It has consistently been observed that attention-deficit hyperactivity disorder (ADHD) and conduct disorder commonly co-occur, and family and twin study findings suggest that much of this overlap is due to a common genetic aetiology (Reference Silberg, Rutter and MeyerSilberg et al, 1996; Reference Faraone, Biederman and MenninFaraone et al, 1998). A separate question is whether the sub-group with both ADHD and conduct disorder (ADHD+CD) is distinct from those with pure ADHD. Children who fulfil ICD-10 diagnostic criteria for both hyperkinetic disorder and conduct disorder (World Health Organization, 1992) are separately categorised under hyperkinetic conduct disorder. There is some clinical support for this distinction in that most studies suggest that ADHD+CD is a more severe condition than either disorder alone (Reference Bark ley, Fischer and EdelbrockBarkley et al, 1990; Reference Jensen, Martin and CantwellJensen et al, 1997; Reference Kuhne, Schachar and TannockKuhne et al, 1997), although findings have not been entirely consistent (Reference Leung, Ho and LukLeung et al, 1996; Reference Taylor, Chadwick and HeptinstallTaylor et al, 1996). Family study findings also suggest that ADHD+CD represents a distinct familial subtype of ADHD (Reference Faraone, Biederman and MonuteauxFaraone et al, 2000) although twin data are needed to test whether ADHD+CD is genetically distinct or a more severe category of ADHD in terms of genetic loading.
METHOD
Our aims were first to replicate and extend previous twin work on hyperactivity and conduct symptom scores (Reference Silberg, Rutter and MeyerSilberg et al, 1996) by examining whether common genetic and environmental risk factors account for the comorbidity between categorically defined ADHD-related problems and conduct problems, and second to use a liability threshold model to examine whether a comorbid category of ‘ADHD +CD’ represents a genetically more severe variant of ADHD-related behaviours.
Participants
The sample consisted of school-aged twins (aged 5-17 years) included on the population-based Greater Manchester twin register. This is a register of 2846 live twin births who were identified from community child-health databases for nine health districts in Greater Manchester and Lancashire. The characteristics of the twin register and responding families have been described in detail elsewhere (Reference Thapar, Ross and HarringtonThapar et al, 2000). The final sample for whom data were available included 767 female twin pairs, 715 male twin pairs and 600 opposite-sex twin pairs. Of the 1920 pairs for whom zygosity could be assigned, 731 (38.1%) were monozygotic, 590 (30.7%) were same-gender dizygotic and 599 (31.2%) were opposite-gender dizygotic; 162 twin pairs could not be assigned zygosity because of incomplete or ambiguous responses.
Measures
Questionnaires were mailed to the families of the twins; non-responders were sent two postal reminders and given one telephone reminder. The overall response rate was 73% (2082/2846). A standard twin similarity questionnaire that has over 90% accuracy (Reference Cohen, Dibble and GraweCohen et al, 1975) was used to assign zygosity. Parent ratings of ADHD symptoms for each twin were obtained using a modified version of the DuPaul ADHD rating scale (Reference DuPaulDuPaul, 1981). The original scale included the 14 DSM—III—R symptoms of ADHD (American Psychiatric Association, 1987) and we added a further four items to cover additional ICD-10 symptoms of hyperkinetic disorder. Each symptom can be rated on a four-point scale of severity, and each item was summed to generate a total ADHD score. Hyperactivity was then defined categorically using the 80th centile as a cut-off point, chosen on the grounds of previously reported validating data (Reference Thapar, Ross and HarringtonThapar et al, 2000), and in accordance with the approach adopted by others when analysing extreme scores in twins.
Parents were also asked to complete the Rutter A scale (Reference Rutter, Tizard and WhitmoreRutter et al, 1970). This scale includes five conduct items, and the scores for these were summed to obtain a total conduct symptom score. A categorical measure of conduct problems was then generated by again using a cut-off above the 80th centile. Children who were assigned to both the ‘ADHD+CD’ and ‘CD’ categories were labelled as ‘ADHD+CD’ for the liability threshold model analyses.
Although diagnostic criteria were not used, for the purposes of simplicity the following discussion refers to the broadly defined study categories as ‘ADHD-related behaviours’ and ‘Conduct problems’, and for the subgroup analysis the terms ‘pure ADHD’, ‘pure CD’ and ‘ADHD+CD’ are given in quotation marks to emphasise that these are not diagnostic categories.
Genetic analysis
Univariate and bivariate genetic model fitting
Monozygotic (MZ) and dizygotic (DZ) probandwise concordance rates were calculated and the raw categorical twin data were then summarised in the form of polychoric and asymptotic covariance matrices which were generated using PRELIS (Reference Joreskog and SorbomJoreskog & Sorbom, 1988). Genetic models were then fitted to these matrices with the structural equation modelling package Mx (Reference NealeNeale, 1997), using the asymptotic weighted least squares method (Reference Neale and CardonNeale & Cardon, 1992).
The full univariate genetic model for a single phenotype includes additive genetic factors (A), shared environmental factors (C) and non-shared environmental factors (E). The overall goodness of fit of a model is given by a chi-squared value, with a smaller value indicating a better fit. Reduced models where additive genes, shared environment and both of these factors are dropped (CE, AE, E models) can then be tested and compared against the fit of the full model by examining the difference in the χ2 goodness-of-fit values.
This method can then be extended to examine to what extent shared genetic and/or environmental influences explain the covariation or correlation between two phenotypes. Bivariate model fitting using a Cholesky decomposition (see Reference Neale and CardonNeale & Cardon, 1992) was used to examine the overlap of categorically defined hyper-activity and conduct problems. The full bivariate model (see Fig. 2) includes a common genetic influence and common non-shared environmental influence on both ‘ADHD’ and ‘CD’ (shared environment was not included, given the results of the univariate analysis for ‘ADHD’; Reference Thapar, Ross and HarringtonThapar et al, 2000), and specific genetic, specific shared environmental and specific non-shared environmental factors for conduct problems. Reduced models where the common factors and specific factors were dropped in turn were tested. The model accepted as the most satisfactory explanation of the data was chosen on the basis of the goodness of fit and parsimony (simplicity of the model). In the results section we also present Akaike's Information Criteria (AIC; Reference Neale and CardonNeale & Cardon, 1992) for each model, which provides an indication of goodness of fit and parsimony. The model with the lowest AIC value is chosen as the most satisfactory (Reference Neale and CardonNeale & Cardon, 1992).
Using a liability threshold model to examine the relationship between ‘ADHD+CD’ and ‘pure ADHD’
Twins were assigned to a broad category if they were either ‘ADHD+CD’ or ‘pure ADHD’ and to a narrow category if they had been assigned to the ‘ADHD+CD’ group.
Probandwise concordance rates for the broad and narrow categories were first calculated. For categorical data, the correlation between twins (tetrachoric correlation) can be estimated from the twin concordance and prevalence rates of the disorder assuming an underlying continuously distributed liability. A two-threshold liability model (Fig. 1) (Reference Reich, Rice and CloningerReich et al, 1979) was fitted using the FORTRAN program TWIN2. This uses the GEMINI subroutine (Reference Lalouel, Morton, Rao and LalouelLalouel, 1983) to minimise a goodness-of-fit χ2. Applying the two-threshold model entails estimating four correlations (broad proband—broad co-twin, broad proband—narrow co-twin, narrow proband—broad co-twin and narrow proband—narrow co-twin). We assume no shared environmental effects, so that the MZ correlations are double the DZ correlations. First, a general model was tested in which all four correlations were estimated. A nested isocorrelational model (Reference Reich, Rice and CloningerReich et al, 1979), which tests whether ‘ADHD+CD’ is a more extreme genetic variant than ‘pure ADHD’ but lies on the same continuum of liability, was then fitted (Fig. 1). In this model, as the name implies, the four correlations are constrained to be equal. An independent model was also applied to test whether ‘pure ADHD’ and ‘ADHD+CD’ are genetically distinct. Here the narrow—broad and broad—narrow twin correlations are constrained to be zero. The models were compared using the difference in χ2 (see Reference Reich, Rice and CloningerReich et al, 1979, for detailed explanation).
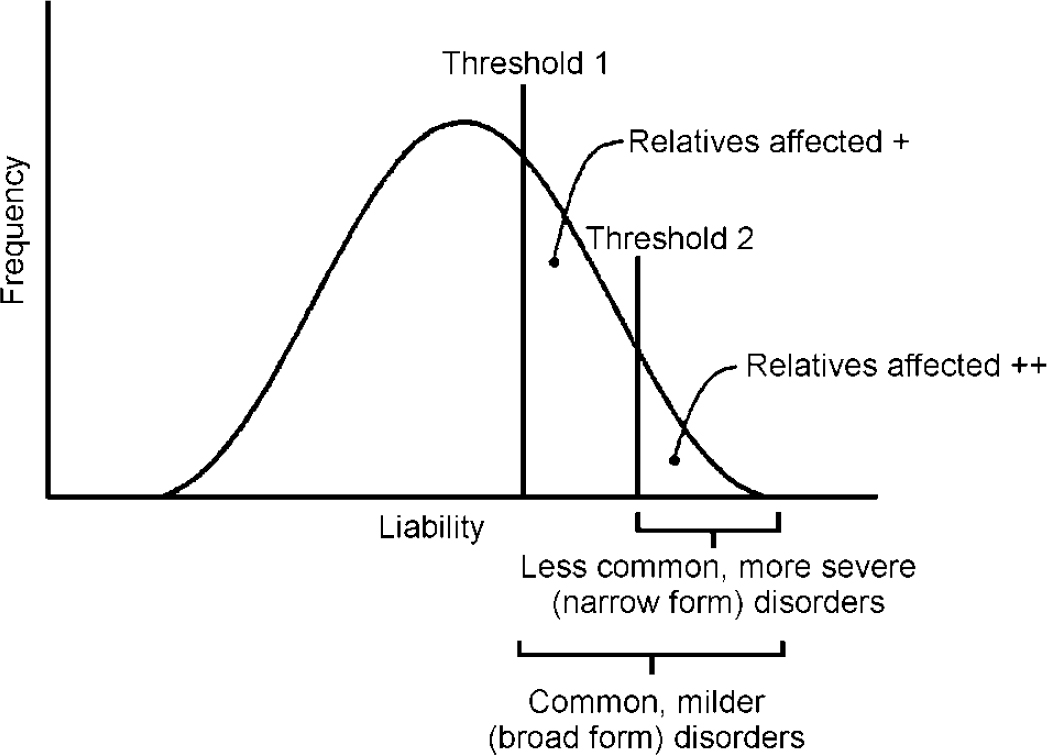
Fig. 1 Liability threshold model: the two-threshold (isocorrelational) type.
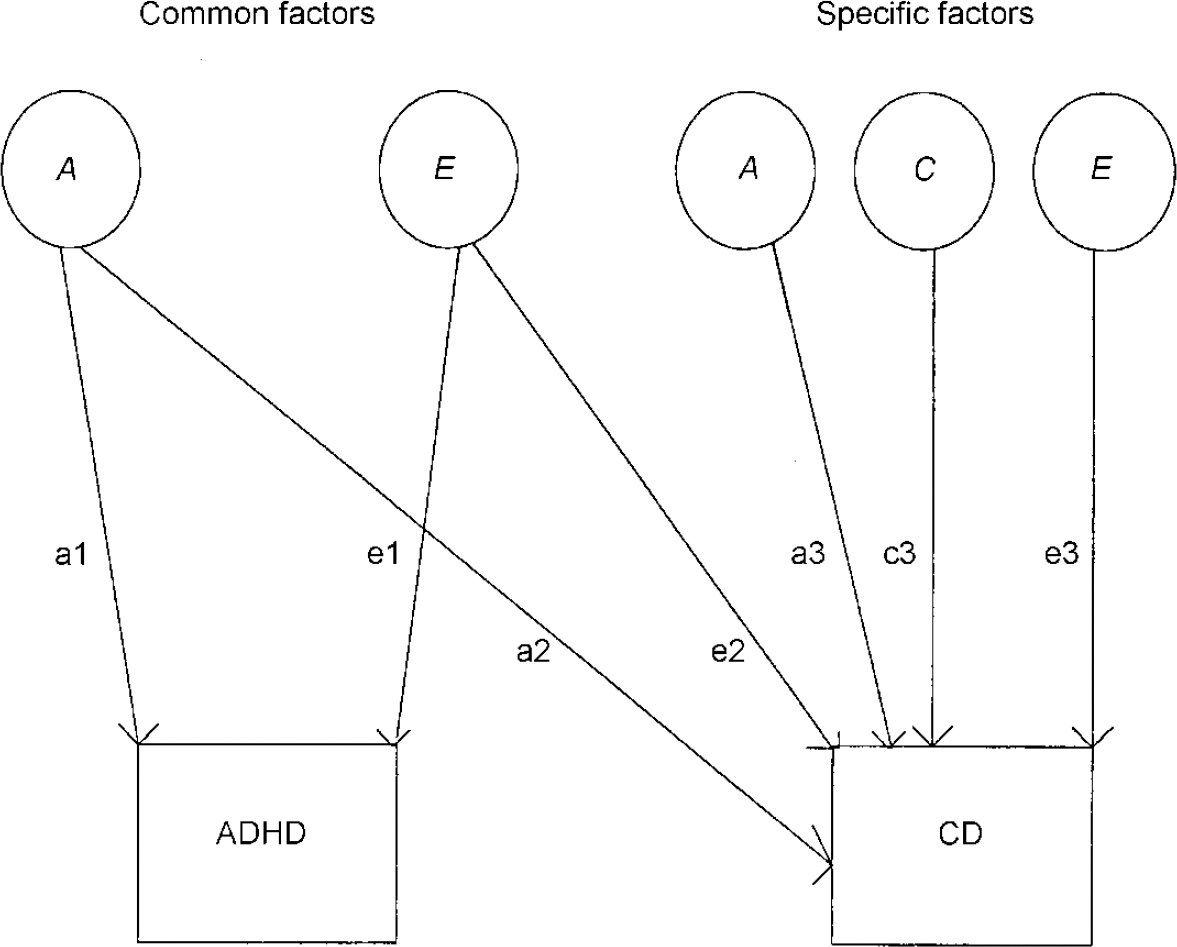
Fig. 2 Full bivariate model for ADHD-related problems and conduct problems; ADHD, attention-deficit hyperactivity disorder; CD, conduct disorder; A, additive genetic factors; C, shared environmental factors; E, non-shared environmental factors.
RESULTS
Univariate genetic models
Previous analyses suggested no significant gender effects (Reference Thapar, Ross and HarringtonThapar et al, 2000). Given that this study focused on categorical data, opposite-gender DZ twin pairs were not included.
Table 1 shows the results of univariate genetic model fitting for ADHD-related behaviours and conduct problems (for more detailed results for ADHD-related behaviours such as teacher ratings and continuous data, see Reference Thapar, Ross and HarringtonThapar et al, 2000). For conduct problems, an AE model where shared environmental effects are dropped results in a significant deterioration in fit compared with the full model (χ2=8.3, d.f.=1). A CE model also provides a poorer fit (χ2=11.65) and a model of no familial transmission (E) results in a very much worse fit. We thus accept the ACE model as providing the most satisfactory explanation for conduct problems with a heritability estimate of 47%, a shared environment component of 36% and the non-shared environment component estimated as 17%. For ADHD-related behaviours the most acceptable model is an AE model with a heritability estimate of 80%.
Table 1 Univariate genetic model fitting for ADHD-related problems and conduct problems

| Model | h2 | c2 | e2 | χ 2 | d.f. | P | AIC |
|---|---|---|---|---|---|---|---|
| Conduct problems | |||||||
| ACE * | 0.47 | 0.36 | 0.17 | 0 | 0 | - | 0 |
| CI | 0.20-0.75 | 0.11-0.60 | 0.08-0.26 | ||||
| AE | 0.86 | [0] | 0.14 | 8.3 | 1 | 0.004 | 6.3 |
| CE | [0] | 0.77 | 0.23 | 11.65 | 1 | 0.001 | 9.65 |
| E | [0] | [0] | 1 | 654 | 2 | <0.001 | 650 |
| ADHD-related problems | |||||||
| ACE | 0.80 | 0.00 | 0.20 | 0.056 | 0 | - | 0.056 |
| AE * | 0.80 | [0] | 0.20 | 0.056 | 1 | 0.812 | -1.94 |
| CI | 0.73-0.87 | 0.11-0.29 | |||||
| CE | [0] | 0.72 | 0.28 | 28.9 | 1 | 0 | 26.9 |
| E | [0] | [0] | 1 | 561 | 2 | 0 | 557 |
Bivariate genetic model fitting
The results of the bivariate model fitting (see Fig. 2 for full model) are shown in Table 2.
Table 2 Bivariate genetic model fitting for ADHD-related problems and conduct problems (see Fig. 2 for full model)
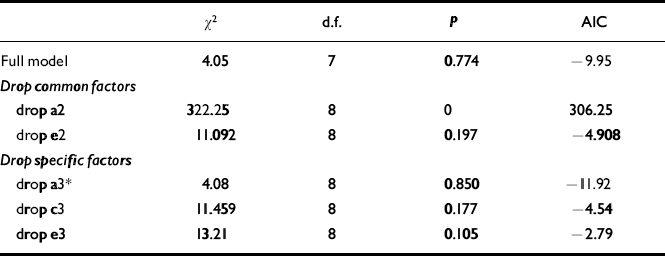
| χ 2 | d.f. | P | AIC | |
|---|---|---|---|---|
| Full model | 4.05 | 7 | 0.774 | -9.95 |
| Drop common factors | ||||
| drop a2 | 322.25 | 8 | 0 | 306.25 |
| drop e2 | 11.092 | 8 | 0.197 | -4.908 |
| Drop specific factors | ||||
| drop a3* | 4.08 | 8 | 0.850 | -11.92 |
| drop c3 | 11.459 | 8 | 0.177 | -4.54 |
| drop e3 | 13.21 | 8 | 0.105 | -2.79 |
It can be seen that neither the common genetic (a2) nor the non-shared environmental component (e2) for conduct problems can be dropped without a significant deterioration in fit. However, the specific additive genetic factor (a3) for conduct problems can be removed. Given the results of the univariate model fitting for conduct problems, not surprisingly a reduced model without a shared environment component (c3) for conduct problems results in a deterioration of fit. Similarly, a specific non-shared environment component (e3) for conduct problems is also needed to explain the data. The accepted model on grounds of fit and parsimony (lowest AIC value) is shown in Fig. 3.
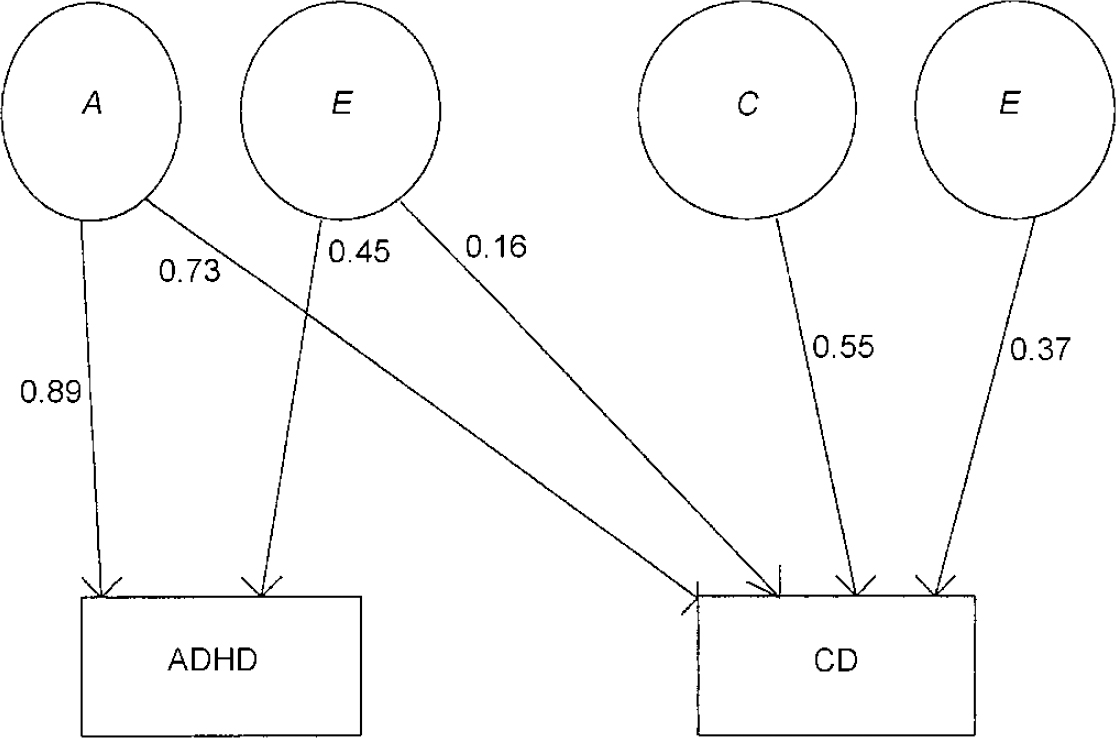
Fig. 3 Accepted bivariate model for ADHD-related problems and conduct-related problems; ADHD, attention-deficit hyperactivity disorder; CD, conduct disorder; A, additive genetic factors; C, shared environmental factors; E, non-shared environmental factors.
Examining ADHD+CD using a liability threshold model approach
In Table 3, the probandwise concordance rates for the broad and narrow categories are presented for MZ and DZ twins. The results of threshold analysis are shown in Table 4. For the isocorrelational model testing whether ADHD and ‘ADHD+CD’ lie on the same continuum of liability, a single correlation was estimated and this resulted in no significant deterioration in fit compared with the general model where four correlations were estimated (χ2=2.48, d.f.=3, P=0.48). However, the independent model, which tests whether the broad and narrow categories are transmitted independently, that is whether they are genetically distinct, provided a very poor fit (χ2=229, d.f.=2, P<0.001). On grounds of parsimony we thus accept the isocorrelational model, where ‘ADHD+CD’ (the narrow category) represents a genetically more extreme variant of ADHD, as providing the most satisfactory explanation of the data.
Table 3 Probandwise concordance rates for broad and narrow categories of ADHD-related problems

| Proband | Concordance rates (%) | |
|---|---|---|
| Broad | Narrow | |
| MZ twins | ||
| Broad | 79 | 53 |
| Narrow | 83 | 74 |
| DZ twins | ||
| Broad | 54 | 37 |
| Narrow | 60 | 47 |
Table 4 Results of liability threshold model fitting
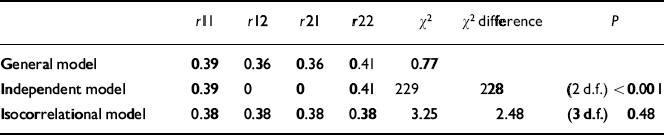
| r11 | r12 | r21 | r22 | χ 2 | χ 2 difference | P | |
|---|---|---|---|---|---|---|---|
| General model | 0.39 | 0.36 | 0.36 | 0.41 | 0.77 | ||
| Independent model | 0.39 | 0 | 0 | 0.41 | 229 | 228 | (2 d.f.) < 0.001 |
| Isocorrelational model | 0.38 | 0.38 | 0.38 | 0.38 | 3.25 | 2.48 | (3 d.f.) 0.48 |
DISCUSSION
Conduct problems and ADHD share a common genetic aetiology
We first considered ADHD-related behaviours and conduct problems as two separate phenotypes and examined the genetic and environmental contribution to the comorbidity of these two categories. Bivariate genetic analysis showed that the genetic contribution to conduct problems was entirely explained by the same genetic factor influencing ADHD-related behaviours. The comorbidity between the two categories was mostly explained by this common genetic risk factor although a common non-shared environmental factor also contributed to the phenotypic overlap. These findings are consistent with family studies based on clinical diagnoses of ADHD, where ADHD and conduct disorder have been shown to share a common familial risk (Faraone et al, Reference Faraone, Biederman and Keenan1991, Reference Faraone, Biederman and Mennin1998). The twin study findings suggest that this common familial risk is genetic in origin. Our findings are similar to those of Silberg et al (Reference Silberg, Rutter and Meyer1996), who found that a common genetic influence accounted for the covariation in symptoms of ADHD and conduct disorder. It is interesting that genetic findings on the comorbidity of ADHD-related behaviours and conduct problems from these three studies have been so consistent, despite phenotypic definition having ranged from questionnaire symptom counts and questionnaire-derived categories to interview-based clinical diagnosis.
Additional environmental influences on conduct problems
Despite the overlap of ADHD-related behaviours and conduct problems, inspection of our twin data strongly supported the distinction of these two categories, in that for both MZ and DZ twins ADHD-related behaviours in one twin rarely predicted conduct problems in the other, and vice versa. The bivariate genetic findings more formally support a degree of aetiological distinction of ADHD-related behaviours and conduct problems in that additional shared environmental and non-shared environmental factors were found for conduct problems. A considerable amount of clinical research has focused on examining the differences between ADHD and conduct disorder. Although there have been some inconsistencies in findings, most results suggest that ADHD and conduct disorder differ in terms of outcome (Reference Taylor, Chadwick and HeptinstallTaylor et al, 1996) and in their pattern of correlates, with ADHD being more commonly associated with neurodevelopmental problems while conduct disorder shows a greater association with social adversity (Reference Taylor, Sandberg and ThorleyTaylor et al, 1991; Reference Schachar and TannockSchachar & Tannock, 1995; Reference Leung, Ho and LukLeung et al, 1996). Our finding of a shared environmental component for conduct problems is in keeping with clinical and epidemiological research, which has consistently shown that conduct disorder is strongly associated with social and family adversity (Reference Rutter, Giller and HagellRutter et al, 1998).
Examining the subgroup of those with both ADHD and conduct problems
The second aim of this study was to examine how ‘ADHD+CD’ is genetically related to ADHD-related behaviours. Using a liability threshold model approach, our results suggest that ‘ADHD+CD’ is a quantitative variant of ADHD-related behaviours and represents a category associated with a higher genetic loading. Twin data have not been used previously to examine this issue, although there have been a series of family study-based papers on this topic (Faraone et al, Reference Faraone, Biederman and Keenan1991, Reference Faraone, Biederman and Mennin1998) and a number of studies have focused on the clinical severity, correlates and outcome of ‘ADHD+CD’ (Reference Jensen, Martin and CantwellJensen et al, 1997; Reference Kuhne, Schachar and TannockKuhne et al, 1997). Most of these studies have shown that ‘ADHD+CD’ is a more severe variant of ADHD (and conduct disorder alone) in so far as it is associated with more severe symptoms (Reference Jensen, Martin and CantwellJensen et al, 1997; Reference Kuhne, Schachar and TannockKuhne et al, 1997) and a worse outcome (Reference Bark ley, Fischer and EdelbrockBarkley et al, 1990; Reference Jensen, Martin and CantwellJensen et al, 1997). Family study findings have suggested that ‘ADHD+CD’ represents a more familial subtype (Reference Faraone, Biederman and MonuteauxFaraone et al, 2000) and our twin findings add a further dimension to this by suggesting that ‘ADHD+CD’ is also a more severe variant of ADHD in terms of genetic loading. One possibility that has to be considered is that our finding of a higher genetic loading for ‘ADHD+CD’ is an artefact of higher ADHD symptom scores in this subgroup. Although we found that ADHD symptom scores were significantly higher in the ‘ADHD+CD’ group compared with the ‘pure ADHD’ group, further analysing twin concordance rates for the ‘ADHD+CD’ group suggested that the genetic findings did not vary according to symptom severity. Moreover, there is now good evidence from twin studies that the genetic aetiology of high ADHD symptom scores is no different from that for scores across the normal range (Reference StevensonStevenson, 1992; Reference Levy, Hay and McStephenLevy et al, 1997). Thus, our results add genetic validational support for the ICD-10 classificatory system whereby ADHD+CD is considered as a category which is related to ADHD, in that hyperkinetic conduct disorder is classified under hyperkinetic disorders, but because it appears to be a more severe genetic (as well as clinical) variant it warrants a separate category.
Limitations
Finally, we have to consider the limitations of this study and how these might have influenced our findings. The results reported here are entirely based on categories defined using questionnaire data, which cannot be equated with clinical diagnoses. Our sample is also population-based, and for these two reasons caution is required in extrapolating the results to clinical populations. Nevertheless, it also has to be emphasised that twin studies based on systematically ascertained population samples are free from the referral and selection biases of clinically referred twin samples. Given that even the largest of population-based twin samples will not yield a sufficient number of twins who meet clinical diagnostic criteria for ADHD and ADHD+CD, at present we have to resort to this sort of phenotypic approximation. However, from an aetiological — and more specifically a genetic — perspective, this may not be such an important problem, given that for ADHD at least there is little evidence to suggest genetic discontinuities between extreme and more broad categories and scores (Reference Levy, Hay and McStephenLevy et al, 1997).
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• Attention-deficit hyperactivity disorder (ADHD)-related behaviours and conduct problems share a common genetic aetiology.
-
• Conduct problems are influenced by additional environmental factors.
-
• When combined with conduct problems, ADHD behaviour appears to represent a genetically more severe variant of ADHD, which supports the validity of the ICD—10 approach to classifying hyperkinetic disorder.
LIMITATIONS
-
• Questionnaire measures were used.
-
• Categories were defined using cut-offs on questionnaires.
-
• A general population sample was used, so the results may not apply to clinical populations.
Acknowledgements
Lydia White, Nicole Perrin Trent, Hilary Hood, Amanda Tew and Justine Porter assisted with establishing the twin register and data collection. Kenny Ross assisted with questionnaire design and data coding. We thank Eric Taylor of the Institute of Psychiatry for his comments on the potential confound of more severe symptoms in the ADHD+CD group.










eLetters
No eLetters have been published for this article.