1. Introduction
It has been over 65 years since Skoog and Miller published their seminal work on the chemical regulation of organ growth in tissue culture (Skoog & Miller, Reference Skoog and Miller1957). After so many years, their paper remains highly cited and relevant to multiple aspects of plant biology. Although this paper is perhaps the best known and most cited from Skoog’s laboratory, Folke Skoog had a long history of studying plant growth substances. Originally from Sweden, Skoog earned his undergraduate and PhD degrees at Caltech where he worked on auxin physiology. Later joining the University of Wisconsin-Madison as a faculty member in 1947, he published over 170 papers during his career that largely focused on phytohormones (Armstrong, Reference Armstrong2002). Several years after starting in Wisconsin, his lab recruited a postdoctoral associate, Carlos Miller, to continue working on hormone physiology. Miller had an ambitious task, to identify the substance(s) responsible for cell divisions in plant tissue. These years leading up to the 1957 paper showed enormous growth and strong enthusiasm for hormone biology with plant physiologists searching for new factors and characterising recently identified ones (Thimann, Reference Thimann1974). In vitro techniques had been previously established and the role of auxin was being studied intensely. Miller succeeded to identify compounds that promoted cell division, and together with previous work on auxin and in vitro techniques, these formed the basis for the 1957 paper with Skoog. Here, I discuss the background, the paper and the implications that stemmed from the seminal work of Skoog and Miller.
2. Establishing de novo organ formation
The ability of plants to form organs and modify their development in response to the environment has been an intense research focus for over a century. However, fundamental to our understanding of such developmental plasticity has been the discovery of the growth hormone auxin (Went, Reference Went1928). This discovery provided an explanation for how plants grow and allowed the exogenous application of this hormone to a multitude of species and tissues. Often, however, phenotypes from these exogenous assays were difficult to reconcile. For instance, auxin could promote root primordia formation yet repress root elongation whereas auxin could also inhibit bud activation yet promote tissue elongation (Skoog & Miller, Reference Skoog and Miller1957). Today these observations have been reconciled, but in the 1930s and 1940s, these results seemed contradictory. To address these challenges, several groups focused on using simplified experimental systems including stem segments of Nicotiana. One such system, White’s tobacco callus, was a cross between Nicotiana glauca and Nicotiana langsdorffii that could spontaneously form masses of undifferentiated cells and galls (White, Reference White1939). Taking stem cuttings from these hybrids and culturing them on nutrient-rich media without hormones allowed an unlimited proliferation of growth with little differentiation (White, Reference White1939). Skoog and colleagues used White’s tobacco callus for early experiments but, due to difficulties obtaining cultures, developed instead a system that used stem cuttings from Nicotiana tabacum that underwent cell proliferation at sites of wounding on nutrient-rich media (Skoog & Tsui, Reference Skoog and Tsui1948). Excising the inner pith tissues from such stem cuttings grew little but would undergo limited proliferation and extensive expansion in the presence of exogenously applied auxin (Jablonski & Skoog, Reference Jablonski and Skoog1954). Thus, by using a Nicotiana stem or pith-derived callus, Skoog and colleagues established a system that was relatively simplified and whose cell growth could be modified by the amounts of exogenous auxin treatment. However, auxin alone was not sufficient for the pith-derived callus to proliferate and instead a second factor was needed.
One hint about this cellular proliferation factor was that Nicotiana stem segments needed their vascular tissues to proliferate callus (Jablonski & Skoog, Reference Jablonski and Skoog1954). Several heterogeneous substances, including coconut milk, were found to induce cell proliferation but the first homogeneous chemical to show this effect was adenine (Skoog & Tsui, Reference Skoog and Tsui1948). It behaved like a weak cytokinin (Amasino, Reference Amasino2005) but required high concentrations to promote cell proliferation (Skoog & Tsui, Reference Skoog and Tsui1948). However, the results were clear from adenine and auxin treatments on Nicotiana stem cuttings: high adenine concentrations induced shoot formation whereas high auxin concentrations induced root formation (Skoog & Tsui, Reference Skoog and Tsui1948). Treatments with both auxin and adenine caused cell proliferation but with neither root nor shoot formation (Skoog & Tsui, Reference Skoog and Tsui1948). These results demonstrated that varying hormone concentrations could modify regenerative fates and determine organ identity. However, although these findings were published 9 years before the seminal 1957 paper, the 1948 paper did not reach the impact of Skoog and Miller’s later work, perhaps due in part due to the challenges of working with adenine and the narrow concentration range at which this substance was active (Amasino, Reference Amasino2005).
In the 1940s and 1950s, groups were actively looking for robust chemical(s) that promoted cell proliferation. Through a combination of luck, hard work and carefully planned experiments, 6-furfurylaminopurine was found to promote cell proliferation at extremely low concentrations (Miller et al., Reference Miller, Skoog, Okumura, Von Saltza and Strong1955a, Reference Miller, Skoog, Von Saltza and Strong1955b). This compound was renamed kinetin and was instrumental to revisiting the work done 9 years earlier with auxin and adenine. Using the in vitro Nicotiana system developed earlier and this recently discovered synthetic cytokinin (kinetin), Skoog and Miller employed these to make several fundamental discoveries. Firstly, they found that the presence of both auxin and cytokinin was required for cell proliferation; the presence of only one compound resulted in stem tissues growing poorly (Das et al., Reference Das, Patau and Skoog1956; Skoog & Miller, Reference Skoog and Miller1957). Secondly, the effects of cytokinin and auxin were quantitative, that is, varying their concentrations would lead to vastly different morphological phenotypes. High levels of auxin and low levels of cytokinin promoted cell expansion and the formation of roots from callus tissues (Figure 1; Skoog & Miller, Reference Skoog and Miller1957). High levels of cytokinin and low levels of auxin promoted bud and shoot formation from callus. Similarly high levels of cytokinin and auxin differentiated neither shoot nor root, but instead favoured callus growth (Figure 1; Skoog & Miller, Reference Skoog and Miller1957). This effect is exemplified in Plate 4 of their 1957 paper (Figure 1a) when varying auxin and cytokinin concentrations promoted callus or shoot growth (Skoog & Miller, Reference Skoog and Miller1957). In addition, there was a clear repressive effect of cytokinin upon root initiation and a clear repressive effect of auxin upon shoot formation (Skoog & Miller, Reference Skoog and Miller1957). Although these experiments were performed in Nicotiana stem segments and callus cultures, Skoog and Miller wisely speculated that these quantitative interactions between auxin and cytokinin might extend more broadly and cover all types of cellular growth and organ formation in plants (Skoog & Miller, Reference Skoog and Miller1957).

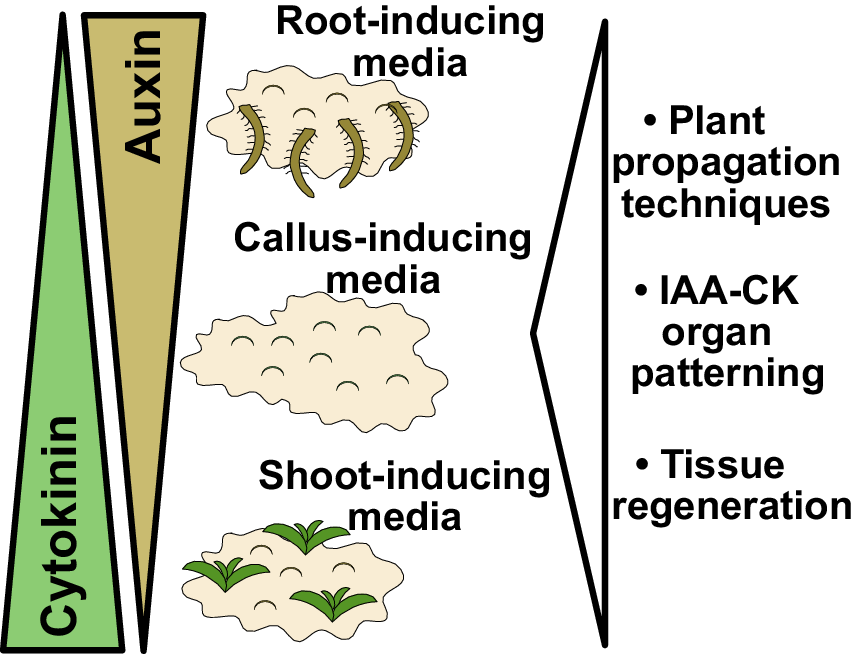
Figure 1. Findings and outcomes from Skoog and Miller. (a) A central finding from Skoog and Miller (Reference Skoog and Miller1957) demonstrated that varying levels of auxin (indole-3-acetic acid; IAA) and cytokinin (kinetin) result in shoot formation or callus formation from Nicotiana stem segments. In this experiment, kinetin levels were too high to allow root formation. Image taken from Plate 4 of Skoog and Miller (Reference Skoog and Miller1957). (b) Auxin and cytokinin are both required for organogenesis but also antagonise each other to promote either root or shoot formation. High auxin and low cytokinin, the basis for root-inducing media (RIM), promote root formation. High cytokinin and low auxin, the basis for shoot-inducing media (SIM), promote shoot formation. Equal levels of auxin and cytokinin, the basis for callus-inducing media (CIM), promote callus formation. These in vitro organ formation experiments have proven critical for understanding tissue regeneration and hormone-mediated organ patterning, whereas the use of CIM, SIM and RIM has been critical for both plant propagation and the regeneration of transgenic plants. Figure 1(a) © Cambridge University Press, 1957. Please note, the Open Access licence covering this article does not apply to this image.
3. The implications of Skoog and Miller
Our mechanistic understanding of plant development and developmental plasticity has made quantum leaps since the work of Skoog and Miller in 1957. Here, I focus on three aspects that have led to their paper becoming a classic in plant biology and remaining a highly cited paper in the field.
The idea that qualitative interactions of growth hormones, for instance, the presence of a hormone regardless of amount, controlled growth was put into doubt by the findings of Skoog and Miller. Instead, their results indicated that small differences in hormone concentrations could modify the fate of organ development and growth. Two notable examples are mentioned in the 1957 paper that are still relevant and widely studied today. Firstly, they observed that auxin enhanced root formation while cytokinin repressed it (Skoog & Miller, Reference Skoog and Miller1957). Although unknown to Skoog and Miller, today we know that in dicots lateral roots emerge from the pericycle cells adjacent to the xylem. There, pericycle founder cell specification requires the activation of a local auxin response to promote lateral root formation (Dubrovsky et al., Reference Dubrovsky, Sauer, Napsucialy-Mendivil, Ivanchenko, Friml, Shishkova, Celenza and Benkova2008). Treatment with cytokinin blocks lateral root initiation by perturbing the expression of the PIN auxin transport genes necessary for the formation of an auxin gradient in the lateral root founder cells (Laplaze et al., Reference Laplaze, Benkova, Casimiro, Maes, Vanneste, Swarup, Weijers, Calvo, Parizot, Herrera-Rodriguez, Offringa, Graham, Doumas, Friml, Bogusz, Beeckman and Bennett2007). Auxin promotes PIN expression and stability (Adamowski & Friml, Reference Adamowski and Friml2015), thus the balance between auxin and cytokinin influences PIN expression and ultimately either promotes or represses auxin response in the pericycle to determine lateral root formation. The second notable example from Skoog and Miller is that they observed cytokinin promoted bud formation whereas auxin repressed it (Skoog & Miller, Reference Skoog and Miller1957). Increasing cytokinin levels activates the expression of WUSCHEL and SHOOT MERISTEMLESS, positive regulators of shoot meristem cell fate (Gordon et al., Reference Gordon, Chickarmane, Ohno and Meyerowitz2009; Rupp et al., Reference Rupp, Frank, Werner, Strnad and Schmülling1999). Overexpressing both WUSCHEL and STM is sufficient to form ectopic shoots (Gallois et al., Reference Gallois, Woodward, Reddy and Sablowski2002; Lenhard et al., Reference Lenhard, Jurgens and Laux2002) suggesting that high cytokinin induces shoot formation via this pathway. How high auxin levels repress shoot formation is less clear. Auxin response factors suppress SHOOT MERISTEMLESS expression (Chung et al., Reference Chung, Zhu, Wu, Simonini, Kuhn, Armenta-Medina, Jin, Ostergaard, Gillmor and Wagner2019), whereas auxin also activates the cytokinin signalling inhibitor AHP6 (Besnard et al., Reference Besnard, Refahi, Morin, Marteaux, Brunoud, Chambrier, Rozier, Mirabet, Legrand, Laine, Thévenon, Farcot, Cellier, Das, Bishopp, Dumas, Parcy, Helariutta, Boudaoud, Godin and Vernoux2014). Thus, it is possible that high auxin levels inhibit shoot formation by decreasing STM expression and repressing cytokinin signalling.
A second outcome from Skoog and Miller’s paper was to establish tissue regeneration that could be quantitatively manipulated. Although previous papers had developed in vitro methods to culture plant tissues, the combination of auxin and cytokinin facilitated the widespread study of tissue regeneration. Such an ability for somatic tissues to form new tissues and whole plants after wounding raised important questions for how such de novo organ formation might occur. One idea is that tissues like Nicotiana stems contain a subset of stem cells that can divide and differentiate to give rise to any cell-type, tissue, or whole organism (Birnbaum & Sanchez Alvarado, Reference Birnbaum and Sanchez Alvarado2008). In contrast, another idea is that differentiated cells such as epidermis, mesophyll or root hairs can change their fate to form new cell types that can give rise to organs (Birnbaum & Sanchez Alvarado, Reference Birnbaum and Sanchez Alvarado2008; Morinaka et al., Reference Morinaka, Sakamoto, Iwase and Sugimoto2023). Such fate changes could involve the change of one differentiated cell to another, a process known as trans-differentiation, or could involve the de-differentiation of cells followed by a re-differentiation process (Sugimoto et al., Reference Sugimoto, Gordon and Meyerowitz2011). Likely both concepts are important, for instance, treatments with exogenous auxin specifically cause xylem pole pericycle cells to divide and give rise to lateral root-like meristems that transition to shoots after cytokinin treatment (Atta et al., Reference Atta, Laurens, Boucheron-Dubuisson, Guivarc’h, Carnero, Giraudat-Pautot, Rech and Chriqui2009; Che et al., Reference Che, Lall and Howell2007; Gordon et al., Reference Gordon, Heisler, Reddy, Ohno, Das and Meyerowitz2007). Transcriptional analyses of these callus masses, whether derived from roots or aerial tissues, revealed that they had a similar identity to lateral root tips (Sugimoto et al., Reference Sugimoto, Jiao and Meyerowitz2010). Thus, not all plant cells give rise to callus but instead these data indicated that only a subset of the pericycle cells adjacent to the xylem do and such masses are not undifferentiated but instead transcriptionally resemble root tips regardless of their tissue origin (Atta et al., Reference Atta, Laurens, Boucheron-Dubuisson, Guivarc’h, Carnero, Giraudat-Pautot, Rech and Chriqui2009; Sugimoto et al., Reference Sugimoto, Jiao and Meyerowitz2010). It seems appropriate then to consider xylem pole pericycle cells as having totipotency that, upon auxin and cytokinin treatment, transdifferentiate or de-differentiate/re-differentiate to other cell types that can give rise to whole plant regeneration.
Lastly, a third outcome from Skoog and Miller’s paper was the establishment of in vitro conditions that allowed for efficient plant propagation. By taking cut tissues and placing them on varying concentrations of auxin and cytokinin, Skoog and Miller had invented callus inducing-media (CIM), shoot-inducing media (SIM) and root-inducing media (RIM) (Figure 1b). Such media were crucial for allowing organ regeneration from cut tissues, processes that have been developed and studied in hundreds of plant species. These media allowed the asexual propagation of plants, such as orchids, using micropropagation techniques. With the advent of transgenesis, T-DNAs could be transferred by Agrobacterium or biolistics directly into CIM-derived callus and transgenic plants regenerated using SIM and RIM techniques. Skoog and Miller in their 1957 paper observed the formation of roots and shoots directly from stem segments (Skoog & Miller, Reference Skoog and Miller1957), a process we refer to today as direct regeneration. However, work 30 years after Skoog and Miller revealed that such a process was less efficient than indirect regeneration. With indirect regeneration, plant segments were incubated on CIM for several days to gain regeneration competency, after which they were transferred to SIM to induce shoot formation (Feldmann & David Marks, Reference Feldmann and David Marks1986; Valvekens et al., Reference Valvekens, Van Montagu and Van Lijsebettens1988). Regeneration rates were substantially higher with indirect techniques and the time it took for shoots to form was dramatically reduced. Although Miller and Skoog established the fundamental concepts behind modern tissue culture, most regeneration protocols today involve modifications of these techniques to incorporate treatments and transfers between different inducing media.
4. Concluding remarks
The work from Skoog and Miller (Reference Skoog and Miller1957) remains a classic and parts, including Plate 4 (Figure 1a), represent one of the most visually striking images from that generation of papers. Their paper was at the right time and place: in vitro techniques were already established, the effects of auxin upon growth were well known and a potent synthetic cytokinin had just been discovered two years prior by Miller, Skoog and colleagues. With a combination of well-planned experiments and visually striking outcomes, the work of Skoog and Miller will remain a classic and likely continue to be highly cited. Today, our understanding of phytohormones has expanded massively and we know that auxin and cytokinin work together in both synergistic and antagonistic functions at the cellular and tissue level. Furthermore, not only is hormone concentration relevant, but so too is the location of hormone response. This complexity confounded early developmental biologists, and today continues to present both a highly interesting research question but also a challenge. Perhaps we should take advice from Skoog and Miller and continue advocating for techniques, such as in vitro cultures, that simplify complex biological systems and help us dissect the complexities of plant growth and developmental plasticity.
Competing interest
The author declares none.
Author contribution
C.W.M. conceived and wrote the article.
Funding statement
Work in the Melnyk lab is supported by a Wallenberg Academy Fellowship (2016-0274) and a European Research Council starting grant (GRASP-805094).




Comments
Dear Editor,
Please see attached my classics review. I apologise this is coming so late!
Best regards,
Charles