Hypovitaminosis D has become a global health problem and high prevalence of inadequate serum vitamin D has been reported even in tropical countries such as Brazil( Reference Wacker and Holick 1 , Reference Maeda, Borba and Camargo 2 ). During childhood, an appropriate serum concentration of vitamin D (25-hydrovyvitamin D; 25(OH)D) is essential for bone health, since its deficiency can lead to rickets, growth retardation and muscle weakness( Reference Holick 3 ).
Vitamin D is a steroid hormone synthesized in the skin from exposure to sunlight, which is its main source, but it can also be obtained from food sources such as cod-liver oil and fatty fish (salmon, sardine, tuna). The risk factors for vitamin D deficiency are related to its decreased cutaneous synthesis caused by low sun exposure, high latitudes, colder seasons, older age and skin pigmentation( Reference Wacker and Holick 1 , Reference Holick 4 ).
Recent behavioural, social and cultural changes favour sedentary behaviour, limiting physical activity – especially outdoor activities – and, as result, sun exposure, which are also important factors related to vitamin D insufficiency/deficiency( Reference Absoud, Cummins and Lim 5 ). Furthermore, overweight and obese children generally spend less time outdoors because of a sedentary lifestyle( Reference Misra, Pacaud and Petryk 6 ).
Vitamin D functions associated with Ca homeostasis and bone health are traditionally recognized, and only after its receptor was found in other tissues such as skin, placenta, brain, breast and pancreas did research begin on the additional effects of vitamin D not related to bone tissue( Reference Holick 4 ). Despite the physiological mechanisms not yet being fully understood, studies have shown the association of hypovitaminosis D with various chronic diseases, including insulin resistance, dyslipidaemia and hypertension, even in children( Reference Guasch, Bulló and Rabassa 7 – Reference Petersen, Damsgaard and Dalskov 9 ). However, we found no reports of studies of this nature in developing countries.
Vitamin D deficiency is associated with several cardiometabolic risk factors that contribute to the development of type 2 diabetes mellitus and CVD, the main causes of morbidity and mortality in the developed world( Reference Balagopal, Ferranti and Cook 10 , Reference Ganji, Zhang and Shaikh 11 ). This association is influenced by body adiposity, since vitamin D is lipid-soluble and its serum concentration tends to be reduced in individuals with excess body fat( Reference Alemzadeh, Kichler and Babar 12 ).
In addition, the inverse association between vitamin D and metabolic syndrome may be influenced by glucose homeostasis( Reference Khan, Kunutsor and Franco 13 – Reference Mousa, Naderpoor and de Courten 15 ). Studies with children and adolescents found an association between serum vitamin D and insulin sensitivity mediated by body fat, as well as that vitamin D supplementation improved insulin resistance and metabolic syndrome score in obese individuals( Reference Alemzadeh, Kichler and Babar 12 , Reference Kelishadi, Salek and Salek 16 ). Thus, vitamin D deficiency in the paediatric age group associated with obesity may lead to increased risk of changes in glucose metabolism. However, other studies have found controversial results( Reference Petersen, Dalskov and Sørensen 17 , Reference Bezrati, Ben Fradj and Ouerghi 18 ).
Excessive adiposity from infancy onwards increases the risk for metabolic disorders related to glucose homeostasis, control of blood pressure and lipid profile( Reference Nadeau, Maahs and Daniels 19 ). Obesity and body fat are also factors associated with lower serum concentrations of vitamin D( Reference Alemzadeh, Kichler and Babar 12 ). Therefore, adjusting for a potential confounding effect of fat mass is important when assessing associations between concentrations of vitamin D and cardiometabolic risk factors. Most studies with children have used BMI, which does not cover information on body composition, and hence more accurate measures of body fat become important in epidemiological studies.
Identifying modifiable cardiovascular risk factors in childhood can help in long-term disease prevention. It is important to consider childhood as a strategic period for assessing serum levels of 25(OH)D and promotion of healthy habits. In Brazil, few studies have assessed the prevalence of vitamin D deficiency and insufficiency and its relationship with cardiometabolic disorders, particularly in children. Thus, the present study aimed to assess the prevalence of vitamin D insufficiency and deficiency and its association with cardiometabolic risk factors, controlled by adiposity, in a representative sample of prepubescent Brazilian children.
Methods
Study design and participants
Participants in the present study came from the Survey of Health Assessment of Schoolchildren (PASE), a cross-sectional population-based study with 378 children aged 8 and 9 years from all public and private schools (n 24) in the urban area of the city of Viçosa, Minas Gerais, Brazil. Viçosa is located in the Zona da Mata Region of Minas Gerais, 227 km from the state capital Belo Horizonte. It features a per capita Gross Domestic Product of R$ 9597·00 ($US 3024·00) and a Human Development Index of 0·775, which is considered high, higher than the state (0·731) and national (0·755) indices( 20 ).
The PASE is a cross-sectional population-based study with children enrolled in urban schools to evaluate the cardiovascular health of these children in the city of Viçosa. In 2015, the city had twenty-four urban schools (seventeen public and seven private) that served children aged 8 and 9 years, totalling 1464 children enrolled. The sample size was calculated using the software Epi Open version 3.03 from the total number of children aged 8 and 9 years (n 1464) enrolled in all urban schools in 2015. Considering the analysis of multiple outcomes and the lack of studies on the prevalence of vitamin D insufficiency in children in south-eastern Brazil, the sample was calculated on the basis of 50 % prevalence, 5 % error tolerated, 95 % CI, 5 % significance level and 20 % estimated loss, resulting in the sample size of 366 children.
Currently, there is no consensus on cut-off points for the classification of 25(OH)D deficiency in clinical practice. However, vitamin D deficiency has been defined as below 50 nmol/l (20 ng/ml) by many specialists, while sufficiency values range from 50 to 80 nmol/l (20 to 32 ng/ml)( Reference Maeda, Borba and Camargo 2 , Reference Holick 4 , Reference Heaney 21 ). In the current study, the concentration of vitamin D is expressed as nmol/l (2·5 nmol/l=1 ng/ml) and deficiency, insufficiency and sufficiency were defined when values were <50, ≥50–<75 and ≥75 nmol/l, respectively( Reference Holick 4 , Reference Heaney 21 – Reference Holick, Binkley and Bischoff-Ferrari 23 ).
The schoolchildren were selected by stratified random sampling. The sample from each school met the proportionality ratio of students enrolled by age and gender. The selection of children was done by random simple draw until the necessary number for each school was completed.
The child did not participate in the study if taking medication that interfered with the metabolism of vitamin D (corticosteroids, anticonvulsants and antifungals), glucose and/or lipids, as well as vitamin or mineral supplements. Data collection took place between May and November of 2015.
The study was conducted according to the guidelines established in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee on Human Research of the Federal University of Viçosa (opinion number 663171/2014) and also presented to the Municipal Department of Education, the Regional Superintendent of Education and principals of schools. Informed consent was signed by the parents.
Anthropometry and body composition
Weight and height were measured using an electronic digital scale (Tanita®, model BC 553, Arlington Heights, IL, USA) and a portable stadiometer (Alturexata®, Belo Horizonte, MG, Brazil), respectively.
The nutritional status of children was assessed based on BMI, according to the BMI-for-age Z-scores proposed by the WHO( Reference Onis and Onyango 24 ). Waist circumference was measured at the midpoint between the iliac crest and the last rib using a flexible and inelastic tape measure. Abdominal adiposity values above the 90th percentile according to age and gender were considered excessive, as proposed for Brazilian children( Reference Barbosa-Filho, Lopes and Fagundes 25 ). Waist-to-height ratio was calculated by dividing waist (in centimetres) by height (in centimetres).
Total and regional body composition, android fat and gynoid fat were assessed by dual-energy X-ray absorptiometric scanning, in the morning, after an overnight fast, with children in a supine position. The total and regional body fat are reported as percentages and expressed as fat mass index (FMI), an index of fat mass-for-height (kg/m2). Excess body fat was classified according to the cut-off points proposed by Lohman( Reference Lohman 26 ).
Biochemical and clinical assessment
Blood samples were collected by venepuncture into serum gel tubes after 12 h of fasting for further analysis. Glucose, total cholesterol, HDL cholesterol, LDL cholesterol and TAG were determined by the enzymatic colorimetric method using the commercial kit Bioclin® (Belo Horizonte, MG, Brazil), following the manufacturer’s instructions, and measured in an automatic biochemistry analyser (Mindray BS-200®, Nanshan, China).
Insulin, calcidiol (25(OH)D) and intact parathyroid hormone were determined by chemiluminescence immunoassay at the Clinical Analysis Laboratory of the Health Division, Federal University of Viçosa.
25(OH)D was determined by the Architect® 25-OH Vitamin D assay, which was developed to have correlation coefficient ≥0·80 for serum samples when compared with the LIAISON® DiaSorin 25-OH Vitamin D Total assay. The Architect 25-OH Vitamin D assay has been developed to have an imprecision of ≤10 % (within-laboratory total CV).
Values were considered increased when fasting glucose was ≥100 mg/dl and fasting insulin was ≥15 mU/ml( 27 ). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated. There is no default setting for insulin resistance, particularly in children, but it has been shown that a HOMA-IR cut-off of about 3 could be used for this age group( Reference Tresaco, Bueno and Pineda 28 ). Therefore, we considered HOMA-IR ≥3·16 according to the I Atherosclerosis Prevention Guideline on Childhood and Adolescence of the Brazilian Society of Cardiology( 27 ).
The lipid profile was classified using specific cut-off points for children. Values were considered altered when total cholesterol was ≥3·9 mmol/l (150 mg/dl), LDL cholesterol was ≥2·6 mmol/l (100 mg/dl), TAG was ≥1·1 mmol/l (100 mg/dl) and HDL cholesterol was <1·2 mmol/l (45 mg/dl)( 27 ).
Blood pressure was measured with the child in sitting position after resting for at least 5 min at three different times. The mean values were used to classify children. Systolic or diastolic pressure was considered altered when greater than the 90th percentile, proposed by the VI Brazilian guidelines of hypertension by the Brazilian Society of Cardiology, according to age, gender and height percentile( 29 ). Measurements were carried out using an automatic inflation blood pressure monitor (OMRON® HEM 907, Vernon Hills, IL, USA).
Dietary assessment
Dietary intake was assessed by three 24 h recalls, on non-consecutive days, including one weekend day. The interval between the recalls was 15 to 20 d. The children responded to the dietary survey accompanied by their parents or guardians who were also interviewed, preferably the one directly involved with the child’s diet.
The quantities of foods consumed in the portion sizes were converted to grams or millilitres for further chemical composition analysis. The analysis of dietary data was performed using the Diet Pro® 5i software, version 5.8.
Vitamin D intake was compared with the Estimated Average Requirement, proposed by the US Institute of Medicine, of 10 μg/d (400 IU/d)( 30 ). Adjustment for the effect of energy consumption on macro- and micronutrients was carried out using the residual method proposed by Willett and Stampfer( Reference Willett and Stampfer 31 ).
Questionnaire
Information on demographic and socio-economic characteristics was collected by personal interview with the child and parents. Per capita income was obtained by dividing the total income of the family by the number of dependants. Maternal education was measured by the number of years of schooling completed by the mother.
The parents/guardians declared the child’s ethnicity and the skin colour was categorized as white, brown or black. The season of the year was evaluated during the time of blood collection.
Based on the child’s life habits, sedentary behaviour was defined as activities that do not increase energy expenditure substantially above rest, such as watching television or engaging in other forms of screen-based entertainment( Reference Santos, Mascarenhas and Satler 32 ). Sedentary behaviour was classified as screen time ≥2 h/d( 33 ). The time of sun exposure was defined according to the outdoor activities that the children performed.
Statistical analyses
Statistical analyses were performed with the statistical software packages IBM SPSS Ststistics version 20.0 and Stata version 13.1. Normality was assessed by the Kolmogorov–Smirnov test. Mean and sd are presented for variables with normal distribution, and median and interquartile range for variables without normal distribution. Pearson’s χ 2 test, Fisher’s exact test and the χ 2 linear trend test were used to analyse the relationship between categorical variables. Differences in quantitative variables between two groups were assessed by Student’s t test or the Mann–Whitney U test.
Poisson regression with robust variance was performed to estimate the association of cardiometabolic risk factors with deficiency and insufficiency of vitamin D. The prevalence ratio (PR) with 95 % CI was used as a measure of effect. The analyses were conducted having the cardiometabolic changes as the dependent variable. Multivariate models were adjusted for age, gender, season, ethnic group, parathyroid hormone, per capita income, maternal schooling, vitamin D intake, sedentary behaviour and percentage of body fat. Additional adjustment for different measures of adiposity as a potential confounding factor was also done, replacing the percentage of body fat in those models that had initially shown significant associations. The goodness-of-fit test was used to evaluate the adjustment of the final model. We considered the group with the lowest concentration of vitamin D as reference.
Finally, the association between the prevalence of insufficiency and deficiency of vitamin D and the number of cardiometabolic alterations (excessive body and abdominal adiposity, hypercholesterolaemia, hypertriacylglycerolaemia, high LDL cholesterol, low HDL cholesterol, hypertension, insulin resistance) was assessed using simple linear regression analysis. The prevalence of vitamin D insufficiency/deficiency was considered as dependent variable, while cardiometabolic alterations were considered as independent variables.
The significance level of 0·05 (α=5 %) was adopted for all tests.
Results
About half of the children were 9 years old (51·6 %) and female (52·1 %). Regarding skin colour, 55·8 % were brown, 32·8 % white and 11·4 % black. The prevalence of deficiency and insufficiency of vitamin D was 12·2 and 43·4 %, respectively (prevalence of sufficiency was 55·6 %). No child had 25(OH)D below 25 nmol/l.
Sedentary behaviour was more prevalent in boys (P=0·026). The mean vitamin D intake (1·68 µg/d) was 83·2 % lower than the Estimated Average Requirement, and it is noteworthy that no child reached the Estimated Average Requirement. Girls had higher mean body adiposity while boys had higher mean HDL cholesterol (P<0·05). There was no difference in the nutritional status of 25(OH)D and anthropometric measurements between genders (Table 1). We also found no difference in the time of sun exposure in relation to sedentary behaviour (P=0·134); however, a longer sun exposure was observed in the autumn (P<0·001).
Table 1 Characteristics of the study population of Brazilian children (n 378) aged 8–9 years, Viçosa, Minas Gerais, Brazil, 2015

IQR, interquartile range; 25(OH)D, 25-hydroxyvitamin D; HOMA-IR, homeostasis model assessment of insulin resistance; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; WC, waist circumference; WHtR, waist-to-height ratio; BF, body fat; FMI, fat mass index; AF, android fat mass; GF, gynoid fat mass.
Variables with normal distribution (mean and sd) were evaluated by Student’s t test and variables without normal distribution (median and IQR) were evaluated by the Mann–Whitney U test.
* P<0·05.
† χ 2 linear trend test.
The mean values of anthropometric measurements, body composition, biochemical variables and blood pressure did not differ by vitamin D intake tertile, and no association was found between serum concentrations and vitamin D intake. It is also noteworthy that the insufficiency/deficiency of 25(OH)D was compared between children from public and private schools and no statistical difference was found (data not presented).
The prevalence of insufficiency/deficiency of vitamin D (<75 nmol/l) was higher in black children (68·3 %) than in brown (56·4 %) and white (50·0 %) children. The prevalence of insulin resistance and the average values of anthropometric measurements and body composition were higher in children with insufficiency/deficiency of vitamin D (Table 2). After adjustment for confounding factors, in multiple regression analysis, the results showed that the prevalence of insulin resistance was lower in children with sufficient serum vitamin D concentration (PR=0·11; 95 % CI 0·03, 0·45) and this association remained significant after adjustment for the percentage of body fat (PR=0·25; 95 % CI 0·08, 0·85). The prevalence of hypertriacylglycerolaemia was lower in children with concentration of vitamin D≥50 nmol/l (PR=0·49; 95 % CI 0·30, 0·81) than in deficient children and this association also remained after adjustment for body fat (PR=0·61; 95 % CI 0·37, 0·99; Table 3). However, after additional adjustment for other indicators of body adiposity in multiple regression analysis, only insulin resistance remained independently associated. The association with TAG was lost after adjustment for central adiposity (Table 4).
Table 2 Characteristics in relation to vitamin D status (sufficiency, insufficiency/deficiency) of the study population of Brazilian children (n 378) aged 8–9 years, Viçosa, Minas Gerais, Brazil, 2015

25(OH)D, 25-hydroxyvitamin D; IQR, interquartile range; HOMA-IR, homeostasis model assessment of insulin resistance; BP, blood pressure; TC, total cholesterol; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; WC, waist circumference; WHtR, waist-to-height ratio; BF, body fat; FMI, fat mass index; AF, android fat mass; GF, gynoid fat mass.
Variables with normal distribution (mean and sd) were evaluated by Student’s t test and variables without normal distribution (median and IQR) were evaluated by the Mann–Whitney U test.
* P<0·05.
† χ 2 linear trend test.
‡ Fisher’s exact test.
Table 3 Poisson regression models estimating the association between cardiometabolic alterations and insufficiency/deficiency of vitamin D in Brazilian children (n 378) aged 8–9 years, Viçosa, Minas Gerais, Brazil, 2015

25(OH)D, 25-hydroxyvitamin D; %BF, body fat percentage; PR, prevalence ratio; HOMA-IR, homeostasis model assessment of insulin resistance; BP, blood pressure; TC, total cholesterol; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol.
PR are presented for higher concentration of vitamin D (≥50 or ≥75 nmol/l) and the group with lower concentration of vitamin D was used as reference. All models were adjusted for age, sex, season, ethnic group, parathyroid hormone, per capita income, maternal education, vitamin D intake and sedentary behaviour.
* P<0·05.
† Inclusion of %BF as an adjustment in the regression model.
Table 4 Poisson regression models estimating the association of deficiency/insufficiency of vitamin D with insulin resistance and hypertriacylglycerolaemia, according to different body measurements, in Brazilian children (n 378) aged 8–9 years, Viçosa, Minas Gerais, Brazil, 2015
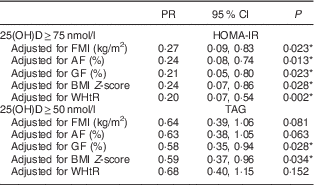
PR, prevalence ratio; 25(OH)D, 25-hydroxyvitamin D; FMI, fat mass index; AF, android fat mass; GF, gynoid fat mass; WHtR, waist-to-height ratio; HOMA-IR, homeostasis model assessment of insulin resistance.
PR are presented for higher concentration of vitamin D (≥50 or ≥75 nmol/l) and the group with lower concentration of vitamin D was used as reference. All models were adjusted for age, sex, season, ethnic group, parathyroid hormone, per capita income, maternal education, vitamin D intake and sedentary behaviour.
* P<0·05.
High prevalence of cardiometabolic risk factors was observed in the children, including overweight (32·8 %), excessive body (49·7 %) and abdominal adiposity (29·6 %), insulin resistance (2·4 %), hypercholesterolaemia (23·4 %), low HDL cholesterol (29·8 %), high LDL cholesterol (4·0 %), hypertriacylglycerolaemia (7·7 %) and hypertension (4·8 %). The prevalence of insufficiency/deficiency of vitamin D was associated with the number of cardiometabolic alterations in children (β=5·62; 95 % CI 0·92, 10·32; P=0·036; Fig. 1).

Fig. 1 Prevalence of insufficiency/deficiency of vitamin D (serum 25-hydroxyvitamin D<75 nmol/l) according to the number of cardiometabolic alterations in Brazilian children (n 378) aged 8–9 years, Viçosa, Minas Gerais, Brazil, 2015 (β=5·62; 95 % CI 0·92, 10·32; P=0·036)
Discussion
The present study found a high prevalence of vitamin D insufficiency/deficiency among the children and insulin resistance was the main cardiometabolic alteration associated with this condition, regardless of body fat location. Although restricted to one city, the present study is the first, to our knowledge, which relates the nutritional status of vitamin D to cardiometabolic risk factors in a population-based sample of Brazilian children.
The prevalence of hypertriacylglycerolaemia and insulin resistance was lower in children with higher serum concentrations of vitamin D. The associations were independent of potential confounding factors and effects of adiposity on HOMA-IR but not on TAG. Central adiposity, but no other measures of adiposity, removed the explanatory power of vitamin D deficiency related to hypertriacylglycerolaemia.
The city of our study is located in the south-east of the country. In Brazil, higher prevalence of hypovitaminosis D was found in the south of the country, with 90·6 % of inadequacy and 63·7 % of deficiency in children and teenagers( Reference Santos, Andaki and Amorim 34 ), and lower prevalence in the north of the country, with 32·0 % of inadequacy among children( Reference Lourenço, Qi and Willett 35 ). In developed countries, studies have shown that prevalence reaches 93 % of insufficiency in American children( Reference Delvin, Lambert and Levy 36 ), 51·6 % of insufficiency in French children( Reference Beuzit, L’Hour and Roudaut 37 ) and 28·4 % of deficiency in Danish children( Reference Guasch, Bulló and Rabassa 7 ). Tunisia, although not a tropical country, has a sunny climate and has also been reported to have a high prevalence of vitamin D deficiency in children and teenagers (84·9 %)( Reference Bezrati, Ben Fradj and Ouerghi 18 ). In the Brazilian population, it is observed that younger age, outdoor physical activities, oral supplementation of vitamin D, and living in coastal cities and at lower latitudes are related to better nutritional status of vitamin D( Reference Maeda, Borba and Camargo 2 ).
We found a higher prevalence of inadequate vitamin D (<75 nmol/l) in black- and brown-skinned children. In general, this has been attributed to the competition of melanin for UVB radiation, which is responsible for the photochemical fragmentation of the vitamin D precursor synthesized in the skin. However, it has been proposed that, despite the lowest total serum vitamin D concentrations, the black-skinned population has similar concentrations of bioavailable 25(OH)D, due to the lower concentrations of vitamin D-binding protein( Reference Powe, Evans and Wenger 38 ). No child reached the Estimated Average Requirement for vitamin D and this can be due to lower average consumption of vitamin D food sources by Brazilians. Low intake of this vitamin among Brazilian and foreign children was also observed in other studies( Reference Díez-Gañán, Labaca and Domínguez 39 – Reference Anta, González-Rodríguez and Ortega 41 ). Additionally, there was no association between intake and serum level of vitamin D, because it is estimated that 5–10 % of bioavailable vitamin D is derived from food intake, the largest proportion being acquired by cutaneous synthesis( Reference Holick 42 ). This is consistent with data from other studies( Reference Absoud, Cummins and Lim 5 , Reference Petersen, Damsgaard and Dalskov 9 ) and strengthens the fact that sunlight exposure is the main source of vitamin D.
Previous studies in the paediatric population also found no association with total cholesterol, LDL cholesterol( Reference Rodriguez-Rodriguez, Ortega and Gonzalez-Rodriguez 43 , Reference Williams, Fraser and Sayers 44 ), HDL cholesterol( Reference Petersen, Damsgaard and Dalskov 9 , Reference Delvin, Lambert and Levy 36 ) and blood pressure( Reference Peters, Roque and Fisberg 45 ). However, there are reports of an inverse association between vitamin D and TAG levels after adjustment for BMI( Reference Petersen, Damsgaard and Dalskov 9 , Reference Delvin, Lambert and Levy 36 , Reference Rodriguez-Rodriguez, Ortega and Gonzalez-Rodriguez 43 ). In a study with Danish children, Petersen et al.( Reference Petersen, Damsgaard and Dalskov 9 ) performed the adjustment for FMI in addition to the adjustment for BMI, and the inverse association between concentration of vitamin D and TAG remained. Yet, our study showed that the inverse relationship between vitamin D and hypertriacylglycerolaemia is indeed confused by adiposity, particularly central adiposity. The loss of association between vitamin D and TAG was observed after adjustment for gynoid fat, waist-to-height ratio (central adiposity) and also FMI (general adiposity). However, the association with FMI was probably prompted by the impact of central adiposity.
BMI, as a measure of adiposity, is often used to control confounding effects of obesity on the relationship of nutrients with diseases( Reference Alemzadeh, Kichler and Babar 12 , Reference Saneei, Salehi-Abargouei and Esmaillzadeh 46 ). Nevertheless, more accurate measures of adiposity such as assessment by dual-energy X-ray absorptiometry, computed tomography or MRI show that there is a direct relationship between body fat distribution and concentrations of 25(OH)D, and central obesity as being a strong predictor of vitamin D serum concentration( Reference Hao, Ma and Shen 47 ).
In the present study, the prevalence of insulin resistance was lower in children with adequate concentrations of 25(OH)D (≥75 nmol/l). Studies have shown that hypovitaminosis D predisposes individuals to increased insulin resistance risk through mechanisms in pancreatic β-cells that can affect the insulin response to glucose stimulation( Reference Zeitz, Weber and Soegiarto 48 , Reference Snijder, Van Dam and Visser 49 ).
Besides changing the synthesis and secretion of insulin, vitamin D deficiency may play an important role in the prevention and treatment of diabetes by acting to reduce insulin resistance also via its immunomodulatory and anti-inflammatory action( Reference Marcotorchino, Gouranton and Romier 50 ). Epidemiological studies have shown that children deficient in 25(OH)D had higher risk of developing diabetes, and that its supplementation may reduce this risk as well as having beneficial effects on the control of some complications caused by childhood obesity( Reference Kelishadi, Salek and Salek 16 , Reference Zipitis and Akobeng 51 ). The association between vitamin D and insulin resistance was independent of adiposity, indicating potential independent mechanisms of extra-bone action by vitamin D. The relationship between overweight, insulin resistance and vitamin D can be explained by the release of NEFA from adipose tissue, which can induce the resistance to insulin, while vitamin D improves the NEFA-induced insulin resistance by neutralizing these fatty acids( Reference Zhou, Hou and Guo 52 ).
In this sense, the serum 25(OH)D concentrations categorized were independently associated with cardiometabolic risk markers, which reinforces the possibility that the serum vitamin D concentration may not be linearly associated with these changes, but may have an effect from a threshold. Randomized clinical trials with long follow-up periods are necessary to establish the causal relationship, as well as proposed cut-off points of serum vitamin D concentration for protection against cardiometabolic changes.
It is important to note that there was an inverse association between the prevalence of deficiency/insufficiency of vitamin D and the number of cardiometabolic risk factors in children in the present study. There is evidence that deficiency of 25(OH)D is associated with the metabolic syndrome, even in children and adolescents( Reference Ganji, Zhang and Shaikh 11 , Reference Kelishadi, Salek and Salek 16 ). Although the specific mechanism of how vitamin D may protect individuals from cardiometabolic changes is not well understood, its function as a hormone has been increasingly recognized( Reference Bouillon, Carmeliet and Verlinden 53 ). Mechanisms such as the regulation of renin–angiotensin system activity in pancreatic β-cells and suppression of inflammation have been suggested( Reference Snijder, Van Dam and Visser 49 , Reference Marcotorchino, Gouranton and Romier 50 , Reference Bland, Markivic and Hilss 54 ).
The American and Brazilian Societies of Endocrinology do not recommend the assessment of serum concentration of 25(OH)D for the general population due to the measurement cost. However, laboratory investigation is recommended in subjects at risk for hypovitaminosis D, including those who are obese( Reference Maeda, Borba and Camargo 2 , Reference Norman, Bouillon and Whiting 22 ).
Recently, in Brazil, the Brazilian Society of Pediatrics published recommendations for vitamin D supplementation only for children under 2 years of age, including exclusive breast-feeding infants. It is important to encourage the adoption of healthy eating and lifestyle habits for children and teenagers, such as intake of food sources of vitamin D and outdoor physical activities associated with sun exposure( 55 ).
It is noteworthy that the present study was conducted with homogeneous sample regarding physiological characteristics, consisting of prepubescent children. Moreover, it is one of the few studies in developing countries that has assessed the serum concentration of vitamin D and its association with cardiometabolic risk factors in childhood; the first population-based study with prepubertal Brazilian children. Additionally, all confounding variables cited in the literature were used for adjustments in the statistical analysis of the study.
Conclusion
In conclusion, the results of the current investigation show that insufficiency/deficiency occurred in more than half of children and none of them reached the recommended intake of vitamin D. It is important to highlight that adequate serum concentration of vitamin D was inversely associated with the prevalence of insulin resistance, regardless of body fat location. The current lifestyle of children, with low intake of vitamin D and few outdoor activities, may explain the high prevalence of insufficiency/deficiency of 25(OH)D even in a tropical climate country like Brazil. Considering the importance of preventing chronic diseases beginning in childhood, strategies for adopting healthy habits to improve vitamin D nutritional status in this age group can be really relevant in order to avoid the repercussions of long-term cardiometabolic alterations.
Acknowledgements
Acknowledgments: The authors are grateful to the National Council for Scientific and Technological Development (CNPq) for financial support (process number 478910/2013-4); BIOCLIN® for providing material for biochemical analysis; and the Coordination for the Improvement of Higher Education Personnel (CAPES) for the granting of a master’s scholarship. Financial support: This work was supported by the CNPq (grant number 478910/2013-4). The funders had no role in the design, analysis or writing of this article. Conflict of interest: The authors declare no conflict of interest. Authorship: L.C.M. conceived and designed this work and the analysis of the data, conducted the literature search, as well as wrote the manuscript. N.P.R. assisted with data collection, and revised and approved the final manuscript as submitted. M.S.F. assisted with data collection, and revised and approved the final manuscript as submitted. F.M.A. assisted with data collection, and revised and approved the final manuscript as submitted. A.P.P.C assisted with data collection, and revised and approved the final manuscript as submitted. M.C.P. supervised data analysis, assisted in the interpretation of results and approved the final manuscript as submitted. M.C.G.P. assisted in the interpretation of results and approved the final manuscript as submitted. J.F.N designed the study including the data collection, coordinated and supervised and approved the final manuscript as submitted. Ethics of human subject participation: This study was conducted according to the guidelines defined in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee on Human Research of the Federal University of Viçosa (opinion number 663171/2014) and also presented to the Municipal Department of Education, the Regional Superintendent of Education and principals of schools. Informed consent was signed by parents.








