Introduction
Agricultural mulch membranes are a cropping system input manufactured from a variety of synthetic or biobased polymers. Mulches are widely used in annual specialty crop production systems and are increasingly common in cereal production (Gao et al., Reference Gao, Yan, Liu, Ding, Chen and Li2019). The primary function of mulch is to manage weeds and soil moisture. Additional benefits can include soil warming or cooling, reduced nutrient leaching and disease management (Kasirajan and Ngouajio, Reference Kasirajan and Ngouajio2012). Mulch use is widespread because it can intensify crop productivity and profitability. In 2006, mulch was used across 162,000 ha of US farmland and global consumption was estimated at 2.6 million tons annually (Briassoulis and Dejean, Reference Briassoulis and Dejean2010; Hayes et al., Reference Hayes, Dharmalingam, Wadsworth, Leonas, Miles, Inglis, Khemani and Scholz2012). The most widely used mulch is low-density polyethylene film (PE) because it is durable, flexible, low-cost and available in different specifications. The PE mulch films can also provide valuable ecosystem services including reduced irrigation requirements, pesticide and fertility inputs, and nutrient leaching (Lippert et al., Reference Lippert, Takatori and Wilding1964; Bhella, Reference Bhella1988; Yang et al., Reference Yang, Sun, Feng, Zheng, Chi, Meng, Hou, Bai and Li2015).
Despite its many benefits and services, PE mulch does have agronomic and environmental drawbacks. First, PE mulch film should be removed from the field, usually every year. Costs associated with removal (labor, hauling and disposal fees) range from US$100 to 400 per ha (Goldberger et al., Reference Goldberger, Jones, Miles, Wallace and Inglis2015; Moore and Wszelaki, Reference Moore and Wszelaki2016). Second, landfilling is the most common means of disposal, but sometimes mulch is stockpiled, buried or burned on the farm. Recycling technologies for PE exist, but recycling rates are low (<1%) because mulch is considered a low-value polyethylene feedstock for recycling due to photodegradation and contamination (Kasirajan and Ngouajio, Reference Kasirajan and Ngouajio2012). The removed PE mulch often contains 50–80% of soil adhered to the mulch (Kasirajan and Ngouajio, Reference Kasirajan and Ngouajio2012; Ghimire and Miles, Reference Ghimire and Miles2016). Thus, mulch hauling and disposal can be a form of soil erosion—an ecologically and agronomically detrimental outcome. Third, PE mulch could also contaminate soil (Steinmetz et al., Reference Steinmetz, Wollmann, Schaefer, Buchmann, David, Tröger, Muñoz, Frör and Schaumann2016). Field removal of mulch inevitably results in tearing of the weathered product, and some weathered plastic is left behind, depending on soil conditions during removal. These mulch fragments could negatively impact soil physical and biological properties, persist for centuries and potentially reduce soil productivity (Liu et al., Reference Liu, He and Yan2014, Reference Liu, Yang, Liu, Liang, Xue, Chen, Ritsema and Geissen2017; Zhang et al., Reference Zhang, Hu, Zhang and Li2015). Overall, the short-term benefits of PE mulch use generally come at the price of long-term negative effects on the environment and soil productivity (Steinmetz et al., Reference Steinmetz, Wollmann, Schaefer, Buchmann, David, Tröger, Muñoz, Frör and Schaumann2016).
Among the consumer and commercial products contributing to global plastic waste, mulch membranes are an ideal candidate for replacement with biodegradable alternatives. In lieu of removal and disposal, biodegradable mulch (BDM) can be tilled into the soil to facilitate degradation. Because tillage is often used in specialty crops to incorporate crops residues, end-of-life BDM management does not require any additional time or labor. While BDM mulch is more expensive than PE, some growers are motivated by end-of-life management cost savings and its environmental benefits. Organic growers, in particular, are motivated for alternatives to PE mulch because its use conflicts with the philosophy and values underpinning organic agriculture. Nevertheless, PE mulch is an allowable input under the United States Department of Agriculture (USDA) National Organic Program and is still widely used among organic vegetable and small fruit growers. While BDM is technically an allowable input under the same program, the definition employed by the USDA for what constitutes a ‘BDM’ effectively precludes the use of all commercially-available BDM films on certified organic farms in the USA. For soil incorporation on an organic farm, any mulch film must be manufactured from 100% biobased feedstock and meet ASTM D5988/ISO 17556 standards for 90% biodegradation under typical field soil conditions in less than 24 months. Paper mulches are the only commercially available mulch membranes that meet this definition, but paper mulch often degrades too early, is relatively expensive and does not provide soil warming benefits that promote crop earliness (Tofanelli and Wortman, Reference Tofanelli and Wortman2020). For organic and conventional growers, slow and unpredictable soil degradation rates of BDM are a significant barrier to adoption. Slow degradation of a mulch can lead to the accumulation of residue that pollutes the visual aesthetic of the farm (Dentzman and Goldberger, Reference Dentzman and Goldberger2020), interferes with normal field operations (e.g. planting) and creates an imbalance of C:N that contributes to N immobilization (Martens, Reference Martens2001). Thus, research is needed to develop new biobased mulch membrane products and soil management strategies that will reliably accelerate mulch degradation rates in soil under field conditions.
Currently, the most widely used BDM films are formulated from biobased-polyester blends, and include the polymer tradenames MaterBi® (starch-polyester blend; Novamont, Novara, Italy) and Ecovio® (polylactic acid-polyester blend; BASF, Ludwigshafen, Germany). Both polymers are certified compostable and biodegradable in soil, but they are not 100% biobased (and cannot be incorporated into soil on organic farms in the USA). Polylactic acid is a promising feedstock for mulch membranes because it is biodegradable, durable and flexible (Hakkarainen et al., Reference Hakkarainen, Karlsson and Albertsson2000; Masaki et al., Reference Masaki, Kamini, Ikeda and Iefuji2005). However, when used as the sole feedstock (not blended with synthetic copolymers as in Ecovio®), polylactic acid products are relatively slow to biodegrade in soil (Miles et al., Reference Miles, Wallace, Wszelaki, Martin, Cowan, Walters and Inglis2012; Siwek et al., Reference Siwek, Domagała-Świątkiewicz and Kalisz2015; Martín-Closas et al., Reference Martín-Closas, Costa, Cirujeda, Aibar, Zaragoza, Pardo, Suso, Moreno, Moreno, Lahoz, Mácua, Pelacho, Martín-Closas, Costa, Cirujeda, Aibar, Zaragoza, Pardo, Suso, Moreno, Moreno, Lahoz, Mácua and Pelacho2016; Wortman et al., Reference Wortman, Kadoma and Crandall2016). As a result, polylactic acid mulches have only been developed and tested on an experimental basis and are not commercially available to growers.
A need exists to better understand whether management practices can accelerate biodegradation of mulches in general, and polylactic acid mulches in particular (Thompson et al., Reference Thompson, Samuelson, Kadoma, Soto-Cantu, Drijber and Wortman2019). Mulch composition and the soil environment influence the degradation rate of xenobiotic polymers in mulch membranes. For example, adding particles of alfalfa and soybean meal to a polylactic acid mulch membrane could accelerate mulch degradation rate (Thompson et al., Reference Thompson, Samuelson, Kadoma, Soto-Cantu, Drijber and Wortman2019). In addition, composition and activity of soil microbial communities can affect mulch biodegradation rates. Indeed, manipulation of microbial communities has been shown to modulate the degradation rate of polylactic acid (Hakkarainen et al., Reference Hakkarainen, Karlsson and Albertsson2000; Karamanlioglu and Robson, Reference Karamanlioglu and Robson2013). Differences in soil properties and climate across diverse agroecoregions will shape microbial community composition and activity (Fierer and Jackson, Reference Fierer and Jackson2006). Li et al. (Reference Li, Moore-Kucera, Miles, Leonas, Lee, Corbin and Inglis2014) reported 98% degradation of BDM films after 24 months in Texas (southern USA), compared to only 11% degradation after 24 months in Washington (northwest USA). Soil temperature, alkalinity and the abundance of soil fungi were greater in Texas than in Washington. Together, this previous research suggests that there may be an opportunity to manage the soil abiotic and biotic environment within locations to favor BDM degradation.
Farmers can influence soil microbial activity and community composition to some degree through routine management practices including irrigation, cover cropping, tillage and compost amendment (Mann et al., Reference Mann, Lynch, Fillmore and Mills2019). However, it is unknown whether the magnitude and duration of these soil microbial changes are sufficient to influence degradation kinetics of BDM. Indeed, cover cropping and compost amendment have been shown to influence soil microbial community composition and function (Toyota and Kuninaga, Reference Toyota and Kuninaga2006; Finney et al., Reference Finney, Buyer and Kaye2017; Barel et al., Reference Barel, Kuyper, Paul, de Boer, Cornelissen and Deyn2019). Moreover, both compost and cover crops represent a source of N that could help to increase residue decomposition if N becomes limiting to microbial metabolism (Parr and Papendick, Reference Parr and Papendick1978). Compost tea—an aqueous suspension of compost used as a biological inoculant—is increasingly popular among growers as a plant and soil amendment, but evidence about whether it can influence soil microbial communities is limited (Scharenbroch, Reference Scharenbroch2013). If short-term soil management can enhance microbial activity and function, it may be possible to leverage one or more practices to accelerate the degradation rate of BDM in the soil.
The objective of this study was to determine the potential influence of compost, compost extract and cover crops on degradation of a polylactic acid biocomposite mulch and a starch-polyester mulch in soil relative to the effects of farm location and mulch composition.
Materials and methods
Experimental location and design
We conducted field experiments from 2017 through 2019 in two ecoregions of Nebraska including the Western Corn Belt, Lincoln, NE USA (LNK), and the Western High Plains, Scottsbluff, Nebraska, USA (SBF) (Table 1; Fig. 1). Fields at both locations had been planted to maize (Zea mays) in 2016 and a diverse rotation of agronomic field crops prior to 2016. The experiment at each location was set up as a split-split-plot, randomized complete block design with three replications. Main plots were mulch type (65.84 m long × 1.83 m separated by 0.30 m buffers): black Bio 360® biodegradable plastic mulch formulated from the Mater-Bi® polymer (abbreviated hereafter as BLK) (Novamont S.P.A.; Shelton, CT, USA); and a prototype bio-based polylactic acid polymer plus fine-grade wood particle biocomposite mulch (abbreviated hereafter as PLA) (3M Company, St. Paul, MN, USA; Table 2; Fig. 2). Mulches were field-applied in main plots for pepper (Capsicum annuum) production during the 2017 growing season. The split-plots (1.83 × 32.92 m) were mulch removal (CTL) or incorporation (INC) of the mulch residue at the end of the 2017 growing season; however, in this study of mulch degradation over time only the INC plots are analyzed. Split-split-plot soil management treatments (1.83 × 5.49 m) included compost (COM), cover crops (COV), compost extract (CEX), a ‘kitchen sink’ management (SNK) comprised of all three management practices and a no amendment control (NA; two replicate plots per block compared to one for the other treatments).
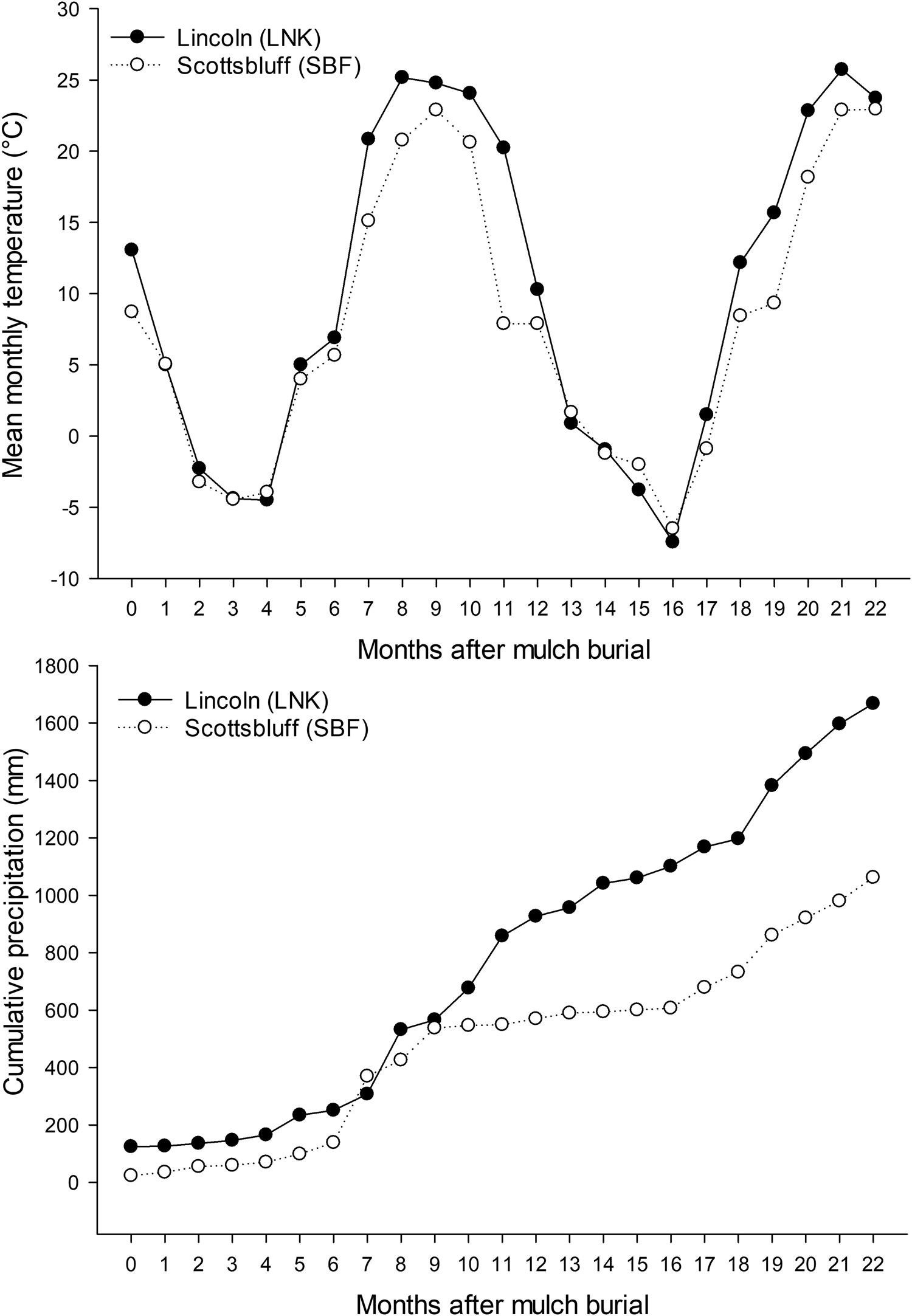
Fig. 1. Observed mean monthly air temperature (top) and cumulative precipitation (bottom) for Lincoln, NE (LNK) and Scottsbluff, NE (SBF) from October 2017 (0 months after mulch burial) through August 2019 (22 months after mulch burial).

Fig. 2. Prototype polylactic acid plus wood particle mulch (PLA) peeled into its component layers. Lighter colored squares are the stronger outer layers of spunbond polylactic acid fiber. The inner, darker speckled layer is meltblown polylactic acid with embedded fine wood particles.
Table 1. General geographic, climatic and soil properties of each experimental location

Table 2. Commercial and physical properties of the two mulch types (main plots) used in the experiment

PLA, polylactic acid plus wood particle biocomposite mulch; BLK, black starch-polyester mulch film.
a 3M Company, St. Paul, MN, USA.
b Novamont, Novara, Italy.
c PLA was comprised of three distinct layers. The inner layer was a black, meltblown micro fiber made from polylactic acid (21% of total mulch mass) with extra fine grade (<0.5 mm in diameter) wood particles (62% of total mulch mass) embedded in the fiber matrix. The two white outer layers were spunbond from polylactic acid fibers and thicker than the inner layer.
Cropping systems management
To expose mulches to a season of weathering by normal use during vegetable production prior to soil incorporation, a mulched crop of sweet pepper (C. annuum var. Carmen) was produced. In the spring of 2017, experimental fields were rototilled to a depth of 20 cm. Both mulch types were field-applied via a raised bed mulch layer (RB448; Nolt's Produce Supplies, Leoloa, PA, USA) with dripline irrigation applied under the mulch. Soil matric potential was monitored weekly (Watermark sensors; Campbell Scientific, Logan, Utah) and all plots were irrigated regularly to maintain matric potentials less than 75 kPa at LNK and less than 25 kPa at SBF (Irmak et al., Reference Irmak, Payero, VanDeWalle, Rees, Zoubek, Martin, Kranz, Eisenhauer and Leininger2016). Sweet pepper was transplanted into the mulch in a single row at 0.46 m spacing. Water-soluble fish emulsion (2.9-3.5-0.3 NPK; OrganicGem, Advanced Marine Technologies, New Bedford, MA, USA) was applied at a rate of 0.3 g N, 0.36 g P and 0.03 g K per planting hole at planting and twice more throughout the season at the same rate. At the second and third fertilization events, blood meal fertilizer (13-1-0 NPK; Earthworks Health, LLC, Norfolk, NE, USA) was added to achieve a cumulative rate of 84 kg ha−1 N. After harvest in the fall of 2017, crop residue was mowed, and mulches were either removed (CTL) or soil incorporated (INC) on 29 September 2017 and 5 October 2017 at LNK and SBF, respectively. Removed mulch was gently cleaned to remove large soil and debris, then stored temporarily for use in buried mesh bags (described below). Soil incorporated mulch (INC) was shredded and tilled into soil by a single pass of a spading implement (Celli Y70 spading machine, Celli SpA, Forlì, Italy).
In the spring of 2018, organic soybean meal fertilizer (7-1-2 NPK; Phyta-Grow, California Organic Fertilizers, Inc., Hanford, CA, USA) was applied at a rate of 67 kg ha−1 N prior to soil sampling and seeding. No mulch was applied and the field was not tilled. Sweet corn (Z. mays cv. ‘Xtra-Tender 2171’) was sown on 24 May and 31 May, at Lincoln and Scottsbluff, respectively, in two rows per plot with approximately 20 cm spacing between seeds and 60 cm between rows. Two rows of drip tape irrigation line were laid adjacent to each row of sweet corn, and plots were watered regularly as in pepper. Weeds were managed with hand hoes as needed. Sweet corn was harvested 23 July and 30 July 2018 in Lincoln and 27 and 28 August 2018 at Scottsbluff. Crop and weed residue was shredded 12 September and 21 September at LNK and SBF, respectively.
Cabbage (Brassica oleraceae var. sabauda cv. ‘Melissa’) was planted at both locations in spring 2019. A white-on-black polyethylene plastic mulch film was applied to flat, non-tilled ground within each plot for weed management. One row of drip irrigation line was laid beneath the plastic mulch film, and cabbage was watered regularly as in pepper and sweet corn. Plots were not tilled, nor were raised beds created (as in 2017), to avoid disturbing mulch residues incubating in mesh bags (details below). Bloodmeal fertilizer (13-1-0) was applied prior to planting at a rate of 34 kg ha−1 N. Cabbage plants were transplanted 16 May at LNK and 5 June at SBF with 0.46 m spacing between plants within a single row. Fish emulsion fertilizer (2-3-1 NPK; Neptune's Harvest, MA, USA) was applied after transplant at both locations at a rate of 0.46 g N, 0.69 g P and 0.23 g K per planting hole. Cabbage was harvested 5 August at LNK and 19 August at SBF.
Soil management treatments
Compost for the COM and SNK treatments was applied and incorporated by tillage along with mulch residues in fall 2017. A second application of compost was made in the COM and SNK treatments in fall 2018 after harvest of sweet corn (Z. mays). Compost applied at LNK was a municipal yardwaste compost applied at a rate of 57 and 60 mg ha−1 (dry weight) in 2017 and 2018, respectively. Compost applied at SBF was a composted beef feedlot manure applied at 42 mg ha−1 in 2017 and 51 mg ha−1 (dry weight) in 2018. Given the different feedstocks between locations, compost rate was standardized by total N rate with a target of 504 kg ha−1 total N.
Compost extract is defined here as a suspension of compost in water including fine particulate and soluble fractions of compost. It is allowable for certified organic production in the USA as long as it is included in a producer's organic system plan and approved by their certifier (Samuelson et al., Reference Samuelson, Wortman and Drijber2019). It is most often used as a microbial inoculant, applied at such low rates as to supply negligible amounts of nutrient fertility. Compost extract for this study was prepared by vigorously kneading vermicompost (from kitchen and yardwaste feedstock) inside of a nylon filter bag with 400 μm openings while submerged in water. A ratio of 60 g fresh (20 g dry equivalent) compost was used per liter of water. After kneading, compost remaining in the nylon filter bag was discarded. Compost extract was applied to CEX and SNK plots by a coarse spray at a rate of 0.37 liter m−2 in fall 2017, spring 2018, fall 2018 and spring 2019. Extract was applied within 48 h of tillage in fall 2017, at the time of cover crop planting in spring 2018, after mowing crop residues in fall 2018, and at the time of cover crop termination in spring 2019.
A cover crop was planted in the fallow period between cash crops in COV and SNK plots in early-spring 2018 and fall 2018. A mustard cover crop (var. Mighty Mustard® Pacific Gold) was broadcast-seeded at a rate of 22.4 kg ha−1 and incorporated by hand raking to a depth of approximately 1 cm. Mustard was sown 23 March 2018 and re-sown (due to poor emergence) 20 April 2018 at LNK, and sown 23 April 2018 at SBF. The cover crop at ~6 cm height was terminated on 23 May at LNK using a flail mower and on 30 May with hoes at SBF. At termination, mustard covered approximately 80% of the soil surface. After the 2018 harvest, a cover crop mixture of cereal rye (Secale cereale) and hairy vetch (Vicia villosa) was broadcast-seeded at a rate of 112 and 44.8 kg ha−1, respectively. Seeds were incorporated in soil by hand rake to a depth of approximately 1 cm. Rye and vetch were sown 28 September 2018 at LNK and 24 September 2018 at SBF. The rye and vetch cover crop was terminated by flail mower on 14 May 2019 at LNK and 4 June 2019 at SBF. At both locations, the cover crop was terminated at the fully emerged head state for rye at ~86 cm tall; vetch was flowering. Soil surface coverage from the rye/vetch mixture (dominated by rye) was close to 100% at both locations.
Mesh bag preparation and burial
To prepare mesh bags, mulch squares measuring 10 cm2 were cut from the gently washed mulch that was removed from the CTL split-plots. Each square was weighed then paired with a methanol-washed aluminum label embossed with a unique ID. Mesh bags were nylon, 26 × 15 cm in size, with 200 μm openings, and a hook and loop closure. Soil for filling mesh bags was collected from CTL split-plots of the same management treatment in fall 2017 after mulch removal, and after application of COM and CEX treatments and tillage. As an example, soil from COM-CTL split-split-plots was removed from the field and used in bags buried in COM-INC split-split-plots. Field-collected soil was sieved to 1 cm and stored at 4°C for less than 1 week before use in mesh bags to be buried in corresponding management split-split-plots. In total, 250 g of soil was added to each bag, then a mulch square was laid flat on this soil, followed by another 250 g soil on top of the mulch square. Eight mesh bags were prepared in this way for burial in each management split-split-plot within INC split-plots. Mesh bags were buried to occupy a depth of 5–10 cm in a grid pattern of two rows with four bags each, spaced 0.61 m between rows and 0.91 m within rows. Mesh bag burial position within split-split-plots was assigned a number (1–8) and two numbers were randomly selected for recovery from each plot and recovery event.
Mesh bag removal and calculation of mulch degradation
Two mesh bags were recovered at approximately 6-month intervals for a total of four recovery events over 2 years. At recovery, bags were placed in one of two plastic bags (one assigned for biological and chemical analysis and one assigned for mulch mass loss determination), stored temporarily in coolers in the field, then transferred to a refrigerator for storage at 4°C for no more than 1 week before processing. To begin processing, bags were cut or carefully ripped open on a 4 mm sieve. Mulch fragments from the biological and chemical analysis bag were recovered and loosely adhering soil was brushed off with gloved hands. This mulch was stored at −20°C for future microbial analyses (Samuelson, Reference Samuelson2019). Remaining soil in the bag was homogenized; 100 g was stored at −20°C for microbial analyses and another 100 g was air-dried and stored at room temperature for chemical analyses. Mulch fragments removed from the second mesh bag were air-dried at room temperature before further processing to determine mass.
Mulch degradation in mesh bags containing soil was measured over time using the mesh litterbag approach common in crop residue and biomulch decomposition studies (Li et al., Reference Li, Moore-Kucera, Miles, Leonas, Lee, Corbin and Inglis2014). Two different approaches were used for determining the mass of recovered mulch fragments: washing and combustion. Mulch fragments recovered in spring of 2018 were weighed after washing. Visible mulch of any particle size that was cohesive enough to be separated from soil was recovered. Mulch washing methods were tailored to each mulch type in an effort to maximize recovery. For the BLK mulch, mulch fragments in 0.25 mm sieves were gently washed under running water until visibly clean. After air-drying at room temperature to constant mass, any remaining soil particles adhered to the mulch were removed by hand or brush, and the mulch was weighed. Washing PLA mulch was more challenging because soil was embedded in the matrix of spunbond fibers. Washing and flotation in a basin was used to separate mulch fibers and soil particles. This resulted in particles of the fine wood fiber and dark middle layer of PLA to detach from the thicker white outer PLA layers. These particles were recovered by: (1) mixing the soil and small mulch particles in a basin of clean water, (2) allowing heavier soil particles to settle for 10 s, and then (3) gently pouring the basin of water over 0.5 mm sieves to collect mulch particles. Recovered mulch particles were washed once more using the same approach to separate plant debris and mulch particles. Recovered mulch was air-dried to constant mass and weighed.
Samples collected in fall 2018 were too fragile to separate from soil by washing; therefore, a combustion method was developed to determine mass for samples collected in fall 2018 and beyond. Ash content of soil, unused mulch and soil-covered mulch samples were measured by loss on ignition (LOI) in order to calculate a mulch mass ratio for the entire sample. First, recovered mulch samples were air-dried with associated soil particles; once dry, any plant root residues were removed from the sample. Next, samples were dried at 60°C in aluminum tins and weighed. Finally, samples were combusted in a muffle furnace and weighed to determine ash content by LOI. The furnace was programmed to ramp to 550°C over a 2 h period, hold at 550°C for 4 h, then cool for 8–10 h before samples were removed.
Ash content of soil ranged from 4.5 to 7.9% and 2.5 to 2.9% at LNK and SBF, respectively. Ash content of unused mulches was 0.45% (0.02% standard deviation) and 0.17% (0.08% standard deviation) for PLA and BLK, respectively. Soil ash content was found to vary significantly between plots within each location. To account for this variability, ash content was determined for mulch-free soil sampled from a mesh bag in each treatment plot. Using this soil and mulch ash content data, mulch mass loss was determined as:
whereby G is the mass (g) of a 60°C oven-dried sample of recovered mulch with adhering soil; P is the fraction of the sample mass (g) remaining after LOI combustion; S is the fraction of soil mass (g) remaining after LOI combustion; and M is the fraction of mulch mass (g) remaining after combustion.
Regardless of the method used to determine mulch mass, percent mulch mass remaining in soil was calculated as:
whereby M o is the original mass of unused mulch placed in mesh bags in fall 2017; and M t is the mulch mass at time t (approximately 6, 12, 18 or 22 months after burial) determined via washing or combustion method.
Microbial biomass and community composition of soil in mesh bags
Saprophytic fungal and bacterial biomass associated with soil in mesh bags were estimated by fatty acid methyl ester (FAME) analysis. Due to limited resources, only the two extreme treatments (NA and SNK) were analyzed. FAMEs were extracted from soil by alkaline methanolysis (Grigera et al., Reference Grigera, Drijber, Shores-Morrow and Wienhold2007; Jeske et al., Reference Jeske, Tian, Hanford, Walters and Drijber2018). Approximately 10 g field-moist soil was extracted in 0.2 M methanolic KOH, partitioned into hexane and quantified by gas chromatography on an Agilent 7890 GC fitted with an HP-Ultra 2 capillary column (50 m, 0.2 mm I.D., 0.33 μm film thickness). Identity of FAMEs was confirmed on an Agilent 7890 GC fitted with an Agilent 5975 mass selective detector. Fatty acids are named by the IUPAC system described in Drijber et al. (Reference Drijber, Doran, Parkhurst and Lyon2000). The biomarker C18:2cis9,12 was used for saprophytic fungal biomass. Bacterial biomass was represented by the sum of 13 FAMES: iC14:0; iC15:0; aC15:0; C15:0; iC16:0; iC17:0; aC17:0, cyC17(9,10), C17:0, 10MeC18:0; 10MeC19:0, cyC19(9,10) and cyC19(11,12).
Data collection for soil properties
At the time of recovery of each mesh bag, eight soil cores (1.9 cm diam. by 20 cm) were collected from each management treatment. Soil cores were homogenized and combined into one sample. A fraction of this sample was analyzed for nitrate (KCl extraction), pH (1:1 dilution) and soil organic matter (loss of weight on ignition) (Ward, Reference Ward2021). Soil temperature was measured every 4 h in all plots to 5 cm depth with temperature sensors and data loggers from fall 2017 through fall 2019 (HOBO Pendant; Onset, Bourne, MA, USA). Sensors were buried to a depth of 5 cm and programmed to log temperature every 4 h. These data were used to calculate mean seasonal soil temperature for the 6 months preceding each mulch recovery event. Gravimetric soil water content was also measured within mesh bags at the time of recovery. Approximately 10 g field moist soil was placed in an aluminum canister, dried at 75°C to constant mass and weighed. Water content was calculated as:

Soil tensile strength was measured in all plots at both locations 6 and 18 months after mulch incorporation in soil with a force meter using a rounded, flat head (FDX Force Ten, Wagner Instruments, Greenwich, CT, USA) following the methods of Öztaş et al. (Reference Öztaş, Canpolat and Sönmez1999) and Dexter and Kroesbergen (Reference Dexter and Kroesbergen1985). Due to variability, 20 air-dried aggregates between 4.75 and 8 mm were crushed individually between a flat metal disk sitting on a metal plate and the force meter head. The force needed to crush each aggregate was recorded. Tensile strength was calculated as:
whereby F is the force required to break the aggregate (N), D is the diameter of the aggregate (m), and 0.576 is the value of the coefficient of proportionality between the applied compressive load and the inner tensile strength of the aggregate (Dexter and Kroesbergen, Reference Dexter and Kroesbergen1985).
Soil penetration resistance as an indicator of compaction was measured in all plots at both locations 6 and 18 months after mulch incorporation in soil using the force meter with an 8 mm diameter cone head. Penetration resistance was measured to a depth of 5 cm with a speed of approximately 1 cm s−1. The readings were converted to MPa based on the basal cone area (Rakkar et al., Reference Rakkar, Blanco-Canqui, Drijber, Drewnoski, MacDonald and Klopfenstein2017). At the time of penetration resistance measurements at each location, soil water content was measured by time domain reflectometry (TDR) (FieldScout TDR 300, Spectrum Technologies, Inc., Aurora, IL, USA) to make corrections for the effects of soil moisture on penetration resistance as needed (Busscher et al., Reference Busscher, Bauer, Camp and Sojka1997).
Statistical analysis
Analysis of variance was conducted with the glimmix procedure in SAS 9.4 (SAS Institute, Cary, NC, USA) to test for the effects of location, mulch type and management treatment on mulch mass remaining over time. Fixed effects were location, mulch type, management and recovery time, and their interactions. Random effects were replicate block and the interaction of block and recovery time (as a repeated measure). To determine treatment effects on soil properties across mulch types, but within locations and sample times, treatment was the fixed effect and block was the random effect. Means separation was determined for significant main effects and interactions using the Tukey's HSD test with a significance threshold of P < 0.05. When an effect was significant, mean separation was performed using Tukey's HSD with the lsmeans function.
Normal distribution of data was confirmed with probability plots in the univariate procedure in SAS. The BLK mulch at LNK was largely unrecoverable and fully degraded at the fall 2018 sampling interval. To avoid violating assumptions of equal variance, BLK data for spring and fall 2019 were omitted from the analysis because all observations were 0% mulch mass remaining (zero variance).
Correlation and canonical discriminant analyses were used to explore relationships between observed soil properties in each management treatment and mulch mass remaining over time for each mulch type and location. Correlation analysis in R (version 3.6.2, R Foundation for Statistical Computing, Vienna, Austria) was used to assess pairwise correlations between mulch mass remaining and soil nitrate, organic matter content, pH, and mesh bag soil bacteria and saprophytic fungi (from NA and SNK treatments only) collected at the corresponding time of mesh bag recovery (spring 2018, fall 2018, spring 2019 and fall 2019). Mean soil temperature in the 6 months preceding a mulch recovery event was used for correlation analysis. For example, mean temperature between 1 October and 31 March was used for correlation analysis with spring measurements of mulch mass remaining in the same year, and mean soil temperature between 1 April and September 30 was used for fall measurements of mulch mass. Soil tensile strength and penetration resistance was measured in spring 2018 and 2019.
Canonical discriminant analysis was used to explore the variation among mulch types and locations as influenced by the combination of observed soil properties in each plot (irrespective of management treatment). For each of the four mulch recovery dates, we created a multivariate linear model. Response variables included mulch mass remaining and SOM, soil nitrate, pH, temperature, aggregate tensile strength and penetration resistance corresponding to the same recovery date (as described above for correlation analysis). The predictor variable was the combination of mulch type by location. Microbial FAME data from soil in mesh bags were excluded from canonical discriminant analysis because data were only collected from the NA and SNK split-split-plots (not COV, COM or CEX plots). Analysis of variance was used to test the hypothesis that the treatment group (mulch type by location) has a significant effect on the collection of response variables (soil properties and mulch remaining). Normal distribution of residuals was evaluated visually with a χ2 QQ plot (cqplot function in R). When the treatment group effect was significant and residuals were normally distributed, canonical discriminant analysis (candisc function in R) of the multivariate linear model was used to identify response variables contributing most to between-group variation. Canonical scores, treatment group means and response variable vectors were plotted for two canonical dimensions using the plot function for the candisc object in R.
Results
Soil properties
Observed soil property changes due to management treatments within locations can be attributed to compost application in the COM and SNK management treatments, and seasonal cover crop growth in the COV and SNK treatments (Tables 3 and 4). There were no effects of CEX on any soil chemical, physical or biological properties. Differences in soil nitrate concentration were usually observed in the spring when cover crops in the COV and SNK treatments were actively growing and assimilating soil nitrate. Compost application in COM and SNK increased soil nitrate concentration by fall 2019 at LNK (and was trending in the same direction at SBF, though not significant). Soil organic matter content (SOM) was greatest in COM and SNK treatments at each location, and differences increased with time (Tables 3 and 4). By 18 months after mulch burial, compost application had increased SOM by 25% at both locations in the COM and SNK treatments relative to the control. Cover crops used alone had no effect on SOM during the course of this experiment. Management did not affect soil pH or soil water content.
Table 3. Soil chemical, physical and microbial properties at Lincoln (LNK) site as influenced by management treatments (COM = compost; COV = cover crop; NA = no amendment control; SNK = all treatment ‘kitchen sink’; CEX = compost extract) and sample time (Spring 2018 = 6 months after mulch burial, Fall 2018 = 12 months after mulch burial, Spring 2019 = 18 months after mulch burial, Fall 2019 = 22 months after mulch burial)

1 SOM = % organic matter content.
2 Temp. = mean soil temperature for the preceding 6 months.
3 SWCg = gravimetric soil water content; data collected from within mesh bags of only the NA and SNK plots.
4 PR = soil penetration resistance; data collected in spring 2018 and spring 2019 only.
5 TS = soil tensile strength; data collected in spring 2018 and spring 2019 only.
6 Bacterial and saprophytic fungi FAME abundance in mesh bag soil were collected from only the NA and SNK plots.
7 Significance of management treatments on a soil property are indicated as: *P < 0.05, **P < 0.01, ***P < 0.001, and ns = not significant. When the effect of management is significant at a given sample time, differences among treatments (P < 0.05) are indicated by different letters within a column.
Table 4. Soil chemical, physical and microbial properties at Scottsbluff (SBF) site as influenced by management treatments (COM = compost; COV = cover crop; NA = no amendment control; SNK = all treatment ‘kitchen sink’; CEX = compost extract) and sample time (Spring 2018 = 6 months after mulch burial, Fall 2018 = 12 months after mulch burial, Spring 2019 = 18 months after mulch burial, Fall 2019 = 22 months after mulch burial)

1 SOM = % organic matter content.
2 Temp. = mean soil temperature for the preceding six months.
3 SWCg = gravimetric soil water content; data collected from within mesh bags of only the NA and SNK plots.
4 PR = soil penetration resistance; data collected in spring 2018 and spring 2019 only.
5 TS = soil tensile strength; data collected in spring 2018 and spring 2019 only.
6 Bacterial and saprophytic fungi FAME abundance in mesh bag soil were collected from only the NA and SNK plots
7 Significance of management treatments on a soil property are indicated as: *P < 0.05, **P < 0.01, ***P < 0.001, and ns = not significant. When the effect of management is significant at a given sample time, differences among treatments (P < 0.05) are indicated by different letters within a column.
Winter and summer soil temperatures were not influenced by management in the first year (Tables 3 and 4). In the second year, the cover crops and resulting cereal rye surface residues that persisted through the summer reduced soil temperatures by 1.1–1.4°C at LNK and 0.5–0.7°C at SBF relative to the control. Management did not affect soil penetration resistance and soil tensile strength at either location. Soil bacterial FAME abundance was 13–19% greater in SNK compared to the control at LNK in the first 12 months, but there was no difference between treatments thereafter. Saprophytic fungal abundance was 68% greater in SNK than the control at LNK after 12 months, but there were no differences at 6, 18 or 22 months. Soil bacterial FAME abundance was consistently 25–67% greater in SNK compared to the control at SBF across all sample intervals, but there were no differences in fungal abundance.
Mulch mass remaining
Management treatment had no effect on mulch degradation. Instead, mulch mass remaining (%) was influenced by the three-way interaction of location by mulch type by time (P < 0.0001). After 6 months of burial, the BLK mulch did not degrade at either location, but the PLA mulch had degraded to 47 ± 4% mulch mass remaining at SBF and 75 ± 4% at LNK (Fig. 3). By 12 months, only 2 ± 4% of the BLK mulch remained at LNK while 68 ± 4% remained at SBF. The PLA mulch degraded to 34 ± 4% mass remaining at SBF and 38 ± 4% at LNK by 12 months after burial. While the BLK mulch was almost completely degraded after 12 months at LNK, degradation of BLK at SBF remained incomplete after 22 months. By 22 months after mulch burial, 48 ± 4% BLK mulch mass remained at SBF. Degradation of PLA mulch slowed significantly after 12 months; 29 ± 4% mulch mass remained at SBF and 33 ± 4% remained at LNK after 22 months.

Fig. 3. Effects of location (SBF = Scottsbluff; LNK = Lincoln), mulch type (PLA = prototype polylactic acid wood particle mulch; BLK = Bio360® black bioplastic film) and recovery date (approximately 6, 12, 18 and 22 months after mulch burial) on mulch mass remaining in soil (%). Error bars represent the standard error of least squares means (n = 18).
Factors driving mulch degradation between locations
Mulch type by location treatment groups significantly influenced mulch remaining and soil properties 22 months after mulch burial (P < 0.0001). Canonical discriminant analysis of the multivariate linear model identified two significant canonical dimensions (P < 0.0001) that explained a combined 99.6% of between-group variation (Fig. 4). The first dimension explained 93.1% of variation and was positively related to soil pH and mulch mass remaining. Soil temperature, organic matter content and penetration resistance were negatively related to the first dimension. Locations were easily segregated by this first dimension with SBF characterized by alkaline soil pH and greater mulch mass remaining (less mulch degradation), and lower soil organic matter content, temperature and penetration resistance compared to LNK. However, the first dimension offered little explanation for differences between mulch types. The second dimension explained considerably less variation (6.5%) and was positively related to mulch mass remaining and soil nitrate and tensile strength (albeit to a lesser degree). Mulch types at LNK were segregated by this second canonical dimension because mulch mass remaining at LNK was greater for PLA than BLK.

Fig. 4. Canonical scores and means for the fall 2019 mulch type by location treatment groups (red d = LNK.BLK = Lincoln, Bio360® black bioplastic film; green V = LNK.PLA = Lincoln, prototype polylactic acid wood particle mulch; purple + = SBF.BLK = Scottsbluff, Bio360® black bioplastic film; blue × = SBF.PLA = Scottsbluff, prototype polylactic acid wood particle mulch) on the first (Can1) and second (Can2) canonical dimensions (explaining 93.1 and 6.5% of variability, respectively). Blue vectors communicate the strength of correlations between response variables [soil temperature, soil organic matter content, pH, mulch remaining (% mulch mass remaining in soil), soil nitrate, soil aggregate tensile strength and soil penetration resistance] and each canonical dimension. Ellipses represent one standard deviation (68%) from the mean of each treatment group.
Factors driving mulch degradation within locations
Soil properties and mulch mass remaining were usually not correlated. Plot-scale variability in nitrate and organic matter concentrations, soil penetration resistance, soil tensile strength and soil fungal FAMEs did not help explain variation in mulch mass remaining within each location (P > 0.05; Supplementary Tables 1 and 2). At SBF, soil temperature was positively correlated with mulch mass remaining of PLA after 6 months burial (P = 0.001; r = 0.71); however, there was no relationship between soil temperature and mulch degradation observed for any other mulch or recovery period at SBF or LNK. Soil pH was negatively correlated with mulch mass remaining for only PLA after 6 months at LNK (P = 0.04; r = −0.47). Soil water content was negatively correlated with BLK mulch remaining after 12 months at LNK (P = 0.02; r = −0.90). Soil bacterial FAMEs were positively correlated with mulch mass remaining of PLA after 18 months (P = 0.03; r = 0.87) and BLK after 22 months (P = 0.05; r = 0.95), but the two variables were unrelated for the other 11 location by mulch by time combinations tested.
Discussion
Intensive soil management did not accelerate mulch degradation
Despite significant plot-scale changes in soil thermal, chemical and biological properties (Tables 3 and 4), intensive use of compost, compost extract and cover crops did not influence mulch degradation rates. Biobased mulch degradation can be faster in compost compared to bare soil (Sintim et al., Reference Sintim, Bary, Hayes, Wadsworth, Anunciado, English, Bandopadhyay, Schaeffer, DeBruyn, Miles, Reganold and Flury2020), but in this study, the high rate (42–60 mg ha−1) and frequency (every fall) of compost and compost extract (every spring and fall) applications to soil did not influence degradation. Thus, it seems the commercial composting environment (i.e., sustained high temperatures and enhanced microbial activity) drives degradation, not the resulting chemical, biological or physical properties of the compost when applied to soil. This is consistent with the results of Thompson et al. (Reference Thompson, Samuelson, Kadoma, Soto-Cantu, Drijber and Wortman2019) where biodegradation of PLA or BLK mulches in response to compost extract, mineral nitrogen fertilizer or commercial biostimulants was inconsistent.
The lack of management effects is also consistent with Li et al. (Reference Li, Moore-Kucera, Miles, Leonas, Lee, Corbin and Inglis2014) where degradation of starch-based and polylactic acid-based mulches was not different between field and high-tunnel vegetable production environments. Soil temperature and mineral N were consistently greater in the high tunnel than the open field, and soil in the tunnel was irrigated regularly to mirror soil water conditions in the field. Given the importance of soil temperature and nitrogen in driving microbial metabolism, it is surprising that degradation rates were not different in the high tunnel. These results by Li et al. (Reference Li, Moore-Kucera, Miles, Leonas, Lee, Corbin and Inglis2014) highlight the challenge of leveraging management to accelerate mulch degradation within farm locations. Although we observed small treatment differences in soil temperature in this study, primarily due to the presence of cover crop residue in the spring (Tables 3 and 4), these modest temperature differences did not help to explain mulch degradation rates.
Looking beyond the categorical treatment structure, plot-scale variability in soil properties did not consistently correlate with mulch mass remaining for either mulch type at either location. Microbial degradation of BDM can immobilize soil nitrate (Moon et al., Reference Moon, Song, Shin, Cho, Bae, Heo, Kang and Lee2016), but we did not observe a relationship between mulch remaining and soil nitrate at any of the four recovery intervals (6, 12, 18 or 22 months after burial). PLA mulch was 62% fine wood particles by weight so we had expected nitrogen immobilization during the initial degradation of this mulch (i.e., 0–12 months). Despite rapid degradation of PLA and its embedded wood particles during the first 6 months of burial, especially at SBF, there was no relationship between remaining mulch mass and available soil nitrate.
Intensive use of compost and cover crop soil amendments has been shown to increase soil water holding capacity, particularly in sandy soils (Brown and Cotton, Reference Brown and Cotton2011; Minasny and McBratney, Reference Minasny and McBratney2018); and microbial activity and soil decomposition rates are driven in part by soil water content (Thomsen et al., Reference Thomsen, Schjønning, Jensen, Kristensen and Christensen1999). Therefore, we hypothesized that mulch mass remaining and soil water content would be negatively correlated within locations due to variability resulting from compost and cover crop use among treatments. This relationship was apparent for one mulch type at one location and sampling interval (BLK at LNK after 12 months) but was otherwise not a useful explanatory variable for mulch degradation within locations. Soil water content was not influenced by management treatments within locations (Tables 3 and 4), so it seems that variability in soil water content was not adequate to drive significant changes in mulch degradation. Instead, larger differences in soil water content among diverse locations resulting from differences in precipitation and soil physical properties are more likely to influence mulch degradation rates (i.e., SB vs LNK in this study). However, the majority of biomulch research is conducted in irrigated specialty crop systems where soil water content is less likely to limit microbial activity during the growing season when mulch degradation rates will be greatest (Li et al., Reference Li, Moore-Kucera, Miles, Leonas, Lee, Corbin and Inglis2014).
Given the essential role of microbes in mulch degradation (Dharmalingam et al., Reference Dharmalingam, Hayes, Wadsworth, Dunlap, DeBruyn, Lee and Wszelaki2015), we had hypothesized that microbial abundance extracted from soil around the mulch would be useful predictors of mulch degradation. We expected that the wood particles in the PLA mulch would cultivate greater abundance of saprophytic fungi and help to explain variation in degradation among plots. Saprophytic fungi abundance was indeed greater in and around PLA mulch (data not shown; Samuelson, Reference Samuelson2019), but it did not help explain degradation. Likewise, Sintim et al. (Reference Sintim, Bary, Hayes, Wadsworth, Anunciado, English, Bandopadhyay, Schaeffer, DeBruyn, Miles, Reganold and Flury2020) did not observe any correlation between mulch remaining in soil and bacterial or fungal abundance over time. They did observe correlations between seasonal degradation rates and soil temperature and microbial activity but point measurements of microbial abundance did not explain degradation. Overall, the apparent lack of microbial influence on mulch degradation adds uncertainty to the possibility of using biostimulants or biological inoculants to increase biomulch residue decomposition.
Location explained degradation of BLK
Variability in soil properties within locations was minor compared to variability between locations tested. The magnitude of climatic and soil property differences between locations was sufficient to drive different degradation rates but leveraging those forces to accelerate degradation within a location was likely constrained by the resilience of soil. The rate of BLK mulch degradation was far greater at LNK than at SBF (Fig. 3), which could be explained by higher precipitation (Fig. 1), soil water content and SOM, warmer soil temperature, and less alkaline soil at LNK (Tables 3 and 4). Compared to soil nutrients, SOM and pH can be relatively slow to change in response to short-term management interventions and are instead more influenced by soil parent material and long-term land use history (Wortman et al., Reference Wortman, Galusha, Mason and Francis2012). Soil temperature and moisture can be manipulated by management, including through the use of mulch films and high tunnels (Wortman et al., Reference Wortman, Kadoma and Crandall2016), but those site-specific differences are often minimal compared to differences among diverse geographical locations (Li et al., Reference Li, Moore-Kucera, Miles, Leonas, Lee, Corbin and Inglis2014).
Li et al. (Reference Li, Moore-Kucera, Miles, Leonas, Lee, Corbin and Inglis2014) observed drastically different degradation rates of starch-based bioplastic films among diverse geographic locations, ranging from 98% degradation in Texas to 11% degradation in Washington after 24 months. The faster degradation rates in Texas were accompanied by warmer soil temperatures, alkaline soil and greater abundance of soil fungi. Degradation of the BLK mulch in this study, one of the starch-based films tested by Li et al. (Reference Li, Moore-Kucera, Miles, Leonas, Lee, Corbin and Inglis2014), was greatest at LNK. While the environment at LNK was characterized by warmer soil temperatures than at SBF (like Texas in Li et al., Reference Li, Moore-Kucera, Miles, Leonas, Lee, Corbin and Inglis2014), saprophytic fungi abundance was lower and soil pH was less alkaline at LNK (Table 3). Sintim et al. (Reference Sintim, Bary, Hayes, Wadsworth, Anunciado, English, Bandopadhyay, Schaeffer, DeBruyn, Miles, Reganold and Flury2020) observed a similar soil temperature trend where degradation of mulch films after 36 months in soil reached 83% in Tennessee (warmer soil temperatures) and 63% in Washington (cooler soil temperatures).
Overall, the rate of BLK degradation at LNK in this study was notably faster than that reported for the same starch-based bioplastic film in northwestern Washington and eastern Tennessee. Sintim et al. (Reference Sintim, Bary, Hayes, Wadsworth, Anunciado, English, Bandopadhyay, Schaeffer, DeBruyn, Miles, Reganold and Flury2020) found that the BLK mulch degraded by a maximum of 20% after 12 months in soil across locations, whereas at LNK the BLK mulch was almost entirely degraded in that same timeframe. Ghimire et al. (Reference Ghimire, Flury, Scheenstra and Miles2020) found that starch-based bioplastics (including BLK) degraded by 31–59% after 24 months in soil at Washington, compared to 100 and 52% degradation at LNK and SBF, respectively. Soil conditions in Washington reported by Ghimire et al. (Reference Ghimire, Flury, Scheenstra and Miles2020) are more similar to SBF than LNK, and are characterized by cooler soil temperatures, 2.8% organic matter content and a silt loam texture. In contrast, the soil texture at LNK was silty clay loam with 4.6% SOM by fall 2019, and warmer soil temperatures. Incomplete degradation of BLK at SBF and other locations (Ghimire et al., Reference Ghimire, Flury, Scheenstra and Miles2020; Sintim et al., Reference Sintim, Bary, Hayes, Wadsworth, Anunciado, English, Bandopadhyay, Schaeffer, DeBruyn, Miles, Reganold and Flury2020) will contribute to an undesirable farm aesthetic (i.e., plastics blowing in the field and fence rows) and remains a barrier to on-farm adoption of BDM (Dentzman and Goldberger, Reference Dentzman and Goldberger2020). The substantial variation in degradation rates across farm locations, which is still largely unexplained and somewhat unpredictable, is another ongoing barrier to farmer adoption of BLK and similar mulch products (Goldberger et al., Reference Goldberger, Jones, Miles, Wallace and Inglis2015).
Degradation of PLA was incomplete at both locations
The rapid degradation of PLA mulch at SBF in the first 6 months was somewhat unexpected. This initial degradation at SBF was likely driven by increased weathering of the PLA mulch during the 2017 growing season (Sintim et al., Reference Sintim, Bary, Hayes, Wadsworth, Anunciado, English, Bandopadhyay, Schaeffer, DeBruyn, Miles, Reganold and Flury2020). The PLA mulch at SBF was noticeably more weathered at the time of soil incorporation, which was likely the result of greater solar irradiance (Table 1) and more frequent in-season irrigation at SBF to avoid water-limiting conditions (Touchaleaume et al., Reference Touchaleaume, Angellier-Coussy, César, Raffard, Gontard and Gastaldi2017; Anunciado et al., Reference Anunciado, Hayes, Wadsworth, English, Schaeffer, Sintim and Flury2021). Overall, degradation of the PLA mulch at both locations proceeded in a linear fashion for the first 12 months after soil burial, and began to slow significantly after reaching 34–38% mulch mass remaining. Not coincidentally, the PLA mulch was composed of 38% PLA and 62% wood particles by weight. In tandem with visual observations of recovered mulch, this suggests that the degradation of wood particles was responsible for the majority of PLA mulch mass loss in the first 12 months. Remaining degradation of PLA over the final 10 months of the experiment—reaching only 29–33% mass remaining by 22 months—was likely driven by depolymerization and degradation of the polylactic acid component of the PLA mulch. Dharmalingam et al. (Reference Dharmalingam, Hayes, Wadsworth, Dunlap, DeBruyn, Lee and Wszelaki2015) found that amorphous regions of polylactic acid were accessible to soil microbes as a carbon source 4–12 weeks after soil burial, but degradation slowed significantly thereafter.
If we assume that the wood particles were completely degraded by 22 months after soil incorporation as per our visual observation, then we can approximate that the original mass of polylactic acid included in the PLA mulch had degraded by 13 to 24 ± 4% (i.e., 38% polylactic acid by weight minus percent mulch mass remaining after 22 months at each location, divided by the original relative weight of 38%, equals the percent degradation of remaining polylactic acid). This modest level of polylactic acid degradation in soil is consistent with previous research. Polylactic acid has a high glass-transition temperature of 49.2°C (the temperature at which the polymer becomes soft; Hayes et al., Reference Hayes, Wadsworth, Sintim, Flury, English, Schaeffer and Saxton2017), which limits biodegradation rates under ambient field conditions. Most studies have reported less than 10% degradation of polylactic acid mulches under lab and field soil conditions (Cowan et al., Reference Cowan, Inglis and Miles2013; Li et al., Reference Li, Moore-Kucera, Miles, Leonas, Lee, Corbin and Inglis2014; Dharmalingam et al., Reference Dharmalingam, Hayes, Wadsworth, Dunlap, DeBruyn, Lee and Wszelaki2015; Satti et al., Reference Satti, Shah, Marsh and Auras2018). The additional degradation observed here, though modest, could point to the potential benefits of blending polylactic acid with other biobased polymers or composite particles (like the fine wood particles added in this study). For example, combining polylactic acid (72% by weight) with polyhydroxy alkanoate (28% by weight) has been shown to increase biodegradability compared to polylactic acid alone (Dharmalingam et al., Reference Dharmalingam, Hayes, Wadsworth, Dunlap, DeBruyn, Lee and Wszelaki2015). Similarly, adding soybean meal or alfalfa meal particles to a polylactic acid mulch can accelerate depolymerization and degradation of polylactic acid in soil (Thompson et al., Reference Thompson, Samuelson, Kadoma, Soto-Cantu, Drijber and Wortman2019).
Conclusions
This study investigated degradation of two potentially BDM membranes [a nonwoven polylactic acid fabric mulch with embedded wood particles (PLA), and a starch-polyester mulch film (BLK)] under a typical certified organic field vegetable production system across two distinct agroecological zones [Lincoln (LNK) and Scottsbluff (SBF), Nebraska] and different management practices including the use of compost, compost extract, cover crops and a combination of all three of these. Mulch mass loss over time was not different among management treatments despite large differences in carbon and biological inputs, and significant changes in soil thermal, chemical and microbial properties. Degradation was instead influenced by location and mulch type. Factors influencing mass loss seemed to differ between mulches, but with only two locations it was not possible to pinpoint the specific characteristics of a location driving degradation. BLK mulch was nearly undetectable after 12 months of burial at LNK, but about half of BLK mulch mass remained at SBF after 22 months. The LNK degradation environment included finer textured soil, lower soil pH, higher SOM, greater precipitation and soil water content, and warmer air and soil temperature compared to SBF. PLA mulch loss was initially more rapid at SBF, but degradation thereafter proceeded similarly across locations. By 22 months after burial, 29 ± 4% PLA mulch mass remained at SBF and 33 ± 4% remained at LNK. The majority of PLA mulch degradation can be attributed to decomposition of the embedded wood particles, but results suggest some limited degradation (13 to 24 ± 4%) of the polylactic acid component.
Future research should seek to understand the considerable variation in mulch degradation that exists across different farms and regions. To accomplish this, we recommend simple mesh bag mulch burial experiments on many farms (>50) that represent a diverse range of soil types and climatic conditions. Observations of degradation for commercially available biomulch products around the USA and world could be used to develop locally relevant predictive models of degradation. Increasing farmer confidence in the degradation potential of a biomulch product on their farm will be critical for improving on-farm adoption of this sustainable alternative to polyethylene plastic mulch film.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1742170521000405
Acknowledgements
This research was supported by the Nebraska Agricultural Experiment Station with funding from the Hatch Act (accession 1014303) through the USDA National Institute of Food and Agriculture (NIFA), and the USDA NIFA Organic Transitions Program (award 2016-51106-25711).
Conflict of interest
I. Kadoma is employed at 3M Company. There are no other competing interests to declare.











