Elevated blood pressure (BP) is a modifiable major risk factor for cardiovascular morbidity. Estimates are that suboptimal BP (systolic BP > 115 mmHg) is responsible for 62 % of cerebrovascular diseases and 49 % of IHD, with little variation by sex( 1 ). The National Center for Health Statistics estimates an overall prevalence of hypertension (systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg or taking medications for hypertension) in 2003–2006 of 31·3 %( 2 ), and of 47·3 % in those aged 55 years or more( Reference Schoenborn and Heyman 3 ). This suggests that 50 million or more Americans have high BP. Favourable BP levels are associated with greater probability of survival to age 85 years as well as increased longevity without co-morbidity( Reference Terry, Pencina and Vasan 4 ).
The differences in prevalence rates for hypertension between certain populations and age groups have been partially explained by differences in intakes of particular nutrients, although the evidence for some is weak. These include positive associations with Na, alcohol and total protein, and negative associations with K, Ca and Mg( Reference Suter, Sierro and Vetter 5 , Reference Berkow and Barnard 6 ). Recent work suggests that a diet similar to the Dietary Approaches to Stop Hypertension (DASH) diet, but containing more lean red meat, is still superior to a more typical diet( Reference Nowson, Wattanapenpaiboon and Pachett 7 ) in postmenopausal women, but results of feeding studies of meat on BP are inconsistent( Reference Hodgson, Burke and Beilin 8 , Reference Sacks, Donner and Castelli 9 ). Dietary fats have been examined, with some studies showing no convincing effects on BP, while other studies indicate a possible relationship depending on fat type( Reference Iacono, Judd and Marshall 10 – Reference Sacks, Marais and Handysides 15 ). Fibre has also been studied, and recent meta-analyses of trials have shown small but significant effects mainly on diastolic BP and more pronounced effects in hypertensive individuals( Reference Streppel, Arends and van ‘t Veer 16 , Reference Whelton, Hyre and Pedersen 17 ).
The effects of a vegetarian diet on BP have been evaluated by several previous studies over three decades. Most of these have been small short-term feeding studies( Reference Sacks, Donner and Castelli 9 , Reference Rouse, Beilin and Mahoney 18 – Reference Margetts, Beilin and Vandongen 20 ) or cross-sectional comparisons also of relatively small selected study groups of vegetarians and others( Reference Rouse, Armstrong and Beilin 21 – Reference Burr, Bates and Fehily 24 ). Melby et al. have studied this association in African Americans( Reference Melby, Goldflies and Toohey 25 , Reference Melby, Toohey and Cebrick 26 ) with mixed results. Most of these studied the lacto-ovo vegetarian rather than vegan diet. An exception to limitations of size and diversity of subjects is a very large cross-sectional comparison of British vegetarians (vegans and lacto-ovo vegetarians) and health-conscious non-vegetarians( Reference Appleby, Davey and Key 27 ), where significant effects were found, mainly in the vegans.
In the current paper we present results of a cross-sectional comparison within a relatively large and diverse group where the vegetarian and non-vegetarian habits were generally long term, and where vegetarians were divided to vegans and non-vegans. This study group is representative of a national cohort of Seventh-day Adventists( Reference Butler, Fraser and Beeson 28 ) which spans a wide range of socio-economic status, age and geographic location, and both genders. Alcohol consumption is minimal or absent.
Materials and methods
The Adventist Health Study-2 (AHS-2) is a cohort study of 96 000 Adventists living in the USA and Canada who enrolled between 2002 and 2007( Reference Butler, Fraser and Beeson 28 ). We analysed data from the AHS-2 calibration study which was designed mainly to evaluate the accuracy of collection and reporting of data. The study subjects are a representative sample of 504 non-black individuals (about 3 % Asian), randomly selected from the parent cohort by church, and then subject-within-church. Recruitment and enrolment methods of the parent cohort and calibration study are detailed elsewhere( Reference Butler, Fraser and Beeson 28 ).
Relevant data were gathered from a self-administered FFQ and a 7 d physical activity recall. In addition, BP and BMI were measured and overnight urine collected during a brief clinical examination performed by a travelling team of trained technicians( Reference Chan, Knutsen and Sabate 29 ).
Calibration study subjects came to the clinic fasting, having been instructed to take their usual medications. They were instructed to empty their bladder and were then seated in a quiet room at a comfortable temperature for approximately 10 min before their systolic and diastolic BP were measured using an Omron automated sphygmomanometer( Reference O'Brien, Mee and Atkins 30 ). Three readings were taken 5 min apart. The mean of the second and third readings was used for the present analyses unless there was a difference of more than 5 mmHg, in which case we used the mean of all three readings. We excluded four subjects with a mean recorded systolic BP of less than 80 mmHg, leaving 500 subjects for analysis.
Dietary pattern was determined using information from the FFQ about intake of twenty-five different food items (Table 1) relevant to vegetarian status. Participants were asked to report their usual or average diet during the past year. Each food item allowed up to nine frequency response options, ranging from ‘never or rarely’ to ‘6+ times/d’. The validity of this FFQ is relatively high for most nutrients and foods when compared with the means of the six dietary recalls( Reference Jaceldo-Siegl, Knutsen and Sabate 31 , Reference Jaceldo-Siegl, Fan and Sabate 32 ). Frequencies of intake were converted to daily equivalents and these were used to construct composite food variables that measured intakes of red meat, poultry, fish, eggs and dairy foods. Values of these variables allowed subjects to be assigned to a dietary pattern as shown in Table 2.
Table 1 The twenty-five items from the FFQ used for classification of dietary patterns

Table 2 Definitions of dietary patterns
There were small amounts of missing data in the dietary variables. For about half of these variables a random 10 % subset of initially missing data was later filled-in by telephone. We used this to guide imputation( Reference Fraser and Yan 33 ) of the remaining missing data for these variables. For other variables we assumed that the data were missing at random, which even if not quite correct will cause little bias when the missing data rate is small( Reference Fraser and Yan 33 ). Imputation was performed at the level of the composite variables meat, fish, dairy and eggs, and was also conditional on other covariates and the dependent variable in any particular statistical model. The imputation software used was the Hmisc package for R version 2·6·0 (R Foundation for Statistical Computing, Vienna, Austria)( Reference R 34 ).
Physical activity was measured using a detailed hour-by-hour telephone recall about type, intensity and duration of different physical activities during the preceding week( Reference Sallis, Haskell and Wood 35 ). These activities were summed to produce hours per week of moderate, hard or very hard exercise (metabolic equivalent task levels ≥4·5).
Information on medication use in the present study was obtained along with the 24 h recalls. A cardiologist subsequently identified all medications used for treatment of or known to reduce BP. The use of such medications is reported as none or some. This population largely abstains from alcohol use (6·75 % admit to current use, mostly infrequent), although it was tested as a covariate.
The study was approved by the institutional review board of Loma Linda University and all subjects gave written informed consent.
Statistical analysis
ANOVA was performed to test null hypotheses of equal means between dietary groups for continuous variables, while the χ 2 test was used for categorical variables. These were performed using the SAS statistical software package release 9·2 (SAS Institute Inc., Cary, NC, USA). Linear and logistic regression modelling was performed using statistical software R version 2·10·1 (R Foundation for Statisical Computing). Where required, hypotheses involving interactions were tested using product terms in regression models.
Results
Demographics
The four groups of participants are presented in Table 3. Ten per cent were vegan, 36 % were lacto-ovo vegetarian, 14 % partial vegetarian and 40 % non-vegetarian (Table 3).
Table 3 Main characteristics of the study population: white subjects representing the Adventist Health Study-2 (AHS-2) cohort, USA and Canada

BP, blood pressure.
Data are presented as numbers and percentages or means and standard deviations where appropriate, with significance tests (n 500).
†13 missing BMI data.
‡Mean of last two BP measurements if difference between last two measurements is ≤5 mmHg; mean of three BP measurements if difference between last two measurements is >5 mmHg.
§19 missing data on age at baptism.
∥P value for χ 2 test for dependency among dietary patterns.
¶P value for ANOVA test of equality of means among dietary patterns.
The age distribution was significantly different between the groups with the vegans being older. The average age of the participants was 62·7 years and 64 % were women. There was a large range of educational attainment and subjects lived in all major regions of the USA and Canada. More of the vegetarians had at least 50 years of church membership. The following percentages of the study population came from the stated regions: West 23·8 %; Northwest 16·2 %; Mountain 4·0 %; Midwest 18·2 %; Mid and North East 11·5 %; South 21·6 %; Canada 4·6 %. The proportions in the various dietary categories did not vary significantly by region although there was a tendency towards a higher proportion of vegetarians in the Northwest and Mountain regions.
Blood pressure and hypertension medication
The mean BP levels were relatively low for all subjects (systolic 125·2 mmHg, diastolic 75·2 mmHg), moderately higher for those treated (systolic 133·3 mmHg, diastolic 76·3 mmHg) and lowest in untreated subjects (systolic 122·5 mmHg, diastolic 74·8 mmHg). The proportion taking some medication known to reduce BP was for the whole population 25·2 %; 22·8 % for women and 29·4 % for men. We found no significant differences in BP between the four dietary groups among those taking antihypertensive medication.
Blood pressure, age, gender and physical activity
There were no significant differences in BP by gender. As expected there were strong age effects on systolic BP, but these were not seen for diastolic BP. We could not demonstrate significant effects of physical activity on BP.
Relationship between diet and blood pressure
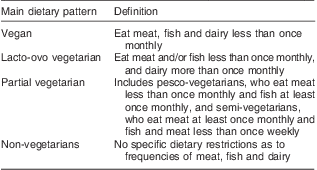
Adjusting for age and gender (Table 4) and including only non-treated individuals, systolic BP was significantly lower in vegans and lacto-ovo vegetarians (β = −6·8 mmHg, P < 0·05 and β = −9·1 mmHg, P < 0·001) when compared with non-vegetarians, and results were broadly similar for diastolic BP (β = −6·9 mmHg, P < 0·001 and β = −5·8 mmHg, P < 0·001; Table 4). There was a significantly higher systolic BP at older ages (0·6 mmHg higher per year, P < 0·001) and there was no discernible effect of physical activity.
Table 4 Parameter estimates (β coefficient, 95 % confidence interval) relating blood pressure (BP) and dietary pattern by antihypertensive medication status, adjusted for gender and age: white subjects representing the Adventist Health Study-2 (AHS-2) cohort, USA and Canada
*P < 0·05; **P < 0·01; ***P < 0·001.
†Hours per week of moderate/hard/very hard physical activity.
BMI was significantly associated with both systolic and diastolic BP (Table 5). Where normal BMI was the reference, overweight subjects (BMI = 25·0–29·9 kg/m2) had systolic BP 3·1 mmHg higher (NS) and obese subjects (BMI ≥ 30·0 kg/m2) on average had systolic BP 11·9 mmHg higher (P < 0·001). For diastolic BP these statistics were respectively 3·5 mmHg (P < 0·01) and 9·6 mmHg (P < 0·001; Table 5). Creating 2-unit wide BMI categories between BMI of <18 kg/m2 and >32 kg/m2 showed a linear association with BP from lowest to highest categories for both systolic BP and diastolic BP.
Table 5 Parameter estimates (β coefficient, 95 % confidence interval) relating blood pressure (BP) and dietary pattern by antihypertensive medication status, adjusted for BMI, age and gender: white subjects representing the Adventist Health Study-2 (AHS-2) cohort, USA and Canada

*P < 0·05; **P < 0·01; ***P < 0·001.
†Hours per week of moderate/hard/very hard physical activity.
When adjusting for BMI the same trends with dietary pattern observed in Table 4 were seen, although the differences were less (Table 5). Clearly BMI is to some extent an intermediary between diet and BP effect. However, significant dietary effects were still seen when the analyses were confined to the 224 subjects with BMI < 25·0 kg/m2. Specifically, with non-vegetarians as the reference, vegans had lower systolic BP by 7·12 mmHg (P = 0·06), lacto-ovo vegetarians were lower by 5·55 mmHg (P = 0·06) and partial vegetarians by 2·75 mmHg (P = 0·47). For diastolic BP vegans were lower by 5·10 mmHg (P = 0·006), lacto-ovo vegetarians were lower by 3·07 mmHg (P = 0·03) and partial vegetarians lower by 0·52 mmHg (P = 0·78). When in addition adjusting for BMI these effects in non-overweight vegetarians diminished by 1–2 mmHg for systolic BP (test for dietary effects: P = 0·17 for vegans, P = 0·11 for lacto-ovo vegetarians) and by about 1 mmHg for diastolic BP (test for dietary effects: P = 0·02 for vegans, P = 0·06 lacto-ovo vegetarians).
Defining hypertension as average systolic BP > 139 mmHg or average diastolic BP > 89 mmHg or taking prescribed antihypertensive medications, logistic analysis showed that the vegetarian categories related to hypertension in a similar fashion to that reported for BP (Table 6). Specifically, vegans, lacto-ovo vegetarians and partial vegetarians had lower estimated odds of hypertension (OR = 0·37 (95 % CI 0·19, 0·74), OR = 0·57 (95 % CI 0·36, 0·92) and OR = 0·92 (95 % CI 0·50, 1·70)) than non-vegetarians and the odds ratios diminished substantially (OR = 0·53 (95 % CI 0·25, 1·11), OR = 0·86 (95 % CI 0·51, 1·45) and OR = 1·22 (95 % CI 0·64, 2·33)) when BMI was added to the model. This again suggests that the effect of diet to reduce BP is partly mediated by dietary effects on BMI.
Table 6 Odds ratio and 95 % confidence interval for hypertensionFootnote † by dietary pattern with and without BMI: white subjects representing the Adventist Health Study-2 (AHS-2) cohort, USA and Canada (n 500)
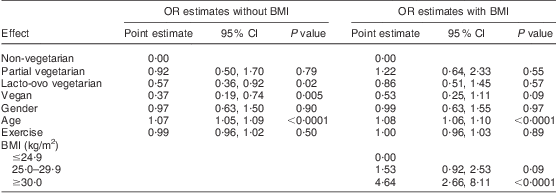
† Hypertension defined as average systolic blood pressure > 139 mmHg or average diastolic blood pressure > 89 mmHg or taking antihypertensive medications.
Adding alcohol intake to any of the above models (not shown) changed results only trivially and the alcohol effect was always far from statistical significance. This is not surprising given the infrequent and small intakes.
In AHS-2, overnight urinary K (but not Na or Ca) excretion correlates well with the corresponding dietary intake (r = 0·55 for K compared with six dietary recalls; GE Fraser, unpublished results). Urinary K results were available from a random thirty-six calibration study subjects who were not taking antihypertensive medications. The latter were excluded as frequent use of diuretics will distort results. The overnight K excretion in vegetarians (vegans and lacto-ovo vegetarians combined) was on average 30·0 mmol/l and 19·8 mmol/l for non-vegetarians (P = 0·10). Although compatible with chance, this suggests a sizeable difference in K intake as would be expected.
Discussion
In this non-black population we report significant differences in both systolic and diastolic BP and the odds of hypertension, depending on vegetarian dietary pattern. These results are from a population that includes a wide variety of age, socio-economic status and both genders, and they represent dietary habits stable over many years for the most part. Subjects summarized their diets over the previous year. Few other studies have been able to compare habitual non-vegetarians with both habitual vegans and lacto-ovo vegetarians. The effect appears to be moderately stronger in vegans as there were fewer vegans taking antihypertensive medications and those not taking such medications had BP as low (approximately) as the lacto-ovo vegetarians. Effects appear to be partially explained by dietary effects on BMI, which are strong in this population( Reference Tonstad, Butler and Yan 36 ). Dietary effects were still evident in those with normal BMI.
The percentage overweight or obese in this population was lower than in the general US population( Reference Tonstad, Butler and Yan 36 ), though still considerable. Our results are adjusted for these factors but it seems likely that the effect of a vegetarian diet to reduce body weight is one mechanism partially responsible for the BP effect. However, some additional effect probably still remains. A partial mediating effect of BMI is consistent with the results of some other observational studies( Reference Rouse, Armstrong and Beilin 21 , Reference Appleby, Davey and Key 27 ). Interestingly, in previous short-term feeding studies there was typically no weight loss during the vegetarian feeding period, despite the well-known long-term large differences in BMI between vegetarians and others. It does seem possible that although no weight changes were seen over a few weeks of the feeding studies, physiological processes (e.g. insulin/glucose metabolism)( Reference Sciarrone, Strahan and Beilin 19 ) resulting in or associated with weight loss over a longer period may have begun and may already have started to affect BP.
As with most other similar studies, we found effects on both systolic and diastolic BP. While a few reported studies found changes in systolic( Reference Sacks, Donner and Castelli 9 , Reference Margetts, Beilin and Vandongen 20 , Reference Armstrong, Clarke and Martin 22 ) pressures only, this may have been due to limited statistical power.
Beyond BMI, which dietary factors in the vegetarian diet may account for effects on BP levels is not well understood. Vegetarians have higher fibre and K intakes as a result of their greater intakes of fruits, vegetables, fruits, nuts and wholegrain products( Reference Haddad and Tanzman 37 – Reference Janelle and Barr 39 ). Recent meta-analyses of randomized trials( Reference Streppel, Arends and van ‘t Veer 16 , Reference Whelton, Hyre and Pedersen 17 ) demonstrate small but significant effects of fibre, particularly in those with higher baseline pressures.
Consumption of a K-rich diet has a natriuretic effect and diets that are high in K usually are low in Na, as long as unprocessed foods are consumed( Reference Suter, Sierro and Vetter 5 ). Low Ca or dairy intake has also been associated with higher BP( Reference Ruidavets, Bongard and Simon 40 – Reference McCarron and Reusser 42 ). Inconsistent with this, our results and those from the European Prospective Investigation into Cancer and Nutrition (EPIC)–Oxford study( Reference Appleby, Davey and Key 27 ) show that vegans who avoid dairy products have the least hypertension. However, Adventist vegans do not have particularly low Ca intakes, perhaps due in part to supplementation but also because of vegetable sources of Ca.
Proposed physiological mechanisms that may mediate the effect of a vegetarian diet include modulation of baroreceptor sensitivity, direct vasodilatory effects, changes in catecholamine and renin–angiotensin–aldosterone metabolism, improvement of glucose tolerance with lower insulin levels( Reference Suter, Sierro and Vetter 5 , Reference Sciarrone, Strahan and Beilin 19 ), and lower blood viscosity in vegetarians( Reference Ernst, Pietsch and Matrai 43 ).
Strengths and weaknesses
We measured BP using an automated sphygmomanometer which provides BP with acceptable validity( Reference O'Brien, Mee and Atkins 30 ). A large amount of apparently random error is associated with BP measurement, despite taking measures to counteract the known influencing factors. In addition to using the digital machine we standardized other environmental factors that may influence BP. Together with the relatively large number of study subjects these factors should reduce the effects of random errors. Most study subjects had been church members for decades, further suggesting stability of dietary habits. Differences in duration of church membership by dietary category seem unlikely to affect results given that only 8–21 % had been members for <25 years across the dietary groups.
It was necessary to assign vegetarian dietary pattern based on the results of an FFQ rather than the repeated recalls, as a small number of recalls will easily miss less frequent consumption of animal products. There is inevitably recall and reporting error in these data. However, compared with the average of six 24 h recalls, correlation coefficients (r) corrected for deattenuation are as follows: red meat (r = 0·76); poultry (r = 0·76); fish (r = 0·53); eggs (r = 0·64); dairy protein (r = 0·77). Thus by usual standards the validity of food frequency questions used in the algorithm assigning vegetarian status is excellent.
Inevitably there were small amounts of missing dietary data (<1 % for most composite variables in this study). However, 9·4 % of subjects were missing at least one of the longer lists of individual dairy items where we needed to assume missing at random for imputation and this may have resulted in a small amount of misclassification.
The proportion of this older study population who were taking antihypertensive medication was 25·3 %, compared with 21·3 % in the US population aged 18 years and above( Reference Ostchega, Yoon and Hughes 44 ). It is interesting that 10·0 % of vegans but 28·8 % of non-vegetarians are taking antihypertensive medications. This could mean that the vegan population is less willing to take medications or that BP is indeed lower in this subgroup. Given the relatively low BP among vegans not taking antihypertensive medications, their need for medications is probably less. The much lower odds of hypertension in this group is thus due both to the lower proportion taking antihypertensive medication and the low BP in those not taking medication.
As this is a cross-sectional study we do not know the stability of dietary patterns over time, meaning that it is not possible to exclude a reverse causation in that some may have changed their diet after they received a diagnosis of elevated BP. However, if this occurred it would likely work against reverse causation as most Adventists with a health problem would actually move towards a plant-based diet.
Conclusions
Our study extends and supports previous evidence that diet affects measured BP levels, both systolic and diastolic, with vegans and lacto-ovo vegetarians having lower BP than non-vegetarians. We show that this appears to be long-lasting as our subjects generally have maintained these dietary habits over at least 1 year. The vegans appear to have the least hypertension, although further evidence should be gathered on this group. Our data represent a diverse population (although all Adventists) by geography, BMI, socio-economic status and gender. Many Americans may benefit from a diet containing more plant foods to prevent hypertension.
Acknowledgements
This study was funded by a grant from the National Cancer Institute, National Institutes of Health ( 5RO1 CA 094594). There are no conflicts of interest to disclose. B.J.P. drafted the manuscript. R.A. wrote the section on statistical analyses, and with J.F. performed the statistical analyses. K.J.-S. and G.E.F. helped collect the data and conceived the study. B.J.P., K.J.-S. and G.E.F. were all involved in study design. All authors reviewed the manuscript on several occasions.








