2.54 cm.
Table 1. Flesh and skin color, germplasm source, and origin of nine sweetpotato clones evaluated at various weed-free periods using a conventional bare ground production system. a, b

a Abbreviations: NCSU, North Carolina State University; USVL, U.S. Vegetable Laboratory.
b Evaluations were carried out in 2018 and 2019 in Charleston, SC.
Weed species and density were recorded on one square meter of row in each subplot 6 wk after planting. The predominant weeds in 2018 were carpetweed (Mollugo verticillata L.), chamberbitter (Phyllanthus urinaria L.), common dayflower (Commelina erecta L.), large crabgrass, common purslane (Portulaca oleracea L.), and yellow nutsedge. In 2019, the predominant weeds were Bermudagrass [Cynodon dactylon (L.) Pers.], carpetweed, chamberbitter, common dayflower, goosegrass [Eleusine indica (L.) Gaertn.], groundcherry (Physalis longifolia Nutt.), large crabgrass, common purslane, Pennsylvania smartweed (Polygonum pensylvanicum L.), spotted spurge (Euphorbia maculata L.), and yellow nutsedge.
At 120 d after planting, plots were mowed with a flail mower to remove foliage, and storage roots were harvested with a single-row potato digger (Model D-10M; U.S. Small Farm Equipment Co., Worland, WY). After harvest, storage roots were cured at 29.4 C and 85% relative humidity (RH) for 5 d and then stored at 14.4 C and 85% RH until roots were further processed for yield estimation and insect damage ratings. Two weeks after curing, storage roots were washed by hand to remove soil and other debris to allow for visual rating of insect damage. To allow storage roots to completely dry after washing, storage root yield and insect damage ratings were conducted 1 mo after harvest. The storage roots were graded and weighed according to the following grades: jumbo (>8.89 cm diameter and/or >22.86 cm long), U.S. No. 1 (5.08 to 8.89 cm diameter and 7.62 to 22.86 cm long), canner (2.54 to 5.08 cm diameter and 5.08 to 17.78 cm long), and cull (badly misshapen, rotted, and/or crack roots; see USDA, 2005). Insect-damaged roots were not grouped with culled roots. For each plot, the number of roots and weight by grade were recorded. Total yield was the summation of U.S. No. 1, canner, jumbo, and cull-grade storage roots. Marketable yield was the summation of U.S. No. 1 roots, canner roots, and jumbo roots. All individual storage roots were visually rated for insect damage by previously published procedures (Jackson et al. Reference Jackson, Harrison and Ryan-Bohac2012; Schalk et al. Reference Schalk, Peterson and Hamalle1987). We calculated the wireworm-cucumber beetle-flea beetle (WDS) severity index (Cuthbert and Davis Reference Cuthbert and Davis1970) by averaging the rating given to each root (1 = 1 to 5 holes or scars; 2 = 6 to 10 holes or scars; 4 = >10 holes or scars). This complex of insect pests consists of several species of wireworms (M. communis and Conoderus sp.), banded and spotted cucumber beetles (D. balteata and D. undecimpunctata howardi), and flea beetles (Systena sp.). Injury by white grubs (primarily Phyllophaga spp.), sweetpotato flea beetle (C. confinis), and sweetpotato weevils (C. formicarius elegantulus) were calculated as the percentage of total roots that had any damage by these insects. The percentages of uninjured roots (undamaged by any of the soil insect pests) also were determined for each clone. Data from each experimental trial were subjected to analysis of variance using the MIXED procedure with SAS software (version 9.4; SAS Institute, Cary, NC) to test the main effect of weed-free interval and sweetpotato clone, and the interaction between the two. Weed-free interval and sweetpotato clone were treated as fixed effects, and year and block and the appropriate error terms were treated as random effects. When significant year-by-clone interactions existed, data were analyzed and presented by year. Mean comparisons were produced using Fisher’s protected least significant difference at the 5% probability level.
Results and Discussion
Year-by-sweetpotato clone interactions were significant, thus variables were analyzed by year. There was no significant weed-free interval by sweetpotato clone interactions for all variables measured, therefore, only significant main effects are presented.
Effect of Weed-free Interval and Sweetpotato Clone on Weed Count
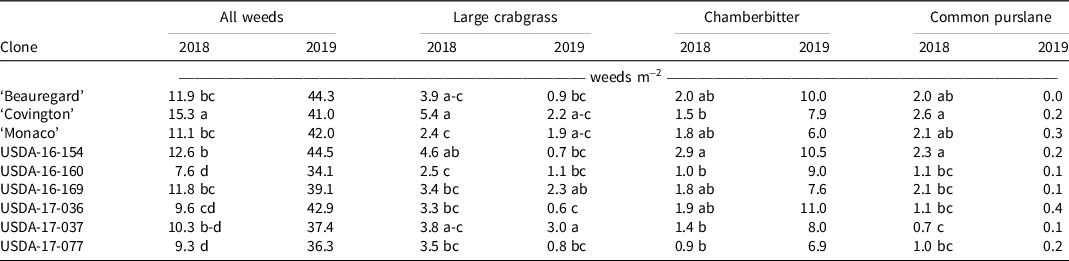
In both field seasons, we observed a reduction in overall weed numbers in response to increased weed-free interval times, with variable effects observed for different weed species. In 2018, 6-wk counts of total weeds, large crabgrass, and chamberbitter were significantly different among weed-free intervals (Table 2), when all weed-free intervals had fewer weeds than the weedy all-season treatment. In 2019, we observed a similar trend in the weed counts, with a reduction in carpetweed in response to increased weeding interval. We also observed a slight, but significant increase in common purslane for the 2-wk weed-free interval. In this specific agroecosystem common purslane was outcompeted by the other weeds in the weedy check plots. No differences in weed count were observed among weed-free interval in either year for common dayflower, yellow nutsedge, Bermudagrass, goosegrass, ground cherry, Pennsylvania smartweed, or spotted spurge.
Table 2. Effect of weed-free interval on weed counts per square meter of row at 6 wk after planting. a, b

a Means followed by the same letter within a column is not significantly different at P < 0.05 according to Fisher’s protected least significance difference test.
b Evaluations were carried out in 2018 and 2019 in Charleston, SC.
We also observed significant effects of sweetpotato clone on weed counts. In 2018, the counts of all weeds (large crabgrass, chamberbitter, and common purslane) were different among sweetpotato clones, whereas only large crabgrass counts were different in 2019 (Table 3). All advanced sweetpotato clones selected for weed competitiveness had significantly lower total weed counts than Covington in 2018. Overall, these results indicate that sweetpotato clones that have vigorous semi-erect or erect plant habit are effective at suppressing weed growth. This is consistent with the findings of Harrison and Jackson (Reference Harrison and Jackson2011), who noted that Carolina Bunch, an erect sweetpotato cultivar, was more effective at suppressing weed growth than Beauregard. Reduction of light penetration through the canopy of the semi-erect or erect sweetpotato clones could have reduced germination of certain weeds and could explain the differences in weed counts observed.
Table 3. Effect of sweetpotato clone on weed counts per square meter of row 6 wk after planting. a, b
a Means followed by the same letter within a column are not significantly different at P < 0.05 according to Fisher’s protected least significance difference test.
b Evaluations were carried out in 2018 and 2019 in Charleston, SC.
Effect of Weed-free Interval and Sweetpotato Clone on Storage Root Yield
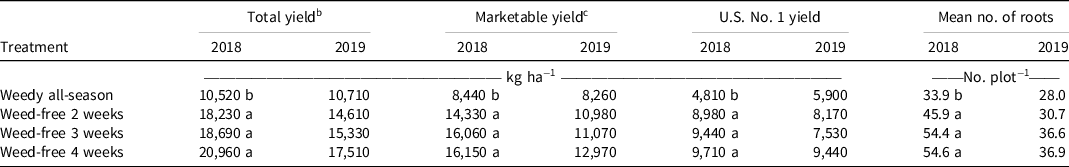
Weed-free interval treatments had a positive effect on sweetpotato yield. Significant differences in yield were observed among weed treatments only in 2018, with yield lower in the weedy all-season treatment (10,520 kg ha−1) compared to the weed-free interval treatments [18,230 to 20,960 kg ha−1 (Table 4)]. A similar trend was observed in 2019, with the lowest yield in each grade observed in the weedy all-season treatment and the highest yield observed in the 4-wk-long weed-free interval. Although these differences did not reach a level of statistical significance, this could be attributed to a lower overall yield in the 2019 field season compared with 2018 and variability in yield data among plots. The decrease yield in 2019 might be attributed to increased weed pressure as well as potential flood stress because there was over 26 cm more rainfall observed in 2019 than in 2018.
Table 4. Effect of weed-free interval on the total yield, marketable yield, U.S. No.1 yield, and mean number of storage roots for nine sweetpotato clones. a, d, e
a Means within a column followed by the same letter is not significantly different at P < 0.05 according to Fisher’s protected least significant difference test.
b Total yield was the summation of U.S. No. 1, canner, jumbo, and cull grade storage roots.
c Marketable yield = U.S. No. 1 roots + canner roots + jumbo roots. U.S. No. 1 = roots 5.08 to 8.89 cm diameter and 7.62 to 22.86 cm long; canner = roots 2.54 to 5.08 cm diameter and 5.08 to 17.78 cm long; jumbo = roots larger than either of the other grades, but marketable.
d Clones were grown in 2018 and 2019 in Charleston, SC.
e Yields have been rounded to the nearest ten.
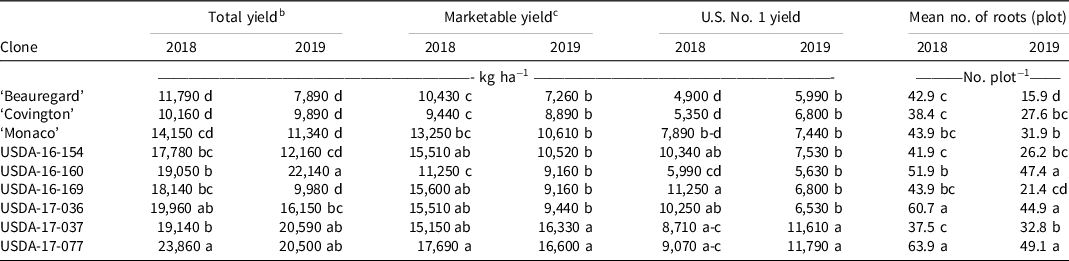
Significant differences were observed among sweetpotato clones for all yield variables in both years (Table 5). In 2018, all USDA clones exhibited higher total yield (17,780 to 23,860 kg ha−1) than Beauregard (11,790 kg ha−1) and Covington (10,160 kg ha−1). For marketable yield and yield of U.S. No. 1 grade storage roots, all USDA clones yielded higher than Beauregard and Covington except for USDA-16-160. USDA-17-077 was the highest yielding clone across all variables in 2018. USDA-16-160 was one of the clones with the highest total yields and was also one of the clones with the lowest marketable yields for both years. This was due to having a high percent of roots culled due to cracking. Across both years and all yield parameters, USDA-17-037 and USDA-17-077 consistently yielded the highest, with USDA-17-077 yielding significantly greater than the control cultivars. Yield in sweetpotato is highly variable, and furthermore, storage root formation in high-yielding cultivars can be strongly influenced by environment (Collins et al. Reference Collins, Wilson, Arrendel and Dickey1987; Manrique and Hermann Reference Manrique and Hermann2000). The differences observed among weed-free intervals in 2018 could be due to differences in rainfall amount received each year during the trial. Recorded rainfall in 2019 was 80.65 cm, whereas in 2018 it was 54.23 cm. The high rainfall in 2019 could have leached fertilizer from the plots and created unfavorable soil conditions for optimal plant growth. This could also be reflected in yield variables for sweetpotato clone because fertility may be more critical for some clones than others (i.e., USDA-16-169 vs. USDA-17-077). Villordon et al. (Reference Villordon, Gregorie, LaBonte, Khan and Selvaraj2018) noted that Beauregard appears to be more sensitive to the absence of phosphorus in temporal treatments compared to ‘Bayou Belle’ and provides a foundation for further studies to validate cultivar-specific requirements. In general, all yield variables were lower in 2019 than in 2018.
Table 5. Effect of sweetpotato clone on the total yield, marketable yield, U.S. No.1 yield, and mean number of storage roots for nine sweetpotato clones. a, d, e
a Means within a column followed by the same letter is not significantly different at P < 0.05 according to Fisher’s protected least significant difference test.
b Total yield was the summation of U.S. No. 1, canner, jumbo, and cull grade storage roots.
c Marketable yield = U.S. No. 1 roots + canner roots + jumbo roots. U.S. No. 1 = roots 5.08 to 8.89 cm diameter and 7.62 to 22.86 cm long; canner = roots 2.54 to 5.08 cm diameter and 5.08 to 17.78 cm long; jumbo = roots larger than either of the other grades, but marketable.
d Clones were grown in 2018 and 2019 in Charleston, SC.
e Yields have been rounded to the nearest ten.
Effect of Weed-free Interval and Sweetpotato Clone on Insect Pest Damage
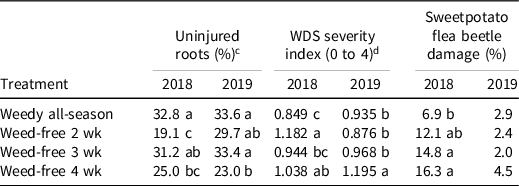
Weeding had a positive effect on insect pest damage. The percentage of uninjured storage roots and WDS severity index were significantly different among weed-free interval treatments in 2018 and 2019, whereas we observed significant differences in sweetpotato flea beetle damage in 2018 (Table 6). In both years, the weedy all-season treatment had the highest percent of uninjured roots (32.8% and 33.6%, respectively), whereas the weed-free-for-4-wk interval had the lowest percent of uninjured roots. For WDS severity index, the weed-free-for-4-wk interval had the highest index values for both years (1.038 and 1.195, respectively). Damage from sweetpotato flea beetle was the lowest in the weedy all-season treatment (6.9%). Weed diversity may be directly influencing damage by insect pests by masking the host plant through visual and olfactory mechanisms or via attracting natural enemies (Root Reference Root1973).
Table 6. Effect of weed-free interval on the uninjured storage roots, wireworm-cucumber beetle-flea beetle severity index, and percent sweetpotato flea beetle damage for nine sweetpotato clones. a, b, e
a Abbreviation: WDS, Wireworm–cucumber beetle–flea beetle severity index.
b Means followed by the same letter within a column is not significantly different at P < 0.05 according to Fisher’s protected least significance difference test.
c The percent of storage roots that were free of insect damage.
d WDS severity index: 1 = 1 to 5 scars, 2 = 6 to 10 scars, and 4 = >10 scars, averaged across all storage roots. Minimum score = 0.0 and maximum score = 4.0. A higher value indicates more damage occurred on the roots.
e Clones were grown in 2018 and 2019 in Charleston, SC.
Specific sweetpotato clones had significantly less insect damage. Significant differences were observed among sweetpotato clones in both years for the percent of uninjured roots, WDS severity index, and percent grub damage, whereas sweetpotato flea beetle damage was significant only in 2018 (Table 7). Beauregard was consistently the most damaged clone across years and insect damage category, whereas USDA-17-077 was consistently the least damaged by insects. Insect damage to ‘Monaco’ was in general lower than to Beauregard and Covington across both years and insect damage category. This is consistent with previously observed WDS resistance in Monaco and the susceptibility of Beauregard and Covington (Wadl et al. Reference Wadl, Williams, Horry and Ward2022), and the reason for these selections as controls in this study. Insect damage to USDA-16-160, USDA-16-169, and USDA-17-036 was inconsistent across years with respect to all insect damage variables, whereas USDA-17-037 and USDA-17-077 can be considered as being insect resistant.
Table 7. Effect of sweetpotato clone on the uninjured storage roots, wireworm-cucumber beetle-flea beetle severity index, and percent sweetpotato flea beetle damage for nine sweetpotato clones. a, b, f
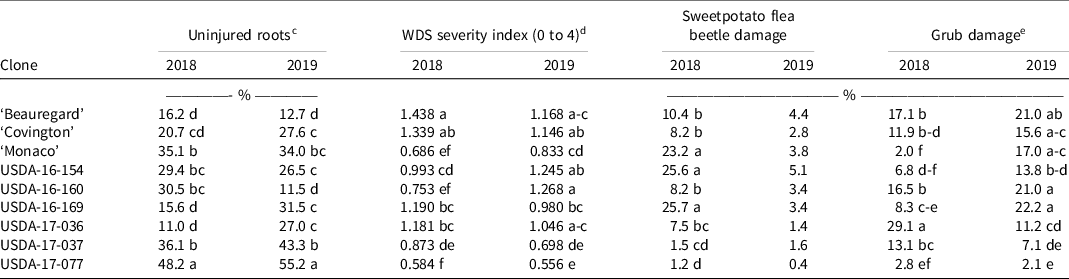
a Abbreviation: WDS, Wireworm–cucumber beetle–flea beetle severity index.
b Means followed by the same letter within a column is not significantly different at P < 0.05 according to Fisher’s protected least significance difference test.
c The percent of storage roots that were free of insect damage.
d WDS severity index: 1 = 1 to 5 scars, 2 = 6 to 10 scars, and 4 = >10 scars, averaged across all storage roots. Minimum score = 0.0 and maximum score = 4.0. A higher value indicates more damage occurred on the roots.
e The percent damaged caused primarily by white grubs.
f Clones were grown in 2018 and 2019 in Charleston, SC.
Practical Implications
Management of weeds and insect pests are of concern to sweetpotato growers, and control options are limited. The predominant cultivars grown in the United States are Beauregard and Covington, both of which have been shown to have severely reduced yields under weedy conditions (Basinger et al. Reference Basinger, Jennings, Monks, Jordan, Everman, Hestir, Waldschmidt, Smith and Brownie2019; Meyers et al. Reference Meyers, Jennings, Schultheis and Monks2010; Meyers and Shankle Reference Meyers and Shankle2015; Smith et al. Reference Smith, Jennings, Monks, Chaudhari, Schultheis and Reberg-Horton2020). Herbicide options are limited, and the increasing frequency of large rainfall events can leach herbicide treatments from the soil rendering them ineffective when herbicides are needed most. Our results support the findings of previous studies that the yield of sweetpotato does not appear to be greatly affected by weed interference when successful control is in place for approximately 3 to 4 wk after planting (Harrison and Jackson Reference Harrison and Jackson2011; Levett Reference Levett1992; Seem et al. Reference Seem, Creamer and Monks2003; Smith et al. Reference Smith, Jennings, Monks, Chaudhari, Schultheis and Reberg-Horton2020). Additionally, both cultivars are susceptible to the major insect pests of sweetpotato. With chlorpyrifos being banned as of 2022 by the U.S. Environmental Protection Agency, insecticide options are further limited for sweetpotato.
Host tolerance/resistance to weeds and insect pests offer an effective sustainable solution to these challenges facing sweetpotato producers, but this option is currently unavailable. Our results indicate that breeding for cultivars and/or germplasm that are competitive with weed interference and resistant to the major insect pests of sweetpotato offers promise. One of the breeding objectives at the USVL is the development of sweetpotato germplasm with erect plant habit combined with insect resistance. The breeding and selection of Carolina Bunch, the only United States cultivar with erect plant habit, demonstrated that the trait is heritable (Dukes et al. Reference Dukes, Jones, Schalk, Harrison and Hamilton1992). Ongoing research in collaboration with the vegetable weed science program at Clemson University’s Coastal Research and Education Center is focused on developing insect-resistant sweetpotato clones with vigorous, erect plant habit and desirable horticultural traits. The competitiveness of the new clones against weeds and their response to weed interference and insect pressure will be assessed to identify those with tolerance to weed interference and resistance to ground dwelling insect pests. In this study we identified two sweetpotato clones, USDA-17-037 and USDA-17-077, that had reduced weed counts, exhibited broad insect resistance, and were the highest yielding entries. The results of this study indicate that development of sweetpotato cultivars that are competitive with weeds through novel plant architecture (erect growth habit) and are also resistant to insects is an effective general pest management strategy, with particular benefit for organic and sustainable growers.
Acknowledgments
We thank Ty Phillips, Lance Lawrence, and Giovanni Caputo for assistance with planting, maintenance, and harvest of field plots. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by Clemson University or the USDA. The USDA is an equal opportunity employer. No conflicts of interest have been declared. This research received no specific grant from any funding agency, or commercial or not-for-profit sectors.










