Published online by Cambridge University Press: 05 August 2004
2K+ to K + K2+ and K to K3+ provide a reaction with a net
enthalpy equal to one and three times the potential energy of atomic
hydrogen, respectively. The presence of these gaseous ions or atoms with
thermally dissociated hydrogen formed a so-called resonance transfer
(rt)-plasma having strong VUV emission with a stationary inverted Lyman
population. Significant line broadening of the Balmer $\alpha $ , $\beta $
, $\beta $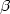 ,
and $\gamma $
,
and $\gamma $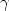 lines of 18 eV was observed, compared to 3–4 eV from a
hydrogen microwave plasma. Emission from rt-plasmas occurred even when the
electric field applied to the plasma was zero. The reaction was exothermic
since excess power of 20 mW cm−3 was measured by Calvet
calorimetry. An energetic catalytic reaction was proposed involving a
resonant energy transfer between hydrogen atoms and 2K+ or K to form
very stable novel hydride ions H−(1/p) called hydrino hydrides having a
fractional principal quantum numbers p = 2 and p = 4, respectively.
Characteristic emission was observed from K2+ and K3+ that
confirmed the resonant nonradiative energy transfer of 27.2 eV and 3 × 27.2 eV from atomic hydrogen to 2K+ and K, respectively. The product
hydride ion H−(1/4) was observed spectroscopically at 110 nm
corresponding to its predicted binding energy of 11.2 eV. The 1H MAS
NMR spectrum of novel compound KH*Cl relative to external tetramethylsilane
(TMS) showed a large distinct upfield resonance at −4.4 corresponding to an
absolute resonance shift of −35.9 ppm that matched the theoretical
prediction of p = 4. A novel peak of KH*I at −1.5 ppm relative to TMS
corresponding to an absolute resonance shift of –33.0 ppm matched the
theoretical prediction of p = 2. The predicted catalyst reactions, position
of the upfield-shifted NMR peaks for H−(1/4) and H−(1/2), and
spectroscopic data for H−(1/4) were found to be in agreement with the
experimental observations as well as previously reported spectroscopic data
for H−(1/2) and analysis of KH*Cl and KH*I containing these hydride
ions.
lines of 18 eV was observed, compared to 3–4 eV from a
hydrogen microwave plasma. Emission from rt-plasmas occurred even when the
electric field applied to the plasma was zero. The reaction was exothermic
since excess power of 20 mW cm−3 was measured by Calvet
calorimetry. An energetic catalytic reaction was proposed involving a
resonant energy transfer between hydrogen atoms and 2K+ or K to form
very stable novel hydride ions H−(1/p) called hydrino hydrides having a
fractional principal quantum numbers p = 2 and p = 4, respectively.
Characteristic emission was observed from K2+ and K3+ that
confirmed the resonant nonradiative energy transfer of 27.2 eV and 3 × 27.2 eV from atomic hydrogen to 2K+ and K, respectively. The product
hydride ion H−(1/4) was observed spectroscopically at 110 nm
corresponding to its predicted binding energy of 11.2 eV. The 1H MAS
NMR spectrum of novel compound KH*Cl relative to external tetramethylsilane
(TMS) showed a large distinct upfield resonance at −4.4 corresponding to an
absolute resonance shift of −35.9 ppm that matched the theoretical
prediction of p = 4. A novel peak of KH*I at −1.5 ppm relative to TMS
corresponding to an absolute resonance shift of –33.0 ppm matched the
theoretical prediction of p = 2. The predicted catalyst reactions, position
of the upfield-shifted NMR peaks for H−(1/4) and H−(1/2), and
spectroscopic data for H−(1/4) were found to be in agreement with the
experimental observations as well as previously reported spectroscopic data
for H−(1/2) and analysis of KH*Cl and KH*I containing these hydride
ions.