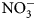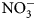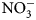Introduction
Intensification of agricultural production systems can lead to natural resource depletion and reduced biodiversity. Although intensively managed agroecosystems can increase food production in the short-term, longer-term outcomes include loss of natural habitats and biodiversity of flora and fauna, water contamination, soil degradation and reduced capacity for crop productivity (Tilman et al., Reference Tilman, Cassman, Matson, Naylor and Polasky2002; Zhang et al., Reference Zhang, Davidson, Mauzerall, Searchinger, Dumas and Shen2015; Imadi et al., Reference Imadi, Shazadi, Gul, Hakeem, Hakeem, Akhtar and Abdullah2016). In particular, low-diversity rotations can lead to soil fertility imbalances and declines in beneficial arthropod habitat, indirectly affecting soil degradation and water pollution (Büchs et al., Reference Büchs, Harenberg and Zimmermann1997; Pender and Mertz, Reference Pender, Mertz, Halberg, Alrøe, Knudsen and Kristensen2006; O'Rourke et al., Reference O'Rourke, Liebman and Rice2008; Bowles et al., Reference Bowles, Mooshammer, Socolar, Calderón, Cavigelli, Culman, Deen, Drury, Garcia y Garcia, Gaudin, Harkcom, Lehman, Osborne, Robertson, Salerno, Schmer, Strock and Grandy2020). Vegetable systems require high nutrient inputs for sustained yields, which may result in overfertilization and surface water eutrophication (Agostini et al., Reference Agostini, Tei, Silgram, Farneselli, Benincasa and Aller2010). Simplification of these systems limits the availability and variety of floral resources in Midwestern farming landscapes (Wäckers et al., Reference Wäckers, Romeis and van Rijn2007; Blaauw and Isaacs, Reference Blaauw and Isaacs2014; Forister et al., Reference Forister, Pelton and Black2019). This, in turn, reduces the ability of the landscape to support beneficial arthropods (O'Connor, Reference O'Connor2013).
Organic farming practices can reduce soil erosion and increase soil organic matter (SOM), thereby enhancing soil structure and stability (Pulleman et al., Reference Pulleman, Bouma, van Essen and Meijles2000; Leithold et al., Reference Leithold, Hülsbergen and Brock2014; Seitz et al., Reference Seitz, Goebes, Puerta, Pereira, Wittwer, Six, van der Heijden and Scholten2019). These outcomes are often achieved via the use of cover crops to meet certified organic system diversification requirements (NOP, 2021), presenting an opportunity for inclusion of a range of cover crop species that meet the dual goals of soil and beneficial insect habitat improvement. This is especially true in Upper Midwest vegetable systems, where a variety of short fallow periods exist between cropping cycles when cover crops could be integrated (Wauters et al., Reference Wauters, Grossman, Pfeiffer and Cala2021). For example, cover crops could be established in spring, maturing during summer, and terminated prior to fall crop planting, or after an early spring cash crop, maturing throughout the late summer months. Upper Midwest summers are increasingly hot and humid with frequent rain events, which increase summer soil erosion and nutrient leaching (NOAA, 2013; Sumiahadi et al., Reference Sumiahadi, Acar, Koç and Özel2019). For organic vegetable farmers in northern regions, cover crops planted during the summer months have the potential to reduce negative impacts of these climate shifts while increasing a range of ecosystem services, including production of aboveground biomass, biomass N, suppression of weeds and floral resources for pollinators (Creamer and Baldwin, Reference Creamer and Baldwin2000; Wilson et al., Reference Wilson, Wong, Thorp, Miles, Daane and Altieri2018).
Cover crop species planted in the summer months, even in the coldest US climates, could provide the multifunctionality needed to meet numerous agroecosystem goals. Cover crops supply critical nutrients to organic vegetable production (Parr et al., Reference Parr, Grossman, Reberg-Horton, Brinton and Crozier2011; Schipanski et al., Reference Schipanski, Barbercheck, Douglas, Finney, Haider, Kaye, Kemanian, Mortensen, Ryan, Tooker and White2014; Blesh, Reference Blesh2017; Blesh et al., Reference Blesh, VanDusen and Brainard2019; Piotrowska-Długosz and Wilczewski, Reference Piotrowska-Długosz and Wilczewski2020). They have been shown to contribute up to 300 kg N ha−1 (Creamer and Baldwin, Reference Creamer and Baldwin2000; Fageria et al., Reference Fageria, Baligar and Bailey2005; Sarrantonio, Reference Sarrantonio and Clark2007; Parr et al., Reference Parr, Grossman, Reberg-Horton, Brinton and Crozier2011; Asik et al., Reference Asik, Uzun and Acikgöz2020), and increase soil fertility through changes in physical, chemical and biological parameters (Drinkwater et al., Reference Drinkwater, Wagoner and Sarrantonio1998; Reicosky and Forcella, Reference Reicosky and Forcella1998; Daryanto et al., Reference Daryanto, Fu, Wang, Jacinthe and Zhao2018). Moreover, when cover crop functional groups are combined, greater multifunctionality can be obtained (Finney et al., Reference Finney, Murrell, White, Baraibar, Barbercheck, Bradley, Cornelisse, Hunter, Kaye, Mortensen, Mullen and Schipanski2017; Kaye et al., Reference Kaye, Finney, White, Bradley, Schipanski, Alonso-Ayuso, Hunter, Burgess and Mejia2019). For example, when legumes and grasses are combined, legumes may fix more N due to competition with grasses in acquiring soil N (Jensen, Reference Jensen1996; Høgh-Jensen & Schjoerring, Reference Høgh-Jensen and Schjoerring2001; Blesh, Reference Blesh2017). These crops also offer an opportunity to add floral resources throughout the growing season. Adding flowering cover crops to the rotation supports diversity and abundance of beneficial arthropods, including bees and natural enemies of pest (O'Connor, Reference O'Connor2013; Redlich et al., Reference Redlich, Martin and Steffan-Dewenter2018). These arthropods can provide ecosystem services to the farm, including pollination and predation and parasitism of pest (Hooks et al., Reference Hooks, Valenzuela and Defrank1998; Landis et al., Reference Landis, Menalled, Costamagna and Wilkinson2005).
Data are scarce regarding the degree to which summer-planted flowering cover crops can support beneficial arthropod communities while simultaneously supporting soil health services, particularly soil nutrient cycling, in vegetable production systems. The objectives of this research were to assess possible contributions of 16 cover crop species and species mixes toward enhancement of (1) soil health factors (biomass production and C:N ratio content, ${\rm NO}_3^-$![]() -N contributions) and (2) beneficial arthropod habitat (population abundance, diversity and richness).
-N contributions) and (2) beneficial arthropod habitat (population abundance, diversity and richness).
Materials and methods
Experimental location and treatments
A summer-planted cover crop trial was established in a single growing season from May to September 2019 at the University of Minnesota Agricultural Experimental Station (MAES), Twin Cities campus, St. Paul, MN (44.990829, −93.175832). Species were selected to represent commonly used Upper Midwest cover crops and their expected contribution of beneficial arthropod floral resources and nutrient cycling. Results from an on-farm preliminary trial and recommendations from the Xerces Society for Invertebrate Conservation and by farmer collaborators further supported species selection (Table 1). Regional mean annual rainfall is 813 mm, and the soil is classified as a Waukegan silt loam (fine-silty over sandy or sandy-skeletal, mixed, superactive, mesic Typic Hapludolls). Treatments were arranged in a randomized complete-block experimental design with four replications, with each treatment plot measuring 3 m2. Cover crops were broadcast sown at two dates (early and late), lightly raked to increase seed to soil contact, and terminated at the time of seed formation (Table 2). Previous fertility management prior to experiment establishment included historical manure additions, as well as regular additions of composted turkey litter. No synthetic or organic fertilizers and pesticides were applied in the 2 years prior to the experiment, or during the experimental growing season.
Table 1. Treatment species and seeding rates

a Consistent for all clover species.
Table 2. Dates of field operations

Biomass and soil sampling
Plant biomass was collected immediately before cover crop termination (Table 2) from a 0.5 m2 sampling area, 0.3 m away from plot borders. To maximize flowering time yet avoid seed-set and cover crops reseeding, cover crops with early [buckwheat (Fagopyrum esculentum Moench)] and medium-length [phacelia monoculture (Phacelia tanacetifolia Bendth), and oat (Avena sativa L.), field pea (Pisum sativum subsp. Arvense L.) and clover (Trifolium incarnatum L., T. alexandrinum L., T. pratense L., T. repens L., T. hybridum L.) mix] flowering were terminated on July 15, sunn hemp (Crotalaria juncea L.) was terminated on July 29 due to low biomass production and no flowering, and late-flowering crops [sunflower (Helianthus annuus L.)] were terminated on August 13. Aboveground biomass samples were categorized and sorted as cover crops and weeds, then oven-dried at 60°C for at least 48 h. Samples were then weighed, and ground to 2 mm for C:N ratio determination. One sunn hemp treatment plot had no biomass collected due to low germination; this plot was excluded from analysis. Cover crop termination was performed with a walk-behind tractor (BCS) and flail mower attachment. Due to unfavorable weather conditions and wet soils, biomass could not be incorporated in the soil with rotary tillage until 2 weeks after termination. Soils were sampled at cover crop termination and again approximately 2 weeks following tillage (Table 2). Ten soil cores were collected to 15 cm depth, using 2.5 cm diameter stainless steel soil probes, and homogenized. Samples were stored in paper bags and oven-dried at 35°C for at least 48 h. Dry soil samples were ground to 2 mm for soil inorganic N (${\rm NO}_3^-$![]() -N) analysis. Inorganic ${\rm NO}_3^-$
-N) analysis. Inorganic ${\rm NO}_3^-$![]() -N was measured using a KCl extraction (modified from Robertson et al., Reference Robertson, Sollins, Ellis, Lajtha, Robertson, Coleman, Bledsoe and Sollins1999). Briefly, 8 g of dry soil was weighed into 50 mL centrifuge tubes, 40 mL 1 M KCl was added, and tubes were shaken for 1 h at approximately 240 rpm. Samples were allowed to settle for at least 1 h, and supernatant was filtered through #1 Whatman papers and collected in scintillation vials. Vials were frozen for analysis, which was later performed using a colorimetric nitrate assay (Doane and Horwath, Reference Doane and Horwath2003). Biomass was analyzed for C and N content using a dry combustion analyzer (Elementar VarioMax CN analyzer).
-N was measured using a KCl extraction (modified from Robertson et al., Reference Robertson, Sollins, Ellis, Lajtha, Robertson, Coleman, Bledsoe and Sollins1999). Briefly, 8 g of dry soil was weighed into 50 mL centrifuge tubes, 40 mL 1 M KCl was added, and tubes were shaken for 1 h at approximately 240 rpm. Samples were allowed to settle for at least 1 h, and supernatant was filtered through #1 Whatman papers and collected in scintillation vials. Vials were frozen for analysis, which was later performed using a colorimetric nitrate assay (Doane and Horwath, Reference Doane and Horwath2003). Biomass was analyzed for C and N content using a dry combustion analyzer (Elementar VarioMax CN analyzer).
Floral and arthropod sampling
Flower units, measurement used to quantify flower abundance, for each individual species were quantified and recorded within a 0.5 m2 area, beginning when ten or more open inflorescence were observed within each plot (Table 2). Flower units were categorized depending on the species. For buckwheat and phacelia, each bifurcated stem was considered one flower unit. Flower units for sunflower, clovers and field peas were determined by each inflorescence. Cover crop peak blooms were categorized as early, early-mid and late bloom time (Fig. 1). Plots were terminated when buckwheat started producing mature seeds (i.e., dark-colored filled pods), even if other species from the treatment were not in bloom (Table 2). Beneficial arthropod communities were sampled every 7–8 days by sampling the perimeter of each plot with a sweep net for 60 s. Sampling did not occur the week of July 7–17 due to weather conditions. After collection, arthropods were preserved in a −20°C freezer until family-level taxonomic identification, and preserved in an 80% ethanol solution thereafter. Specimens were identified to family using the keys provided by Triplehorn et al. (Reference Triplehorn, Johnson and Borror2005) and Marshall (Reference Marshall2006) as well as photographs and descriptions available in www.BugGuide.net. Selected specimen identifications were verified by Dr Ralph Holzenthal, Department of Entomology, University of Minnesota. Beneficial arthropod abundance, diversity and richness were assessed and categorized by functional groups for all flowering cover crops.

Fig. 1. Mean numbers of open flowers across time on treatment plants that reached peak bloom (a) early, (b) mid-early and (c) late.
Statistical analysis
For soil and cover crop biomass, we report dry biomass quantity (kg ha−1), biomass N content (kg N ha−1), and soil N (${\rm NO}_3^-$![]() -N) at 0 and 2 weeks after biomass incorporation. Changes reported in ${\rm NO}_3^-$
-N) at 0 and 2 weeks after biomass incorporation. Changes reported in ${\rm NO}_3^-$![]() -N were calculated by subtracting mean pre-termination soil ${\rm NO}_3^-$
-N were calculated by subtracting mean pre-termination soil ${\rm NO}_3^-$![]() -N values from those taken 2 weeks following termination. Because soil conditions (temperature and moisture) are known to impact N mineralization rates, and times of sampling and termination in this study varied by flowering times (Table 2), reported soil ${\rm NO}_3^-$
-N values from those taken 2 weeks following termination. Because soil conditions (temperature and moisture) are known to impact N mineralization rates, and times of sampling and termination in this study varied by flowering times (Table 2), reported soil ${\rm NO}_3^-$![]() -N values should be considered as a potential of summer cover crops, and not absolute. Cover crop treatment fixed effects on soil properties and biomass production (P < 0.05) were analyzed using a mixed-effects model ANOVA with replication as a random effect (package lme4; Bates et al., Reference Bates, Mächler, Bolker and Walker2015). Floral, beneficial arthropod and predominant group taxa abundance as a function of cover crop treatments were analyzed using a generalized linear model with a Poisson family distribution (package R; R Core Team, 2019). Beneficial arthropod data are reported as the total number and relative abundances of collected families by treatment. To assess the diversity of pollinators and natural enemies, for each experimental unit, Shannon indices were calculated using insect family as a proxy for species (R vegan package; Okansen et al., Reference Okansen, Guillaume Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara and Simpson2019). Linear mixed-effects models with terms for cover crop treatment as the main effect and replicated plot as a random effect were used to compare richness and diversity of natural enemies among treatments (package lme4; Bates et al., Reference Bates, Mächler, Bolker and Walker2015). Response variables of biomass, biomass N, richness and Shannon's diversity indices were square root transformed to normalize error and satisfy the assumption of equal variance. All analyses were conducted with R, version 3.5.3 (The R Foundation for Statistical Computing, 2019) and R Studio, version 1.2.1 (R Studio Team, 2018).
-N values should be considered as a potential of summer cover crops, and not absolute. Cover crop treatment fixed effects on soil properties and biomass production (P < 0.05) were analyzed using a mixed-effects model ANOVA with replication as a random effect (package lme4; Bates et al., Reference Bates, Mächler, Bolker and Walker2015). Floral, beneficial arthropod and predominant group taxa abundance as a function of cover crop treatments were analyzed using a generalized linear model with a Poisson family distribution (package R; R Core Team, 2019). Beneficial arthropod data are reported as the total number and relative abundances of collected families by treatment. To assess the diversity of pollinators and natural enemies, for each experimental unit, Shannon indices were calculated using insect family as a proxy for species (R vegan package; Okansen et al., Reference Okansen, Guillaume Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara and Simpson2019). Linear mixed-effects models with terms for cover crop treatment as the main effect and replicated plot as a random effect were used to compare richness and diversity of natural enemies among treatments (package lme4; Bates et al., Reference Bates, Mächler, Bolker and Walker2015). Response variables of biomass, biomass N, richness and Shannon's diversity indices were square root transformed to normalize error and satisfy the assumption of equal variance. All analyses were conducted with R, version 3.5.3 (The R Foundation for Statistical Computing, 2019) and R Studio, version 1.2.1 (R Studio Team, 2018).
Results and discussion
Biomass production, C:N ratio and soil inorganic N
Cover crop biomass production (kg ha−1) varied across treatments (P < 0.001) with higher-biomass treatments trending toward higher N-contributing treatments. The oat, field pea and clover (OFC) treatment produced the greatest biomass (8747 ± 1600 kg ha−1) and suppressed the most weeds (Table 3). This treatment also produced the highest biomass N (265 ± 73 N kg ha−1), attributed to its high biomass and low C:N ratio. Clover biomass did not reflect the original proportion of clover in the OFC mix, resulting in an over-representation of oats and field peas in this treatment. Although many clovers are slower to establish than either field pea or oat, and often do not flower the first year, crimson clover flowered by week 3. As expected, weed biomass was negatively correlated to cover crop biomass (P < 0.001, R 2 = −0.63). This result supports previous findings that summer-planted cover crops can produce sufficient biomass to be competitive with weed species (Finney et al., Reference Finney, White and Kaye2016; Sharma et al., Reference Sharma, Singh, Kahlon, Brar, Grover, Dia and Steiner2018; Stepanovic et al., Reference Stepanovic, Burr, Peterson, Rudnick, Creech and Werle2018). Sunn hemp, a buckwheat and partridge pea mix (BP), and the most diverse plant family mix (MD) had low cover crop biomass and correspondingly greater amounts of weeds due to poor germination relative to other treatments. Sunn hemp is a warm-climate cover crop that is extensively used in the southern US and the Caribbean (Halbrendt, Reference Halbrendt2010; Morris et al., Reference Morris, Chase, Treadwell, Koenig, Cho, Morales-Payan, Murphy and Antonious2015). Sunn hemp germination may have been affected by cooler air and soil temperatures during the planting dates of May 1 and May 15, when temperatures in Minnesota varied between 7 and 25°C. The mixture treatments, BP and MD, both included buckwheat, known to have rapid establishment and competitive germination, potentially affecting the establishment of other species in the mix. However, the mix containing buckwheat, annual ryegrass and clovers (BAC) had comparable biomass to phacelia and sunflower, second and third highest biomass production species. Clover species’ germination in BAC was not representative of seeding rate.
Table 3. Mean C:N ratio, cover crop and weed aboveground biomass, aboveground biomass N and percent N by treatment

B, buckwheat; BAC, buckwheat, annual ryegrass and clovers; BP, buckwheat and partridge pea; BPhS, buckwheat, phacelia and sunflower; MD, buckwheat, canola, radish, field peas, pearl millet and oats; OFC, oats, field peas and clovers; Ph, phacelia; S, sunflower.
Results followed by the same letter within each column are not different (P < 0.05).
Soil ${\rm NO}_3^-$![]() -N varied across treatments (P < 0.001), increasing from 26 to 55% 2 weeks following biomass incorporation in BP, BPhS and OFC treatments (Fig. 2). Although this is expected for OFC, which produced significantly higher biomass N that likely drove soil N increases, this result was surprising for BPhS and BP, as these treatments did not produce substantially greater amounts of biomass N relative to any other treatment. In these cases, available soil N could possibly have increased via N inputs not quantified in this study, including N from root residue decomposition (Bukovsky-Reyes et al., Reference Bukovsky-Reyes, Isaac and Blesh2019).
-N varied across treatments (P < 0.001), increasing from 26 to 55% 2 weeks following biomass incorporation in BP, BPhS and OFC treatments (Fig. 2). Although this is expected for OFC, which produced significantly higher biomass N that likely drove soil N increases, this result was surprising for BPhS and BP, as these treatments did not produce substantially greater amounts of biomass N relative to any other treatment. In these cases, available soil N could possibly have increased via N inputs not quantified in this study, including N from root residue decomposition (Bukovsky-Reyes et al., Reference Bukovsky-Reyes, Isaac and Blesh2019).

Fig. 2. Effect of cover crop treatments on inorganic N from 0 to 2 weeks after biomass incorporation. B, buckwheat; BAC, buckwheat, annual ryegrass and clovers; BP, buckwheat and partridge pea; BPhS, buckwheat, phacelia and sunflower; most diverse (MD); buckwheat, canola, radish, field peas, pearl millet and oats; OFC, oats, field peas and clovers; Ph, phacelia; S, sunflower; Sh, sunn hemp. Asterisk over the boxes denote statistically different means at *P ≤ 0.05 and **P ≤ 0.001.
Cover crop mixtures that include legumes or low C:N biomass species often increase soil health indicators, including SOM and N (Finney et al., Reference Finney, White and Kaye2016; Kaye et al., Reference Kaye, Finney, White, Bradley, Schipanski, Alonso-Ayuso, Hunter, Burgess and Mejia2019). We observed 73–100% greater biomass N provided by the OFC treatment relative to all other treatments. However, other treatments that also included legumes with non-legume components (Table 1) did not produce comparable amounts of biomass N. These other treatments included buckwheat, known to have earlier and faster growth rates (Hogg et al., Reference Hogg, Bugg and Daane2011), relative to other species included in the evaluated mixtures. This rapid growth of buckwheat may have decreased legume productivity and subsequent N contributions via competitive effects. Legumes generally contribute higher biomass N than buckwheat, with recorded levels of 217 kg ha−1 compared to 21.3 kg ha−1, respectively (Marcinkonis et al., Reference Marcinkonis, Pranaitis and Lisova2007; Parr et al., Reference Parr, Grossman, Reberg-Horton, Brinton and Crozier2011), yet individual species’ productivity in a given environment ultimately controls overall N contributions. Among the buckwheat-containing treatments, the BAC mix trended towards higher biomass N, but was not statistically different from B, BP, MD treatments (P > 0.05). The MD treatment had 51–79% lower biomass production compared to OFC and BAC mixes, and to phacelia and sunflower monocultures, also likely attributed to competition with mixture components (Sarrantonio, Reference Sarrantonio and Clark2007). Results support that selecting appropriate competitive plant traits for mixes, especially favoring legumes, may present a challenge, as greater competitive establishment may benefit weed control but decrease germination of other mix species.
Floral resources
Buckwheat treatments started flowering extensively 42 days after planting date. Buckwheat monoculture produced the highest mean floral peak (flower units = 374 ± 95.9) relative to other treatments containing buckwheat (ranging from 56.2 ± 14.1 to 202 ± 71.6). Phacelia began flowering progressively 42 days after planting date, reaching peak bloom by July 9 (181 ± 53.4), and culminating July 30. Although both buckwheat and phacelia monocultures had started flowering by the first collection sample, buckwheat in mixed culture flowered 14 days earlier than phacelia, providing early floral resources for a wide range of arthropod visitors. The OFC treatment started flowering 42 days after planting date and reached an average peak bloom of 11.2 ± 2.9 flower units. The sunflower treatment had a period of 76 days to flower from planting date, lasting 14 days and with an average peak bloom of 8.9 ± 1.6 flower units. The sunn hemp treatment did not provide flowers during the experimental period.
Beneficial arthropod abundance, diversity and richness
Across all treatments, a total of 2668 arthropod specimens were collected from cover crop treatments, of which 25% (667) were categorized as beneficial arthropods, including natural enemies (predators and parasitoids) and pollinators (bees and hoverflies). The 667 beneficial specimens were subset into 18 taxonomic families with Syrphidae (68.1%) and Apidae (7.3%) comprising approximately 75% of all specimens collected, followed by Coccinellidae (5.5%) and Eulophidae (4.5%). Syrphidae had the highest relative abundance (77.9%), followed by Coccinellidae (21.8%) and Apidae (16.2%) (Table 4).
Table 4. Season-long total number of individuals and relative abundance (RA) for each taxon encountered across nine treatments

B, buckwheat; BAC, buckwheat, annual ryegrass and clovers; BP, buckwheat and partridge pea; BPhS, buckwheat, phacelia and sunflower; MD, buckwheat, canola, radish, field peas, pearl millet and oats; OFC, oats, field peas and clovers; Ph, phacelia; S, sunflower.
Abundance of beneficial arthropods in the OFC and sunflower treatments were lower than all other treatments, likely attributed to low inflorescence production in both treatments. The MD treatment had the highest abundance of total beneficial arthropods (147) relative to both OFC (53) and S (28) treatments (Table 4). However, this was not statistically different from buckwheat, BAC, BP, BPhS and phacelia treatments.
Plant functional diversity has been correlated with higher arthropod diversity in a perennial savannah grassland (Haddad et al., Reference Haddad, Tilman, Haarstad, Ritchie and Knops2001). However, our results demonstrate that higher plant functional diversity does not necessarily result in higher arthropod diversity. Although the MD treatment had the greatest beneficial arthropod abundance, it had lower richness than the monoculture or bi-culture treatments. Buckwheat (diversity/richness, respectively; 0.66 ± 0.14/2.6 ± 0.26) and BP (0.67 ± 0.17/2.6 ± 0.42) treatments had a higher richness than the MD (0.22 ± 0.08/1.3 ± 0.28) and phacelia (0.26 ± 0.10/1.5 ± 0.29) treatment. B, BAC and BP displayed higher richness but no significant difference in diversity relative to OFC (0.35 ± 0.11/1.4 ± 0.29) treatment. BAC (0.68 ± 0.20/3.0 ± 0.60) treatment showed difference in diversity compared to sunflower (0.37 ± 0.12/1.9 ± 0.19), however no difference in richness.
Members of the Syrphidae family are known to be attracted to small, white flowers such as buckwheat, Lobularia (alyssum) spp. and Coriandrum sativum (Hogg et al., Reference Hogg, Bugg and Daane2011; Martinez-Uña et al., Reference Martínez-Uña, Martín, Fernández-Quintanilla and Dorado2014). Considering these specimens composed 68% of the total amount of beneficial arthropods collected, the higher amounts of beneficial arthropods in treatments containing buckwheat (Table 4) are consistent to previous studies and throughout our treatments. Additionally, Phacelia sp. was particularly attractive to native and non-native pollinator groups such as Bombus, Halictidae and honeybees (Apis mellifera) (Table 4). The sunflower treatment flowered the latest and had short flowering length, explaining the low abundance of arthropods captured. However, arthropod diversity in sunflower was equivalent to longer-flowering treatments, demonstrating the capacity of sunflowers to attract a wide range of beneficial arthropods even during a shorter flowering period. Native species are believed to have co-evolved with endemic natural enemies (James et al., Reference James, Seymour, Lauby and Buckley2014). Therefore, sunflowers, which are native to North America, may play a key role in attracting and sustaining endemic beneficial arthropod populations (Fiedler and Landis, Reference Fiedler and Landis2007).
Conclusion
This research explored the effect of summer-planted flowering cover crop mixes on concurrent N provision and presence of floral resources for beneficial arthropods. Nine treatments containing one to seven species of cover crops were evaluated for flowering time, N content, soil N contribution and beneficial arthropod attraction. The oat, field pea and clover mix added the most biomass, followed by phacelia and sunflower monocultures. The OFC treatment also provided the greatest increase in soil ${\rm NO}_3^-$![]() -N from 0 to 2 weeks after biomass incorporation relative to all other treatments. Buckwheat monoculture and mixes demonstrated the shortest time to flower and ranked higher in all three arthropod indices: abundance, diversity and richness. In the Upper Midwest, a short summer fallow period in organic vegetable crop rotations presents a valuable opportunity to introduce cover crop mixes and provide additional ecosystem services. Such mixes typically include a variety of grasses, legumes and brassicas. Expanding cover crop options to include non-traditional flowering species attractive to beneficial insects, such a phacelia, may increase the provision of desired ecosystem services. As these flowering cover crop mixtures are introduced into farmer's rotations, pollinator presence may also be increased. More research is needed to determine optimal termination timing, given that longer growth is needed for flowering, but the physiological changes associated with flowering could lead to less biomass-N availability (Schiltz et al., Reference Schiltz, Munier-jolain, Jeudy, Burstin and Salon2005). This trade-off between ecosystem services is especially challenging to manage when using cover crop mixes. A mix with varying bloom times can extend the degree of pollinator habitat provision, yet leads to inconsistent biomass quality among mix species, as well as potential termination time challenges, as some species may go to seed prior to other species. Further research of ecosystem services trade-offs such as this is required to obtain greater clarity of their interactions and to what extent summer-planted cover crops may supply multifunctionality.
-N from 0 to 2 weeks after biomass incorporation relative to all other treatments. Buckwheat monoculture and mixes demonstrated the shortest time to flower and ranked higher in all three arthropod indices: abundance, diversity and richness. In the Upper Midwest, a short summer fallow period in organic vegetable crop rotations presents a valuable opportunity to introduce cover crop mixes and provide additional ecosystem services. Such mixes typically include a variety of grasses, legumes and brassicas. Expanding cover crop options to include non-traditional flowering species attractive to beneficial insects, such a phacelia, may increase the provision of desired ecosystem services. As these flowering cover crop mixtures are introduced into farmer's rotations, pollinator presence may also be increased. More research is needed to determine optimal termination timing, given that longer growth is needed for flowering, but the physiological changes associated with flowering could lead to less biomass-N availability (Schiltz et al., Reference Schiltz, Munier-jolain, Jeudy, Burstin and Salon2005). This trade-off between ecosystem services is especially challenging to manage when using cover crop mixes. A mix with varying bloom times can extend the degree of pollinator habitat provision, yet leads to inconsistent biomass quality among mix species, as well as potential termination time challenges, as some species may go to seed prior to other species. Further research of ecosystem services trade-offs such as this is required to obtain greater clarity of their interactions and to what extent summer-planted cover crops may supply multifunctionality.
Acknowledgments
We would like to thank the Xerces Society and Sogn Valley Farm for selecting the cover crop treatment species, all the members of our labs for final commentaries and our family and friends. This work was supported by NRCS Conservation Innovation Project (NR203A750008G002) and North Central Region SARE (NC-SARE Project LNC19-423).
Conflict of interest
None.