Over the past 30 years, evidence supporting the importance of a healthy prenatal diet for optimal health outcomes in children has been mounting(Reference Jazwiec and Sloboda1). Diet quality during pregnancy also predicts diet quality in children(Reference Bjerregaard, Halldorsson and Tetens2). In the context of the Developmental Origins of Health and Disease hypothesis, strategies that provide the early foundations for optimal maternal and infant nutrition are a public health imperative for reducing the risk of noncommunicable disease. In many Western countries, including Australia, diet quality (adherence to the dietary guidelines) in pregnant women is often less than optimal, with few meeting the dietary guidelines(Reference de Jersey, Nicholson and Callaway3,Reference Malek, Umberger and Makrides4) . Pregnant women do not typically receive detailed nutritional advice as part of their health care(Reference Lee, Belski and Radcliffe5,Reference Lucas, Charlton and Yeatman6) . A lack of dietary knowledge is one key explanation for the failure for pregnant women to meet the dietary guidelines both in Australia and elsewhere(Reference de Jersey, Nicholson and Callaway3,Reference Bookari, Yeatman and Williamson7,Reference Dundas and Taylor8) . A cross-sectional Australian study reports that pregnant women were highly motivated and confident in their ability to maintain a healthy diet and meet the dietary recommendations; however, their dietary intakes indicated poor knowledge of the dietary guidelines and poor adherence to them(Reference Bookari, Yeatman and Williamson7). Others report that pregnant women are motivated and would like nutritional education(Reference de Jersey, Nicholson and Callaway3). A motivating factor for pregnant women to make dietary behaviour change is to promote infant health(Reference Forbes, Graham and Berglund9). New approaches are needed to harness this motivation and empower pregnant women to improve their diet quality. Pre-clinical evidence demonstrates that the gut microbiota are an important mechanism by which the perinatal diet can modulate immune(Reference Thorburn, McKenzie and Shen10) and brain development(Reference Buffington, Di Prisco and Auchtung11) in offspring. In humans, the gut microbiota respond rapidly to dietary change(Reference David, Maurice and Carmody12,Reference Singh, Chang and Yan13) and are related to health and disease(Reference Maslowski and Mackay14,Reference Cryan, O’riordan and Cowan15) . Hence, the gut microbiota may be used to harness the increased motivation to make dietary improvement during pregnancy(Reference de Jersey, Nicholson and Callaway3). A dietary intervention that specifically targets the gut microbiota may thus improve diet quality whilst helping to support the gut.
In Australia, the Australian Dietary Guidelines (ADG) recommend consuming a variety of vegetables, legumes and fruit, wholegrain cereals, lean meats, nuts and seeds, and mostly reduced fat dairy and alternatives, while limiting intakes of ‘discretionary’ foods (foods with added fats, sugars, salts and other additives)(16). This advice concords with evidence supporting a healthy, diverse gut microbiota(Reference Singh, Chang and Yan13,Reference Telle-Hansen, Holven and Ulven17–Reference Bowyer, Jackson and Pallister19) . Moreover, prebiotic fibre (non-digestible carbohydrates) usually present in plant-based foods may help to support the gut by promoting the growth of beneficial probiotic genera, Bifidobacterium or Lactobacillus(Reference Shortt, Hasselwander and Meynier20). During pregnancy and the perinatal period, probiotic supplementation of Lactobacillus, Bifidobacterium and/or Saccharomyces species has been trialled, and meta-analysis of 4356 women indicates that probiotics reduced the risk of atopic eczema and eczema in infants(Reference Kuang and Jiang21). Regarding safety, no changes in the risk of adverse pregnancy or birth outcomes were reported by a recent meta-analysis of probiotic supplementation trials in 4098 women(Reference Jarde, Lewis-Mikhael and Moayyedi22). A problem with supplementation is that it does not address the underlying diet quality. Given that the majority of women from higher income countries do not generally meet recommendations for energy, macronutrients and fibre and often exceed them for saturated fat(Reference Blumfield, Hure and MacDonald-Wicks23), their nutrition and gut health may not be optimal.
The Healthy Parents, Healthy Kids randomised controlled trial study was a prospectively registered study testing the efficacy of a perinatal educational dietary intervention(Reference Dawson, Craig and Clarke24). Pregnant women participated from gestation week 26 until 4 weeks after birth (henceforth referred to as the ‘perinatal’ period). The dietary intervention targeted the behaviour ‘eating for the gut microbiota’ as an intervention change mechanism(Reference Wight, Wimbush and Jepson25) to promote dietary improvement in participants. It aimed to increase dietary health literacy, self-efficacy and behavioural enactment to improve diet quality. Particular focus was placed on the intervention educational design to ensure that learning could take place(Reference Dawson, Craig and Clarke24). Participants were taught how to meet the ADG(16), to consume prebiotic- and probiotic-containing foods, the gut microbiota respond to diet(Reference David, Maurice and Carmody12), different factors shape gut microbiota in adults(Reference Singh, Chang and Yan13) and infants(Reference Collado, Cernada and Baüerl26) and how gut microbiota may relate to health(Reference Borre, O’Keeffe and Clarke27). The intervention design incorporated 30 of Michie et al.’s behaviour change techniques(Reference Michie, Richardson and Johnston28), these are intervention design components that bring about change by addressing the underlying drivers of dietary behaviour(Reference Michie, Richardson and Johnston28–Reference Atkins and Michie30). The use of behaviour change techniques has been successful for supporting adherence to community-based interventions targeting dietary behaviour amongst adults(Reference Greaves, Sheppard and Abraham29) and pregnant populations(Reference Hill, Skouteris and Fuller-Tyszkiewicz31).
The present study addresses a secondary aim of the Healthy Parents, Healthy Kids randomised controlled trial: to determine the efficacy of the intervention for improving maternal diet(Reference Dawson, Craig and Clarke24). We hypothesised that women in the intervention group would: (i) improve their diet in accordance with the ADG (as measured by total diet quality scores); (ii) consume a wider variety of core foods (such as vegetables, fruit, grains, lean meat, poultry, fish, eggs, tofu, nuts, seeds, legumes, milk, yogurt cheese and alternatives); increase intakes of (iii) prebiotic foods, (iv) probiotic foods and (v) fibre; and reduce intakes of (vi) discretionary foods, (vii) saturated fat and (viii) total energy, compared with the control group, and that these changes would be sustained throughout pregnancy(Reference Dawson, Craig and Clarke24).
Methods
Trial design and participants
The Healthy Parents, Healthy Kids study was a two arm, parallel, single-blind study of pregnant women receiving either dietary intervention v. standard medical care on dietary intake outcomes measured at prenatal weeks 32, 36 and 4 weeks postnatally(Reference Dawson, Craig and Clarke24). The primary outcome was a between-group difference in the diversity of the gut microbiota in infants measured 4 weeks after birth(Reference Dawson, Craig and Clarke24); the results of which will be reported in a subsequent publication. The study details have been described elsewhere(Reference Dawson, Craig and Clarke24). Briefly, women were recruited online or within the Melbourne community (e.g., obstetric clinics, doctor’s surgeries, maternal and child health centres, playgroups, childcare centres, shopping centres, physiotherapists and media). Women provided informed consent prior to study enrolment, commencing the study conditions from week 26 of pregnancy. Women were included if they had the capacity to attend a dietary workshop and did not meet any exclusion criteria: aged under 18 years; a BMI of 30 or higher; diagnosis of diabetes mellitus, mental illnesses or bowel conditions; medically advised exclusion or restriction diets, illicit drug use, prebiotic or probiotic supplementation or antibiotic use in the previous month; and/or lacking dietary autonomy(Reference Dawson, Craig and Clarke24).
Sample size and randomisation
The study was powered for the primary outcome of differences in infant microbial diversity and aimed to recruit ninety women(Reference Dawson, Craig and Clarke24). This target was not met, and forty-five healthy pregnant women were recruited. Participants were randomised to continue receiving treatment as usual as dietary advice from their healthcare provider or to additionally receive the dietary intervention. Randomisation occurred in gestation week 26 after baseline data collection, using a concealed 1:1 ratio with randomly permutated block sizes.
Intervention
The intervention composed of three sessions, an educational dietary workshop delivered by a nutritionist (sd), offered between gestation weeks 26–29 and two scripted dietary support calls in gestation weeks 31 and 36. To address the underlying drivers of dietary behaviour change, the intervention design used the following behaviour change techniques(Reference Michie, Richardson and Johnston28), as outlined in(Reference Dawson, Craig and Clarke24): goal setting (behaviour) (1·1), problem solving/coping planning (1·2), action planning (including implementation intentions) (1·4), review behaviour goal(s) (1·5), discrepancy between current behaviour and goal standard (1·6), behavioural contract (1·8), commitment (1·9), feedback on behaviour (2·2), self-monitoring of behaviour (2·3), social support (general, practical and unspecified) (3·1, 3·2 and 3·1), instruction on how to perform a behaviour (4·1), information about health and emotional consequences (5·1, 5·6), modelling or demonstration of the behaviour (6·1), prompts/cues (7·1), behavioural rehearsal/practice (8·1), behaviour substitution (8·2), habit formation (8·3), use of credible sources (9·1), self-incentive (10·7), non-specific and self-reward (10·3, 10·9), reduce negative emotions (11·2), restructuring the physical environment (12·1), adding objects to the environment (12·5), identification of self as role model (13·1), framing/reframing (13·2) and focus on past success (15·3)(Reference Michie, Richardson and Johnston28). The educational design was underpinned by the theory of constructive alignment(Reference Biggs32),which supports learning by ensuring alignment between intended learning outcomes, learning activities and assessments.
Participants in the intervention group were taught how to align their diet to the ADG(16) and how to substitute foods or ingredients for high-fibre, prebiotic or probiotic alternatives using a list of forty-two prebiotic foods(33) containing high quantities of inulin, fructo-oligosaccharide and/or oligosaccharides(Reference Muir, Rose and Rosella34,Reference Muir, Shepherd and Rosella35) and a list of foods and drinks containing probiotics or postbiotics (i.e., microbial components or their metabolites(Reference Aguilar-Toalá, Garcia-Varela and Garcia36)), such as yogurt containing cultures, sauerkraut, fermented vegetables, kimchi, sourdough bread, tempeh, miso soup, soy sauce, natto, kombucha, fermented kvass, rejuvelac, kefir, probiotic drink, vinegar and apple cider vinegar. By the end of the workshop, participants set three dietary SMART goals (Specific, Measurable, Action oriented, Realistic and Time-bound)(Reference Doran37,Reference Piskurich38) . A trained member of the study team reviewed and discussed goals during support phone calls.
Data collection and dietary assessment
Data were collected online over four time points: gestation week 26 (baseline), weeks 31 and 36, and postnatal week 4 (follow-up) (Fig. 1). All participants reported the types of dietary advice that they received from healthcare providers. Prospectively set dietary goals were recorded for the intervention group by the facilitator during the intervention support calls.
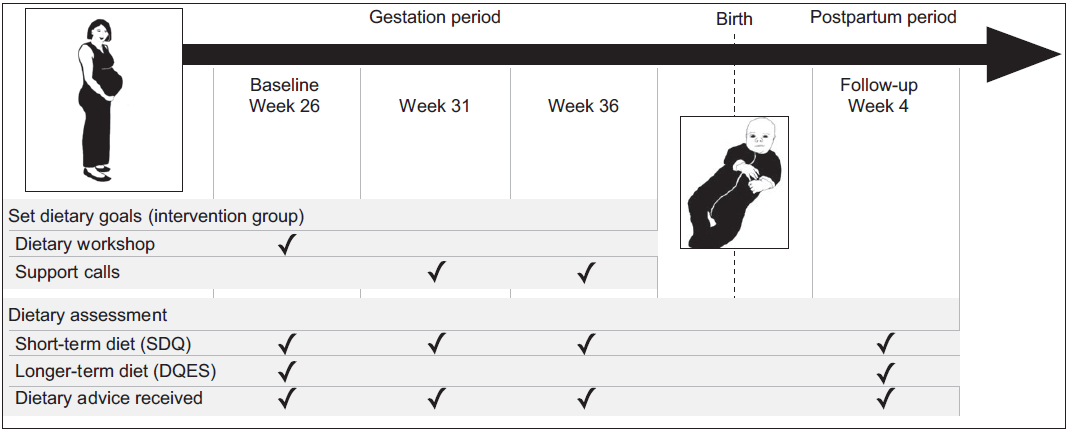
Fig. 1 Time points for the intervention and data collection activities. Short-term dietary assessment represents the previous 2 weeks intake. Longer-term dietary assessment represents the previous 3 months intake
Short-term dietary intake representing the previous 2 weeks was measured at all time points using the Simple Dietary Questionnaire (SDQ)(Reference Parletta, Zarnowiecki and Cho39). Participants recorded intake frequency and variety for a list of foods. We modified this questionnaire to include the prebiotic and probiotic foods presented in the intervention, including questions about how certain starchy vegetables and grains were cooked and whether they were consumed hot or cold. Prebiotic food questions were added to the existing SDQ within their respective food group sections, and these questions were administered at all four time points to all participants. A new section in SDQ was created for probiotic food and drink intake and this was administered at all time points to the intervention group (for monitoring intervention adherence), but only at baseline (week 26) and follow-up (4 weeks postpartum) in the control group. The two intermediate assessments were not administered to the control group to reduce the possibility of influencing the intake of these probiotic foods. The SDQ has been used to measure dietary change in intervention studies amongst clinical populations (HELFIMED)(Reference Parletta, Zarnowiecki and Cho39) and in children(Reference Strugnell, Millar and Churchill40) and adolescent populations(Reference Hayward, Jacka and Skouteris41) and is in the process of being validated.
Longer-term dietary intake data were collected at baseline and follow-up using the validated Dietary Questionnaire for Epidemiological Studies, Version 2, modified to reflect the previous 3 months’ dietary intake(Reference Giles and Ireland42,Reference Hodge, Patterson and Brown43) . The first Dietary Questionnaire for Epidemiological Studies measured typical dietary intake over gestation weeks 13–26, while the second measured dietary intake from 1 month after the workshop (gestation week 31) until postnatal week 4.
Diet quality scoring
To evaluate short-term diet quality across all four time points, we scored the SDQ in accordance with Parletta et al.(Reference Parletta, Zarnowiecki and Cho39). The total SDQ diet quality score (out of 100) comprises eight components rating dietary adherence to the ADG: ‘wide variety’ of core foods (20 points), ‘adequate intakes of the food groups’ (each worth 10 points), ‘water consumption’ (10 points) and ‘limiting discretionary foods’ (20 points). Maximum scores represent the highest compliance. Our scoring for the dietary variety rule deviated from Parletta et al. (Reference Parletta, Zarnowiecki and Cho39) (worth twenty points, comprising of four points per food group), due to our need for precision when relating dietary intake to the microbial endpoints. Instead of scoring one point if any food within each food sub-category was consumed, the proportion of foods consumed within each food sub-category were calculated and scaled to a total of four points for each of the five food groups. Composite scores for intakes of prebiotic and probiotic foods were created from the SDQ by multiplying the daily intake frequency by a count of the variety of prebiotic or probiotic foods. Standardised Z-scores were subsequently applied to the prebiotic and probiotic intake scores.
Longer-term diet quality was evaluated with the Dietary Guideline Index (DGI-13)(Reference Thorpe, Milte and Crawford44) adapted for pregnant women(16) using Dietary Questionnaire for Epidemiological Studies data. The DGI-total score (out of 130) comprises thirteen component scores, each evaluating an ADG (e.g., ‘wide dietary variety’ (ten points), ‘adequate intakes of the five food groups’ (each worth ten points) and ‘water consumption’ (ten points), ‘moderating unsaturated fat’ (ten points) and ‘limiting intakes of discretionary foods, saturated fats, salt, sugar and alcohol’(each worth ten points))(Reference Thorpe, Milte and Crawford44). The maximum total score is 130, representing highest compliance. Component scores were calculated as outlined in online Supplementary Appendix 1.
Study outcomes and measures
The main outcome was a between-group difference in short-term (previous 2 weeks dietary intake using the SDQ) total diet quality over the perinatal period (gestation week 26 to 4 weeks after birth). Secondary outcomes included between-group differences in short-term prenatal (gestation week 26–36) and perinatal measures of total diet quality, dietary variety, prebiotic intakes, probiotic intakes and discretionary foods, and longer-term (previous 3 months intake using the DGI-13) perinatal measures of diet quality, dietary variety, fibre, energy, saturated fat and discretionary food intakes. These study outcomes were specified a priori(Reference Dawson, Craig and Clarke24).
Measures of short-term and longer-term total diet quality were used to evaluate the hypothesis that the intervention would improve total diet quality compared with the control group. Short-term and longer-term dietary variety components of the SDQ and DGI-13 were used to evaluate whether the intervention increased the variety of core foods consumed. Z-score standardised prebiotic and probiotic food intake scores (representing intake and variety of prebiotic and probiotic foods) were used to evaluate whether the intervention increased intakes of these foods compared with the control group. Dietary Questionnaire for Epidemiological Studies-reported fibre (g/d), energy (kJ/d) and saturated fat (kJ/d) intakes were used to determine whether the intervention increased fibre and/or reduced total energy and/or saturated fat intakes. SDQ and DGI-13 component scores for discretionary food intakes were used to determine whether the intervention reduced intakes of discretionary food. To determine whether dietary change was sustained before and after birth, time trends for the SDQ short-term measures were evaluated using a prenatal assessment from baseline to gestation week 36, and a postnatal assessment from baseline to 4 weeks after birth.
Feasibility and participation were evaluated as secondary outcomes using rates of attendance, completion and engagement.
Exploratory analysis included comparing intakes of carbohydrate (kJ/d), protein (kJ/d) and fat (kJ/d) between the two groups.
Statistical analysis
Generalised estimating equation models for continuous outcomes were used to estimate the intervention effects on each outcome measure. An exchangeable correlation structure was used to account for within-individual autocorrelation, and a robust sandwich estimation approach was used for model s e estimation. The generalised estimating equation models contained nominal factors of group allocation and follow-up time points and the two-way interaction between group allocation and time point. The between-group differential change from baseline at each time point was reported as the intervention effect using the generalised estimating equation models’ two-way interaction estimation between-group allocation and time point. For the short-term dietary measures, two sets of analyses were conducted: one included all prenatal assessments (weeks 26, 31 and 36), to determine whether the changes were sustained throughout pregnancy, and another with all four assessments, to determine whether changes were sustained through to the postnatal period. For the generalised estimating equation models, the two-way interaction overall P-value was calculated using χ 2 goodness of fit to test the intervention effect over follow-ups (i.e., comparing a model with the two-way interaction with a simpler model without interaction). Cohen’s d effect sizes for between-group differential changes were reported. No adjustment for multiple testing was performed as the primary hypotheses were all specified a priori(Reference Dawson, Craig and Clarke24). All tests were two-sided with statistical significance considered at P-value < 0·05. Per protocol results are presented in accordance with the CONSORT 2017 guidelines for intervention reporting(Reference Boutron, Altman and Moher45).
Changes to methods after commencement
To assist recruitment, an ethics amendment included a series of new messages for social media and print form. Messages mentioned the ‘gut bacteria’ as a method of capturing public interest; however, the study’s interest in the gut microbiota was already disclosed in the original informed consent statement within the explanation of how the stool samples would be used. Additional community-based locations for distributing recruitment material were added, such as physiotherapists and prenatal yoga centres. As a gesture of appreciation for participating, a raffle for an iPad to be drawn at study closure was introduced and participants were invited to attend an optional results seminar.
Results
Three hundred and seventy-two women visited the online eligibility-screening questionnaire (Fig. 2). Seventy-five women were eligible and forty-five consented, enrolled and completed baseline data and sample collection. The first participant was enrolled in July 2016 and the last was enrolled in March 2017; the final postnatal visit was conducted in October 2017. Due to premature delivery, one participant from the intervention group withdrew after randomisation but prior to biological sample collection and intervention initiation. At follow-up, forty-three participants completed data collection, and forty-four completed sample collection (Fig. 2). There was an average delay of 12 d (range 1–25) between randomisation and initiating the intervention. The intervention workshop was offered 16 times. The intervention followed a script, and neither the content nor procedures were changed during delivery. The trial was not stopped early.
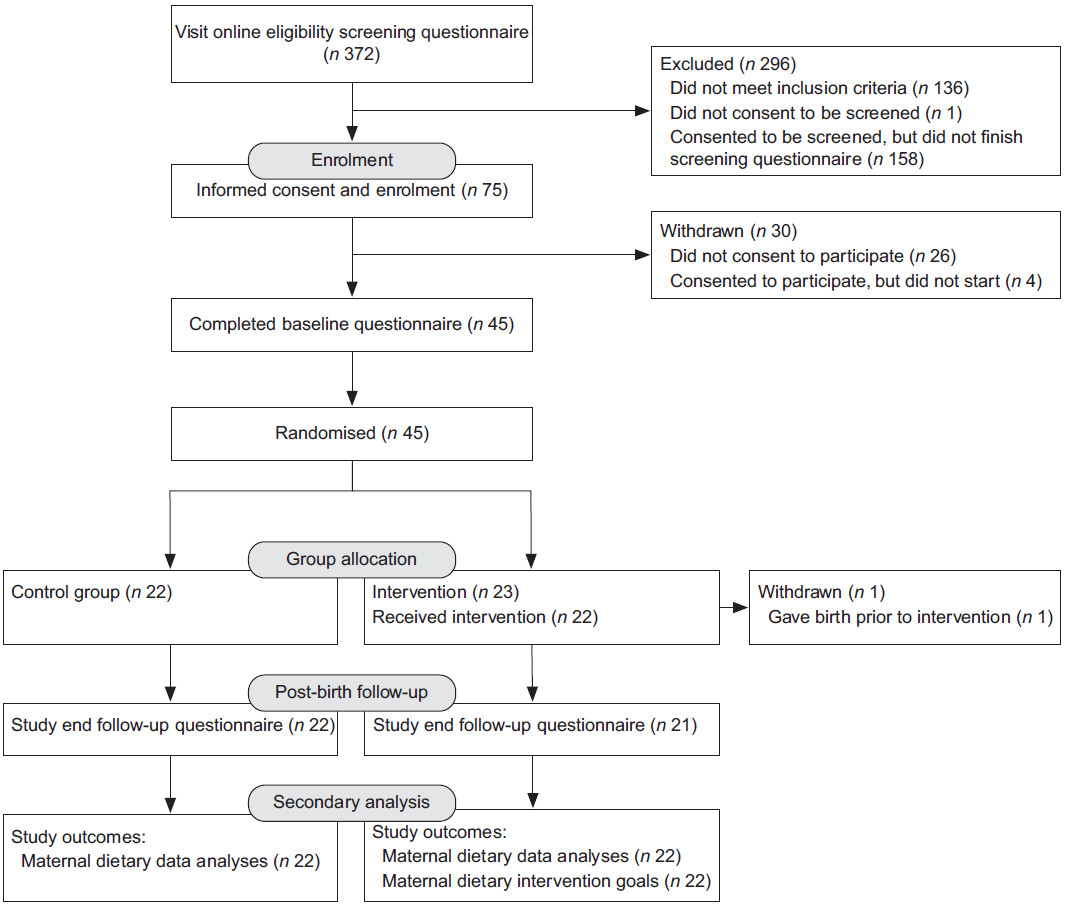
Fig. 2 CONSORT flow chart showing flow of participants through the trial(Reference Boutron, Altman and Moher45)
Participant characteristics
Participant characteristics were similar between groups, with no imbalances in characteristics at baseline (Table 1). The majority of women were born in Australia (72 %) and aged between 30 and 35 years, generally higher educated and in full-time work. The majority of participants were first-time parents. No one individual met all of the thirteen DGI-13 components(Reference Thorpe, Milte and Crawford44) nor met the ADG guidelines for the five food groups(16).
Table 1 Baseline demographics
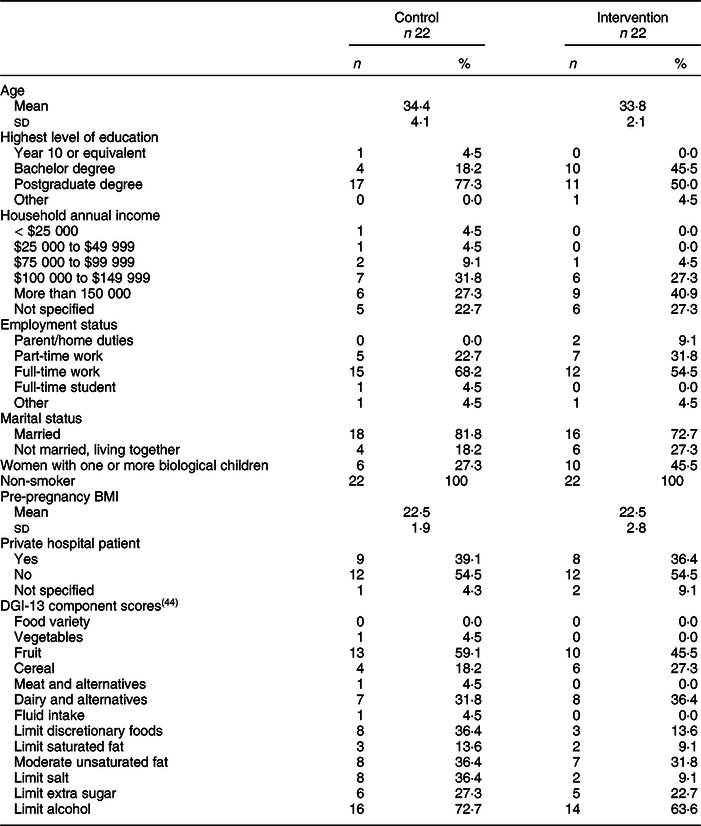
DGI-13 measures diet quality (adherence to the ADG) for the previous 3 months dietary intake.
Dietary change assessment
Dietary adherence to the Australian Dietary Guidelines
Prenatal assessment
Short-term total adherence to the ADG significantly improved in the intervention group from week 26 to weeks 31 and 36 weeks (Table 2, Fig. 3a). Prior to birth, at week 36, the differential change (intervention effect) from baseline between intervention and control group for total adherence to the ADG had increased by 5·66 points and the effect size was large.
Table 2 Between-group change dietary intake over time
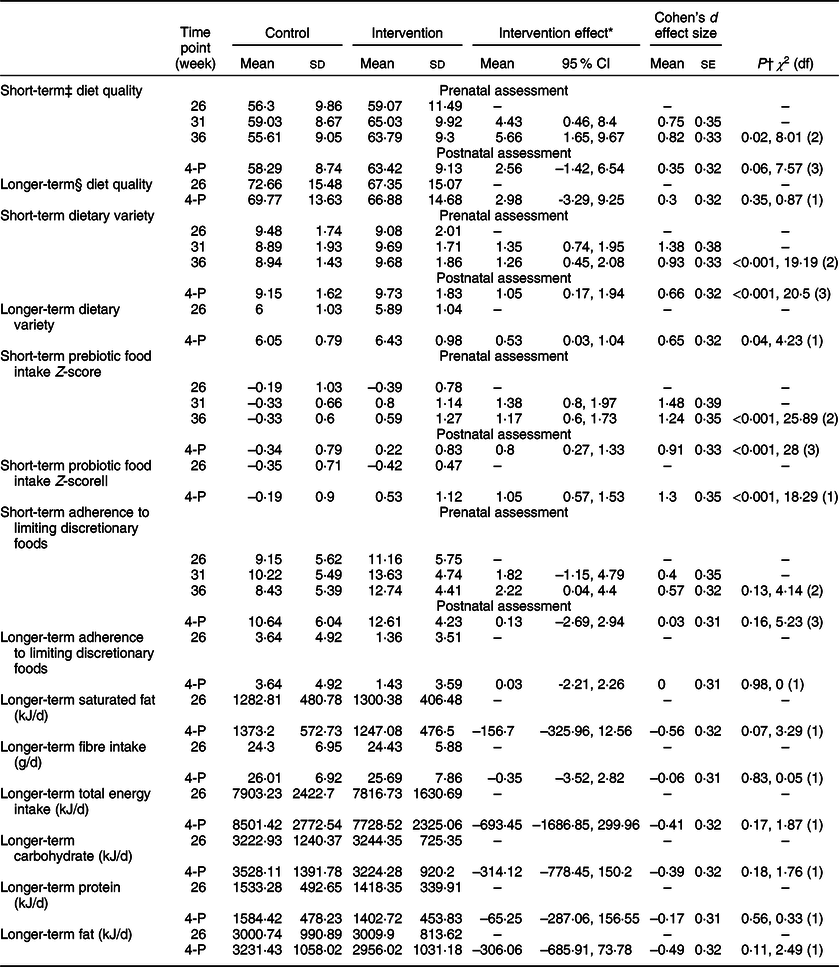
4-P, postnatal week 4.
* From two-way interaction between group allocation and time point.
† Between-group differential change from baseline at follow-ups estimated from two-way interaction between group allocation and time point from GEE model.
‡ Short-term measures represent intake over previous 2 weeks using SDQ FFQ.
§ Longer term is previous 3 months using DQES FFQ.
|| Probiotic intake was collected for the intervention group at all four time points, and at week 26 and 4 weeks postpartum for the control group.
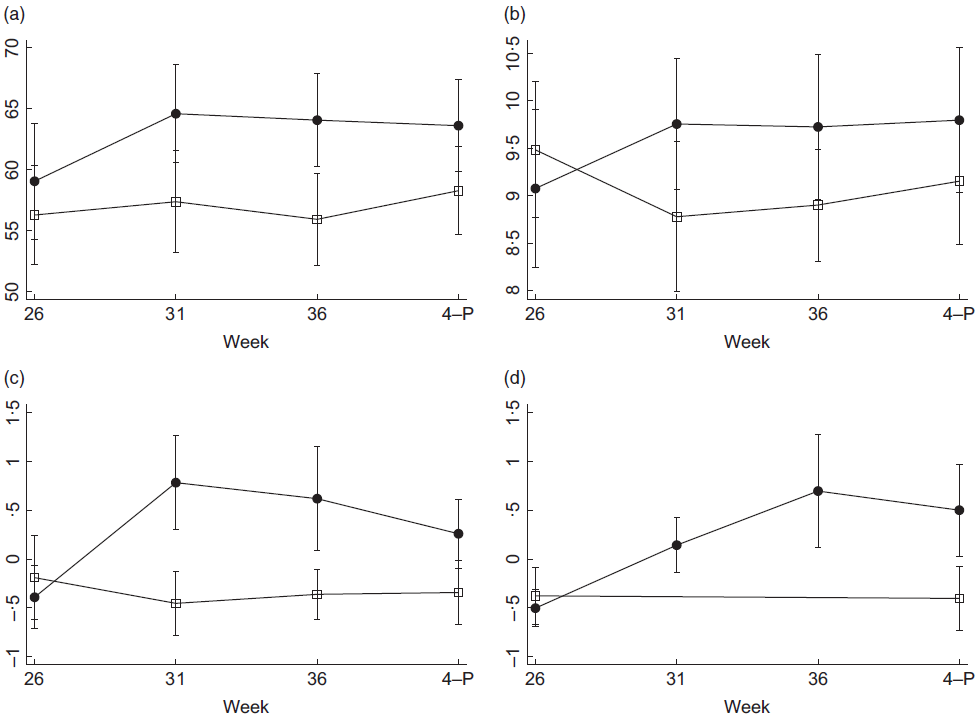
Fig. 3 Margin plots of means and 95 % CI trend across the four dietary assessment time points in the control and intervention groups. 4-P, postnatal week 4. (a) Short-term adherence to the ADG, units are SDQ-total score, where 100 is the highest diet quality. (b) Short-term dietary variety, units are SDQ diet variety score, 20 is the highest dietary variety. (c) Short-term prebiotic intake, units are Z-scores representing frequency of consumption and prebiotic variety. (d) Short-term probiotic food intake, units are Z-scores representing frequency of consumption and probiotic variety. Probiotic intake was collected for the intervention group at all time points, and at gestation week 26, and 4 weeks postnatal for the control group. ![]() , control group;
, control group; ![]() , intervention group
, intervention group
Postnatal assessment
At the 4 week postnatal assessment, there was no longer evidence that short-term adherence to the ADG differed between groups. Similarly, there was no between-group difference for the longer-term measure of adherence to the ADG from week 26 to postnatal week 4 (Table 2).
Dietary variety
Prenatal assessment
During the prenatal assessments, short-term adherence to the ADG ‘Eat a wide variety of nutritious food from the five food groups every day’(16) target was significantly improved from baseline for the intervention group compared with the control group with a large effect size (Table 2, Fig. 3b).
Postnatal assessment
Improvement in short-term dietary variety for the intervention group was also evident at the postnatal assessment. The longer-term measure of dietary variety was also significantly increased in the intervention group compared with the control group from baseline to postnatal week 4 (Table 2).
Intake of prebiotic-containing foods
Prenatal assessment
Short-term intakes of prebiotic foods significantly increased from baseline for the intervention group compared with the control group with a large effect size (Table 2, Fig. 3c).
Postnatal assessment
The significant improvement in short-term prebiotic food intake in the intervention group was maintained through to the postnatal assessment, although the intervention effect was smaller after birth (Table 2).
Intake of probiotic foods
Prenatal assessment
Probiotic intakes increased in the intervention group, with highest levels of consumption evident prior to birth (Fig. 3d).
Intakes of discretionary foods
There were no between-group differences in adherence to guideline 3 ‘limit intake of foods containing saturated fats, added salt, added sugars and alcohol’(16) for the short- or longer-term measures, either pre-or postnatally (Table 2).
Longer-term intakes of energy, macronutrients and fibre
In further exploratory analyses, there were no between-group differences for longer-term intakes of energy, saturated fat, carbohydrate, protein, fat or dietary fibre over the whole study period (Table 2).
Description of participant-selected dietary goals
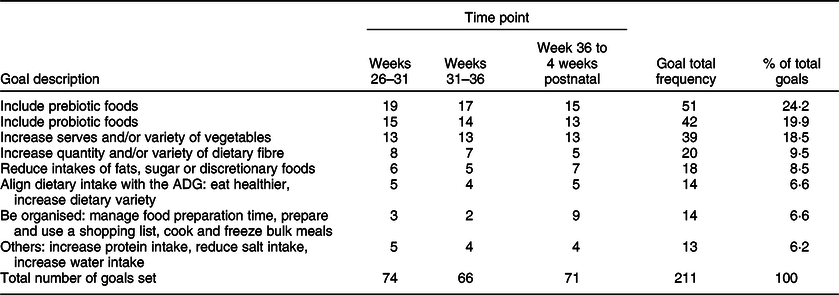
Intervention group participation and engagement were high. All participants attended the workshop and set individual Specific, Measurable, Action oriented, Realistic and Time-bound dietary goals (Appendix 2, see online supplementary material, Supplemental Table 1). Participant dietary goals remained fairly consistent over time (Table 3). Participants set between one to six goals (mean = 3). Increased consumption of prebiotic foods, probiotic foods and vegetables was the most common goals set across all time points. After week 36, there was a greater focus on setting goals for being organised around groceries and meal preparation.
Table 3 Frequency of prospectively set dietary goals over the intervention period
Description of participant-reported dietary advice given by their healthcare providers
Twenty-six women (59 %) reported receiving dietary advice from their healthcare provider. Women in the intervention group reported receiving dietary advice during their pregnancy more frequently than the control group (Appendix 3, see online supplementary material, Supplemental Table 1). The most common messages were related to food safety to minimise listeria risk (Appendix 3, see online supplementary material, Supplemental Table 2). Alcohol avoidance was the second most frequently reported message received. Detailed dietary advice around how to achieve a quality diet was seldom reported.
Discussion
The current study reports on dietary change occurring in response to a dietary intervention targeting the gut microbiota. Dietary intakes significantly improved owing to the intervention for four of the eight main dietary outcomes. Most importantly, the intervention increased adherence to the ADG (a measure of diet quality); however, this was only sustained throughout the prenatal period. Throughout the whole perinatal period (gestation week 26 to 4 weeks postpartum), the intervention increased both short-term and longer-term measures of dietary variety and increased intakes of prebiotic- and probiotic-containing foods. The effect sizes for these changes are generally considered large in accordance with guidelines for interpreting Cohen’s d (Reference Sawilowsky46). The improvement to diet quality was characterised by higher dietary variety, specifically prebiotic and probiotic-containing foods in the intervention group, such as garlic, nuts, rye bread, sourdough bread and yogurt containing cultures.
Our results show feasibility and potential for using dietary intervention focusing on ‘eating for the gut microbiota’ as a mechanism for dietary improvement throughout the perinatal period. The main effects align with the principles for ‘eating for gut microbiota’, as did participant intent, with the most frequently set goals being to increase intakes of prebiotic- (24·2 %) and probiotic-containing foods (19·9 %) and to increase dietary variety (18·5 %). It is plausible that these changes will influence microbial composition due to increased prenatal diet quality(Reference Maskarinec, Hullar and Monroe18,Reference Bowyer, Jackson and Pallister19) , increased dietary variety(Reference Claesson, Jeffery and Conde47) and the symbiotic interactions of prebiotic and probiotic foods(Reference Collins and Gibson48).
Prenatal dietary intakes of saturated fat are above the recommendations across the western world(Reference Blumfield, Hure and MacDonald-Wicks23). In Australia, compliance with the guideline for limiting discretionary foods is consistently low(Reference Hendrie, Golley and Noakes49), with 60 % exceeding three servings per d(Reference Fayet-Moore, McConnell and Cassettari50). There was a lack of intervention effect for limiting discretionary foods. Although this specific guideline was discussed at the workshop, it was seldom the focus of participants’ goal setting. Other explanations for a lack of intervention effect around discretionary food intake may be related to food cravings and nausea(Reference Forbes, Graham and Berglund9).
The intervention effects for short-term dietary improvement measures were highest during the prenatal period but were reduced after birth. This is consistent with other reports of reduced diet quality from the prenatal to postnatal period(Reference Lebrun, Plante and Savard51,Reference Ilmonen, Isolauri and Poussa52) . Similar to barriers reported elsewhere(Reference Tuffery and Scriven53), a lack of time and intense demands of a newborn present barriers to postnatal diet quality. However, we still expected that the intervention effect could persist 2 months after the last support call, as other community-based behaviour change interventions in non-pregnant populations have reported modest effect sizes for dietary change for follow-ups of 6 months or less(Reference Samdal, Eide and Barth54). The use of goal setting and self-monitoring techniques have been associated with positive effects(Reference Samdal, Eide and Barth54). Hence, changes in participant intent may partly explain this postnatal reduction in certain dietary measures. Some participants changed their dietary goals during week 36, placing a greater focus on being organised, including organising groceries or preparing meals for their baby’s arrival.
The intervention was compared against the current level of care in Australia. Only one participant reported receiving detailed advice about how to improve her diet. This is consistent with previous reports of low levels of prenatal dietary advice from healthcare providers(Reference Lee, Belski and Radcliffe5,Reference Lucas, Charlton and Yeatman6) . Food-safety advice was reported as the most frequent practitioner-provided dietary message; this included avoiding raw fish, deli meats and salads, undercooked eggs and soft cheeses for listeria prevention. Indeed, coupling food-safety messages with detailed dietary advice to ensure diet quality could be helpful, especially as women are increasingly prioritising food safety above nutritional adequacy(Reference Pezdirc, Hure and Blumfield55). For example, if dietary advice to avoid raw fish is extrapolated to all seafood(Reference Forbes, Graham and Berglund9), pregnant women may be at risk of n-3 fatty acid deficiency that is critical for infant brain development(Reference Hibbeln, Davis and Steer56).
The strengths of the current study are its design, a priori hypotheses, the use of two FFQ and the high rates of participant engagement and retention. The primary outcome of the Healthy Parents, Healthy Kids randomised controlled trial study was to evaluate the efficacy of the intervention in altering gut microbiota and this relies on participants learning how to make dietary change. Hence, we focused on educational design and used behaviour change techniques to address and support the underlying drivers of behaviour. If the intervention was poorly delivered, and participants did not learn and if we found no change in the gut microbiota, then this may lead to incorrectly interpreting the effects of poor learning design as a lack of effect for ‘dietary targeting of the gut microbiota’. Importantly, our results provide evidence that participants met the intervention learning outcomes because they went home and made dietary change that focused on supporting the gut. Hence, if there are null results for the main outcome, then these dietary results provide a level of confidence that null results would not be due to poor intervention delivery but rather due to other factors, for example, dietary change of this magnitude lacking any effect on the gut microbiota or perhaps a lack of statistical power.
Limitations of the current study include the small sample size and self-reported dietary intake. The study was powered to detect a between-group difference in the primary outcome, infant gut microbiota diversity. Due to the small sample size, the power to detect changes in the secondary dietary outcome measures was low and only large effect sizes (i.e., larger than 0·8) had ≥80 % power. Therefore, the current study may suffer from type two error, being underpowered to detect true differences in the reported dietary measures. To test the primary outcome, the exclusion criteria needed to ensure homogeneity in participant baseline characteristics, but this limits the external validity of the current study, because, for example, the programme remains untested on women with a BMI over 30, which encompasses approximately 21 % of Australian pregnant women(57). The study sample primarily comprised well-educated, high-income women born in Australia, hence the impact, feasibility, uptake and adherence of the dietary intervention would likely be different in women with diverse or disadvantaged backgrounds or different health characteristics. Although recruitment targets were not met, participation and engagement rates were high, and all but one participant completed the study. The SDQ was modified because there are currently no validated Australian dietary questionnaires that include prebiotic and probiotic foods and drinks. Probiotic foods and drinks are not part of the formal dietary guidelines and are relatively uncommon. Hence, to reduce the possibility of influencing probiotic intake in the control group, these questions were only asked at baseline and follow-up – this was a trade-off between accuracy of the results (comparing two time points rather than four) and biasing the results (by influencing intakes within the control group). The modified SDQ is pending validation; however, the results for prebiotic and probiotic intakes were concordant with support phone call data, providing some evidence to support the validity of the modified SDQ for measuring prebiotic and probiotic intakes. Social desirability bias may have influenced these results, for example, participants may have reported their intakes to over-report foods considered to be ‘healthy’ and under-report foods considered to be ‘unhealthy’ foods. However, we saw no evidence of underreporting discretionary foods. Dietary improvement was greater prior to birth compared to the postnatal period, indicating that the postnatal diet may require additional support.
Further larger randomised controlled trials with diverse samples are needed to confirm whether perinatal diet quality can be improved through dietary interventions targeting the gut microbiota, and, importantly, whether this alters the gut microbiota composition of mothers and infants. Strategies to address diet quality during pregnancy are needed because diet quality is less than optimal during this important period(Reference de Jersey, Nicholson and Callaway3,Reference Malek, Umberger and Makrides4) critical for early programming. Supporting women to eat a varied, high-fibre diet with prebiotic- and probiotic-containing food may improve diet quality and variety and offer additional benefits via compositional changes in the gut microbiota and improvement to gut barrier integrity(Reference Sanders, Merenstein and Reid58). The current study supports the notion that teaching women to ‘eat for their gut microbiota’ may be an efficacious way of motivating and empowering them to increase their short-term diet quality, dietary variety and intakes of prebiotic and probiotic foods.
Acknowledgements
Acknowledgements: The authors thank Associate Professor Adrienne O’Neil for reviewing the final manuscript; Professor Graham Giles of the Cancer Epidemiology Centre of The Cancer Council Victoria for permission to use the Dietary Questionnaire for Epidemiological Studies (Version 2), Melbourne: The Cancer Council Victoria, 1996; the study team: Jessica McArdle, Ivy Craw, Victoria Hobbs, Tiril Borge and Sara Campolonghi; Mrs Jann Parkes for the artwork. We acknowledge support from the Victorian Department of Education and Training. Financial support: The current study was funded by Deakin University’s Food & Mood Centre and received in-kind support from the Murdoch Children’s Research Institute and the Royal Children’s Hospital. Conflict of interest: The authors’ competing interests are unrelated to the current study. APC Microbiome Ireland is a research centre funded by Science Foundation Ireland (SFI), through the Irish Government’s National Development Plan (Grant number 12/RC/2273). G.C. is currently in receipt of research funding from the Irish Health Research Board (Grant number ILP-POR-2017-013) and by the US Airforce Office of Scientific Research (Grant number FA9550-17-1-006). G.C. has previously received funding from the Brain and Behavior Research Institute. APC Microbiome Ireland collaborates with a number of industry partners including Dupont Nutrition Biosciences APS, Cremo SA, Alkermes Inc, 4D Pharma PLC, Mead Johnson Nutrition, Nutricia Danone and Suntory Wellness. G.C. has spoken at meetings sponsored by food and pharmaceutical companies including Janssen Ireland and Probi. This neither influenced nor constrained the content of this publication. M.L.K.T. is a past member of the Nestle Nutrition Institute medical advisory board, past member of Nutricia Global Scientific Advisory Board and has received speaker fees from Nestle Health Sciences, Nutricia, Abbott. F.N.J. has received Grant/Research support from the Brain and Behaviour Research Institute, the National Health and Medical Research Council (NHMRC), Australian Rotary Health, the Geelong Medical Research Foundation, the Ian Potter Foundation, Eli Lilly, Meat and Livestock Australia, Woolworths Limited, The A2 Milk Company, Fernwood Gyms, The Wilson Foundation and The University of Melbourne and has received speakers honoraria from Sanofi-Synthelabo, Janssen Cilag, Servier, Pfizer, Health Ed, Network Nutrition, Angelini Farmaceutica, Eli Lilly and Metagenics. F.N.J. has written two books for commercial publication and has a personal belief that good diet quality is important for mental and brain health. These sources of funding and personal beliefs have neither influenced nor constrained the content of this publication. Authorship: All authors met the criteria for authorship, and all approved this final manuscript. F.N.J. conceived and S.L.D. and F.N.J. designed the study. S.L.D. implemented the study, performed data collection, statistical analyses and drafted this manuscript. F.N.J. and J.C. supervised the conduct of the study. M.M. advised and reviewed the statistical methods and results. F.N.J., J.M.C., G.C., M.L.K.T., M.M. and P.D. provided expert input into the study protocol and provided feedback and revisions on this manuscript. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Royal Children’s Hospital Human Ethics (HREC 35200), and Deakin University Human Ethics Committee (DUHREC 2016-036). Written informed consent was obtained from all participants.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020003511









