Iron deficiency (ID) and anaemia are a global public health problem, especially in sub-Saharan Africa, impacting the health and cognitive potential of young children(Reference Zimmermann and Hurrell1–Reference Cook3). Global estimates show that the highest prevalence of anaemia (47·4 %) is among children under 5 years of age, with the prevalence rising to 64·6 % in Africa(Reference Zimmermann and Hurrell1, Reference McLean, Cogswell and Egli2). The 1999 Kenya national micronutrients survey estimated that 70 % of children under the age of 5 years were likely to be anaemic, while nearly half (43·2 %) had ID(Reference Mwaniki, Omwega and Muniu4). High prevalence of inadequate dietary intake, an immediate determinant of micronutrient deficiencies, of up to 77 % has been reported among Kenyan children(Reference Black, Allen and Bhutta5, Reference Grillenberger6). Plant foods have been found to be the major source of energy, of which cereals contribute ∼62–68 % to total daily energy intake, among Kenyan infants and pre-school children(Reference Grillenberger6–Reference Ndiku, Jaceldo and Sabate9). In addition, maize in the form of soft or stiff maize porridge has been shown to be the major complementary food for children aged 12–23 months, contributing over 50 % of Fe intake(Reference Macharia-Mutie, Brouwer and Mwangi7, Reference Ndiku, Jaceldo and Sabate9).
Food-based approaches, including home fortification with low doses of highly absorbable, low-Fe micronutrient powders (MNP) or dietary diversification, may enhance the probability of adequate nutrient intake(Reference Zimmermann and Hurrell1, Reference Johns and Sthapit10) among children. Consumption of MNP together with food has been found to improve Fe status in children within short intervention periods(Reference Osei, Rosenberg and Houser11–Reference Lundeen, Schueth and Toktobaev13). Less studied but widely consumed wholegrain cereals such as grain amaranth (Amaranthus spp.) may have the potential to improve Fe status among children in areas of chronic food insecurity. Grain amaranth is reported to have two to four times more Fe than wheat, high-quality proteins that are easily digested compared with other cereals and to be a drought-resistant fast-maturing crop able to survive in semi-arid areas(Reference Pedersen, Kalinowski and Eggum14, Reference Ologunde, Morris and Shepard15).
Simulating the probable impact of strategies on the potential target population is an essential step in the choice and development of the appropriate actual intervention. Previous studies have simulated intervention effects of Zn and Fe bio-fortification as well as increased use of animal-source foods to reduce the prevalence of inadequate Zn and Fe intakes among women and young children(Reference Grillenberger6, Reference Arsenault, Yakes and Hossain16, Reference Denova-Gutiérrez, García-Guerra and Flores-Aldana17), but none has attempted to estimate the possible effect on Fe status particularly while using plant foods or MNP. The objective of the present study therefore was to use data on food consumption patterns of 12–23-month-old Kenyan children to simulate the potential effect of enriching maize porridge with grain amaranth flour or MNP on the adequacy of Fe intake and the prevalence of ID.
Methods
Study design and participants
Two cross-sectional surveys were carried out in Migwani Division of Mwingi District, Kenya, purposively selected as it falls in a semi-arid area that experiences food shortage for most of the year. In both surveys, multistage sampling was used. Two locations within Migwani Division, namely Migwani and Nzauni, were randomly chosen for the purpose of these studies. The sampling frame consisted of households with children below 5 years of age, but only one child per household within the defined age was randomly selected so as to get a more representative sample population. Households were selected using the random walk method until the desired sample size was achieved(Reference Gibson and Ferguson18). Informed verbal and written consent was obtained from the principal caregiver/parent. Both studies were approved as part of a larger study by the Kenyatta National Hospital/University of Nairobi Ethical Review Committee and the Ministry of Higher Education, Science and Technology in Kenya. A quantitative food consumption survey was carried out in March 2008 involving 197 children aged 12–23 months. Their characteristics have been described elsewhere(Reference Macharia-Mutie, Brouwer and Mwangi7). Data from that survey were used to simulate the effect of fortification of maize porridge with grain amaranth or MNP on Fe intake. A second survey was done in January 2010 including 263 children aged 12–59 months. From a subsample (n 70; aged 12–23 months) it was estimated whether the simulated effect of the intervention on Fe intake as found in the first survey would translate into a reduction of ID prevalence in this age group assuming a similar food consumption pattern for children in the two surveys.
Anthropometry
Weight measurements were taken with either a Salter spring scale (UNICEF) or a digital bathroom scale (Ashton Meyers® model no. 7752, Dubai, United Arab Emirates). The bathroom scale was only used if a child refused to put on the provided weighing pants or could not fit in them. Weight was measured to the nearest 0·1 kg. The children were in minimum clothing which was not corrected for during analysis. Standing height (>2 years) or recumbent length (<2 years) was measured to the nearest 0·1 cm using height/length wooden boards. All measurements were done twice. If the variation between the two measurements was more than 0·5 kg or 1·0 cm for weight or height/length, respectively, the measurements were done a third time(Reference Cogill19) and the mean value calculated. Age was calculated from the birth date reported on clinic cards or, in rare cases, on parent's/principal caregiver's recall.
Blood collection and analysis
Non-fasting venous blood samples (5 ml) were collected from children by venepuncture and divided into two portions. The first portion sample series were stored in EDTA tubes (Becton-Dickinson®, Temse, Belgium) for determination of Hb concentration, while the second portion sample series were stored in plain tubes without anticoagulant for serum separation. The samples were stored in cool boxes and transported to Migwani Hospital. Hb concentration was determined using a KX-21 haematological analyser (Sysmex Corporation, Kobe, Japan). Separation was done within 6 h and serum was transferred into sterile cryovials, flushed with pure white spot N2 gas and thereafter stored in liquid N2 before further analysis in Nairobi. Serum ferritin (SF) was analysed using enzyme-linked fluorescent assay with a Mini-VIDAS® Immunoanalyser (Biomeriux SA, Johannesburg, South Africa), while C-reactive protein (CRP) was analysed using a NycoCard® Reader II at the Centre for Public Health Research, Nairobi. Serum transferrin receptor (sTfR) was analysed with a Cobas Integra® analyser (Roche Diagnostics, Mannheim, Germany) at the Pathologists Lancet Kenya Ltd, Nairobi. Certified reference kits/cell controls were used for precision determination during analysis. All samples were measured once and the CV was <10 %.
Dietary intake
Food consumption was measured using repeated 24 h recall on two non-consecutive days as described previously(Reference Macharia-Mutie, Brouwer and Mwangi7, Reference Gibson and Ferguson18). The observed intakes were adjusted for day-to-day variation using the US Institute of Medicine adjustment procedure to obtain the estimated usual intake for each individual(20). The full probability approach was used to estimate the prevalence of Fe intake inadequacy assuming an average bioavailability of 5 %(Reference Allen, de Benoist and Dary21). The total amount of Fe absorbed after the simulated intervention was obtained by adding the total estimated absorbable Fe from the different treatments to that absorbed from the usual intake. The total amount of actual and simulated absorbable Fe per child was categorized into probabilities of inadequacy ranging from 0 to 1, where 0 was assigned to absorbed Fe that fell above the 97·5th percentile of requirements and 1 was assigned to absorbed Fe that fell below the 2·5th percentile of requirements(Reference Allen, de Benoist and Dary21). The average of the prevalence of inadequacy in the different categories gave an estimate of the total prevalence of inadequacy in the population. Energy requirement was estimated at 3766 kJ/d (900 kcal/d), the average requirement of a girl or boy aged 1–2 years at moderate physical activity(22).
To describe the dietary pattern, each individual's dietary diversity score (DDS) was calculated using the first-day recall data and a 1 d simple qualitative FFQ asking information on the number of meals and types of foods/ingredients in each meal from the second survey. All ingredients mentioned in the 24 h recall or in the qualitative FFQ were assigned into eleven food groups: (i) cereals roots and tubers; (ii) legumes and nuts; (iii) vitamin A-rich fruits and vegetables; (iv) dark green leafy vegetables; (v) other fruits and vegetables; (vi) dairy; (vii) meat, poultry and fish; (viii) eggs; (ix) organ meats; (x) condiments; and (xi) fats and oils. We did not include the fats and oil group when awarding the scores, or the condiments, since our focus was on Fe intake(23). If a food from a certain food group was consumed, this food group received a score of 1; otherwise a score of 0 was given if no food from that group was consumed. To calculate individual DDS, the scores for each food group were summed; hence the minimum score was ‘0’ and the maximum score was ‘9’. The children were further classified into DDS tertiles: low (≤3 food groups), medium (4–5 food groups) and high dietary diversity (≥6 food groups)(23).
Simulation
A simulation model using the deterministic approach was developed to estimate the expected change in the prevalence of Fe intake inadequacy. It was based on three types of porridges: (i) plain maize porridge with 60 g of maize flour; (ii) amaranth–maize porridge with 80 g of flour at the ratio of 70 % grain amaranth (56 g) and 30 % maize (24 g); and (iii) maize porridge (60 g of maize flour) with added 1 g of MNP containing vitamin A (100 μg retinol equivalents), vitamin D (5 μg), vitamin E (5 mg tocopherol equivalents), phylloquinone (30 μg), thiamin (0·5 mg), riboflavin (0·5 mg), pyridoxine (0·5 mg), folic acid (90 μg), niacin (6 mg), vitamin B12 (0·9 μg), vitamin C (60 mg), Fe (2·5 mg as NaFeEDTA), Zn (2·5 mg), Se (17 μg), Cu (0·34 μg) and iodine (30 μg)(Reference de Pee, Kraemer and van den Briel24, Reference Ndemwa, Klotz and Mwaniki25). The amount of flour required to make the plain maize porridge was lower as the consistency was found to be thicker than for a similar amount of porridge made from grain amaranth and maize at 70:30 ratio(Reference Macharia-Mutie, Van de Wiel and Moreno-Londono26). We also desired to give as much Fe from grain amaranth as possible, and a previous sensory study showed a preference for porridges with a lower amount of amaranth flour but no significant difference between 50:50 and 70:30 amaranth–maize mixtures(Reference Macharia-Mutie, Van de Wiel and Moreno-Londono26). It was assumed that these porridges would be added to the children's diet as an extra meal, as previously estimated energy and Fe intakes for children in the area showed that nutrient adequacy was <70 % for both energy and Fe requirements(Reference Macharia-Mutie, Brouwer and Mwangi7).
Simulations of the effect of the intervention on Fe intake were made using data from the first survey. Assumptions made included: (i) wet weight Fe content of maize is 0·061 g/kg and of grain amaranth is 0·20 g/kg based on own analysis of the flours (CW Macharia-Mutie, AM Mwangi and ID Brouwer, unpublished results); (ii) bioavailability of Fe from maize flour in all of the porridges is 4 %(Reference Cook, Reddv and Burri27), bioavailability of the 70 % grain amaranth flour in the amaranth–maize porridge is 3 %(Reference Ologunde, Morris and Shepard15) and bioavailability of the 2·5 mg Fe in the form of NaFeEDTA is 7 %(Reference Troesch, Egli and Zeder28); and (iii) the intervention would last for 80 d, i.e. 16 weeks of feeding at 5 d/week, as treatment effects may be achieved within this time frame and an even shorter period for MNP(Reference Christofides, Asante and Schauer29). We also assumed that the baseline usual Fe intake for children was the median intake (4·9 mg/d) obtained from the food consumption data. The additional absorbable Fe obtained by simulating the different treatments was added to the assumed median usual absorbed Fe intake of 0·24 mg/d calculated from an overall Fe bioavailability of 5 % based on the regular diet containing enhancers such as ascorbic acid and animal-source foods but in limited amounts, a characteristic found in our previous study(Reference Zimmermann and Hurrell1, Reference Macharia-Mutie, Brouwer and Mwangi7). The prevalence of inadequate Fe intake was then estimated for the three groups using the full probability approach as described earlier(Reference Allen, de Benoist and Dary21).
Results of the second survey were used to estimate the expected change in prevalence of ID, SF concentration and body Fe stores. Assumptions made include: (i) total median Fe requirement for growth and basal losses of 0·46 mg/d for a child aged 1–3 years weighing 13·3 kg, equivalent to 34·6 μg Fe/kg body weight per d(30); and (ii) absorbed Fe in excess of the total median requirement for growth and basal losses would be used in the formation of Fe stores at the end of the 80 d intervention. Total Fe stores after the intervention were calculated assuming that the amaranth-enriched and MNP-fortified porridge will provide an extra 13·5 and 8·3 μg Fe/kg body weight per d, respectively, while the non-fortified maize porridge group would have a deficit of 5·3 μg Fe/kg body weight per d. These values were obtained after deducting the daily median requirement of 34·6 μg Fe/kg body weight per d. Further, a linear relationship between log SF and Fe stores per unit body weight was assumed. The equation: Y = 9380X−11260, where Y (μg Fe/kg body weight) is the total body Fe calculated after the intervention and X is log SF, as described by Hallberg et al.(Reference Hallberg, Hulthen and Garby31), was used to estimate the log SF for every child assuming that he/she received the different treatments. The exponential of log SF was assumed to be the extra SF formed or deficit at the end of the 80 d intervention. These values were then added to the original SF values which had been corrected for acute inflammation (see next section) to give the final SF value for the three different treatments.
Data analysis
Anaemia was defined as Hb < 110 g/l. ID was defined as SF < 12 μg/l(32) or sTfR > 8·3 mg/l (Roche kit specific). Body Fe content was calculated using the formula(33): body Fe (mg/kg) = −[log(sTfR/SF)−2·8229]/0·1207. This was after recalculation of Roche kit sTfR values with the regression equation: sTfR = 1·5 × Roche kit values + 0·35, to transform them to ELISA assay values(Reference Pfeiffer, Cook and Mei34). To adjust SF concentration for those children with elevated CRP (>5 mg/l), a correction factor (0·67) was used to yield an adjusted value representative of a child without acute inflammation(Reference Thurnham, McCabe and Haldar35).
Weight-for-height Z-score (WHZ), weight-for-age Z-score (WAZ) and height-for-age Z-score (HAZ) were calculated with ANTHRO version 3·2·2, using the 2006 WHO growth standards. Stunting, underweight or wasting respectively was defined as WHZ, WAZ or HAZ <−2(Reference de Onis, Garza and Onyango36). Statistical analyses were done using the SPSS statistical software package version 16·0 and Microsoft® Excel 2003. Univariate analysis with planned contrasts was done to estimate treatment effects on SF and body Fe concentration relative to the maize porridge-only group after simulated intervention. The level of significance was set at P < 0·05.
Results
Mean energy intake for the 197 children who participated in the first survey was 2902 kJ/d, with 88 % of the children having an intake below the daily energy requirements for children aged 1−2 years (Table 1). Maize contributed more than half (57 %) of the Fe intake and a third of the energy and protein intakes in the children's diet. Nearly half (48 %) of children had a low DDS and only 2 % had a high DDS (data not shown).
Table 1 Adjusted usual dietary intakeFootnote † distributions among children (n 197) aged 12–23 months in Mwingi District, Kenya, 2008 (first survey)
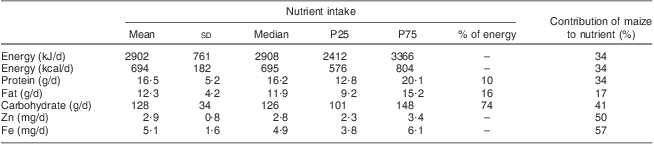
P25, 25th percentile; P75, 75th percentile.
† The estimated nutrient intakes do not account for breast milk intakes as they were not measured.
More than half (60 %) of the children who participated in the second survey were stunted, 49 % were anaemic and 50 % had a low DDS (Table 2). The cumulative prevalence of ID (45·7 %) as indicated by SF and sTfR was indicative of a high prevalence of Fe depletion. Only half of the children with anaemia had Fe-deficiency anaemia (24·3 %). The simulations estimated that amaranth-enriched and MNP-fortified porridges would provide extra body Fe stores of 0·013 and 0·008 mg Fe/kg body weight per d, respectively, while the non-fortified maize porridge group would have a deficit of 0·005 mg Fe/kg body weight per d compared with the absolute median requirement for a child aged 12–23 months (Table 3). The extra Fe would cause a modest increase of Fe stores by 1·1 and 0·7 mg Fe/kg body weight in the amaranth-enriched and MNP-fortified porridge group, respectively, after the intervention. Simulated SF concentration increased by ∼5 μg/l in the amaranth-enriched and MNP-fortified porridge groups compared with baseline. The prevalence of inadequate Fe intake was estimated to decrease to 35 % and 45 % after an assumed consumption of amaranth-enriched maize porridge or maize porridge with MNP, respectively, translating into over 50 % reduction. Based on the second survey the increased estimated SF in the amaranth and MNP groups reduced the prevalence of ID in the two groups to 27 %, but not in the maize porridge-only group. Consumption of amaranth-enriched maize porridge or MNP-fortified maize porridge was estimated to cause a similar and significant improvement in SF concentration (1·82 (95 % CI 1·42, 2·34) μg/l and 1·80 (95 % CI 1·40, 2·31) μg/l, respectively) compared with the maize porridge-only group (P < 0·005).
Table 2 Characteristics of children (n 70) aged 12–23 months in Mwingi District, Kenya, 2010 (second survey)

WAZ, weight-for-age Z-score; HAZ, height-for-age Z-score; WHZ, weight-for-height Z-score; sTfR, serum transferrin receptor; SF, serum ferritin; CRP, C-reactive protein.
†Underweight, stunting and wasting defined respectively as WAZ, HAZ and WHZ <–2.
‡Geometric mean (95 % CI).
§Adjusted for elevated CRP > 5 mg/l(Reference Thurnham, McCabe and Haldar35).
∥Defined as concurrent anaemia and Fe deficiency (SF < 12 μg/l or sTfR > 8·3 mg/l).
Table 3 Simulated impact of amaranth-enriched and MNP-fortified maize porridges on iron intake and status in children aged 12–23 months, Mwingi District, Kenya,

MNP, micronutrient powder; P25, 25th percentile; P75, 75th percentile; SF, serum ferritin.
Data are median (P25, P75) unless otherwise indicated.
*Significant difference compared with maize porridge-only group: P < 0·05.
†This is the existing situation before simulation and the data form the basis of the simulation by assuming that these children did not receive any intervention. Bioavailability of Fe from the diet considered to be 5 %(Reference Zimmermann and Hurrell1).
‡MM: plain maize porridge with 60 g of maize flour (Fe content = 0·061 g/kg wet weight) with 4 % bioavailability(Reference Cook, Reddv and Burri27).
§MA: amaranth–maize porridge with 56 g of grain amaranth flour (Fe content = 0·20 g/kg wet weight) and 24 g of maize flour. Assumed bioavailability of amaranth is 3 %(Reference Ologunde, Morris and Shepard15) and 4 % bioavailability for Fe in maize(Reference Cook, Reddv and Burri27).
∥MM+: plain maize porridge (60 g of maize flour) with added 2·5 mg of Fe in the form of NaFeEDTA(Reference de Pee, Kraemer and van den Briel24). Assumed bioavailability of Fe in the form of NaFeEDTA is 7 %(Reference Troesch, Egli and Zeder28) and 4 % bioavailability for Fe in maize(Reference Cook, Reddv and Burri27).
¶This is the usual Fe intake from other foods (4·9 mg/d) plus the added Fe intake from the intervention. Usual Fe intake has been adjusted for day-to-day variation.
**For the interventions we add the extra absorbable Fe calculated using the intervention respective bioavailability (see footnotes ‡, § and ∥ above) to the usual bioavailable Fe intake calculated using 5 % bioavailability.
††Median absolute requirements for absorbed Fe estimated at 0·46 mg/d for a child aged 1–3 years; equivalent to 34·6 μg Fe/kg body weight per d for a child aged 1–3 years weighing 13·3 kg(30).
‡‡Simulation based on median absorbable Fe intake from the first survey food consumption study and addition of the simulated extra intake of absorbable Fe assuming an 80 d intervention.
§§Body Fe stores after the 80 d intervention assuming that the amaranth-enriched and MNP-fortified porridge will provide an extra 0·013 and 0·008 mg Fe/kg body weight per d, respectively, while the maize porridge group would have a deficit of 0·005 mg Fe/kg body weight per d obtained after deducting the daily median requirement of 0·0346 mg Fe/kg body weight per d.
∥∥SF from intervention calculated using the Hallberg equation: Y = 9380X−11260, where Y is Fe stores expressed as μg Fe/kg body weight and X is log SF(Reference Hallberg, Hulthen and Garby31).
¶¶Data are geometric mean (95 % CI).
***Based on the simulated SF concentration, defined as SF < 12 μg/l. Baseline prevalence is the actual prevalence as measured in the second survey based on biochemical parameters.
Discussion
Simulated results showed that enriching maize porridge with grain amaranth or MNP may comparably reduce the prevalence of inadequate Fe intake and ID among children aged 12–23 months in Mwingi District, Kenya. Giving extra maize porridge only will improve energy intake but not Fe status of the children. The food consumption data show a deficit in energy and Fe intakes. Prevalence of stunting was higher than the national prevalence(37), which is reflective of a process of failure to reach linear growth potential. This could be a result of suboptimal health and/or food and feeding conditions combined with poor household socio-economic conditions indicating the high risk of ID(Reference Black, Allen and Bhutta5). The anaemia prevalence was high in the present study and half of the children with anaemia had Fe-deficiency anaemia (24·3 %). It is likely that other causes such as presence of illnesses and parasite infestations, inaccessibility of health-care services, genetic determinants and environmental factors(Reference Zimmermann and Hurrell1, Reference Zimmermann, Harrington and Villalpando38) contributed to the high prevalence of anaemia.
The present study had several methodological strengths and limitations. The two surveys were conducted in the same area and the dietary diversity was found to be similar in both the first and second survey, with a low intake of Fe-rich food by this age group. This similarity must be interpreted with caution however, as intake data in the two surveys had been collected using different methods. Furthermore, the sample size calculations in the second survey were powered for data on children aged 12–59 months, while we analysed data from only a sub-sample of children aged 12–23 months in the present study. Nevertheless, we assumed that the time interval and methodology would not significantly affect our interpretation of results in the simulation.
A quantitative repeated 24 h recall which has been shown to give acceptable estimates of total intake in rural Kenya was used to collect information on food consumption(Reference Murphy, Gewa and Liang39). The method was considered appropriate since it indicates the habitual intake and gives a valid measure of food intake in a population(Reference Gibson40). The full probability approach was used to estimate the prevalence of inadequate Fe intake in a given intake range, as the Fe requirements of children are not normally distributed(Reference Allen, de Benoist and Dary21). The observed Fe intake was adjusted for day-to-day variation, which is appropriate for use in analyses of the prevalence of inadequate or excess intakes in the group although it does not address problems of systematic bias due to under-reporting of intakes on the recall days or errors in estimation of nutrient intakes(20). A qualitative 24 h recall used in the second survey was preferred above list recall as it allows respondents to freely recall all meals and snacks eaten both at home and outside by the child, thus reducing over- or under-reporting of foods consumed(Reference Kennedy, Pedro and Seghieri41). We did not collect quantitative breast milk intakes and so our estimates of daily Fe intake for breast-feeding children may be slightly underestimated. In infants fed on breast milk however, over 90 % of their Fe requirements should be met by complementary feeding as early as during the second 6 months of life in order to prevent ID. Although breast milk is known to have a high bioavailability of up to 34 %(Reference Dube, Schwartz and Mueller42), its Fe concentration has also been shown to decrease drastically by the fifth month of lactation by up to 0·3 mg/l(Reference Siimes, Vuori and Kuitunen43). Considering that the age of our study children was above 12 months and a reported minimal breast-feeding, we therefore suppose that the fraction of Fe from breast milk is even lower for our study group and the impact on the estimated Fe intakes and status is minimal.
The projected values for prevalence of inadequate Fe intake could be attributed to the assumptions that were made. We used a deterministic approach and the model may result in overestimations of the intake distribution as it does not take into account the uncertainties or variability in concentration or consumption data(Reference Verkaik-Kloosterman, van't Veer and Ocke44). Nevertheless, this model was thought to adequately estimate the expected impact of an intervention to improve dietary Fe intake in this population. The equation used to estimate SF concentrations has been suggested to effectively predict the effects of Fe fortification under different conditions with respect to dietary properties and Fe requirements, as it does not overestimate the amount of Fe stores(Reference Hallberg, Hulthen and Garby31). Evidence has shown that Fe stores tend to be stable or have small changes once the maximum requirement has been reached since the body auto-regulates further Fe absorption(Reference Hallberg, Hulthen and Garby31). The simulated intervention period in the present study was within a time frame that Fe stores would be expected to improve in the majority of the children, as their baseline Fe stores were not high.
We took a conservative bioavailability estimate of 3–4 % for both the amaranth-enriched and plain maize porridge, although the range of bioavailability of non-haem Fe has been reported as 4–12 % in the literature(Reference Zimmermann and Hurrell1). Fe absorption is reduced by high intakes of phytate, which is known to inhibit absorption by forming insoluble Fe–phytate complexes, and the effect has been shown to be dose dependent, starting at very low concentrations of 2–10 mg phytate/meal(Reference Hurrell and Egli45). Our preliminary results (CW Macharia-Mutie, AM Mwangi and ID Brouwer, unpublished results) indicate that while grain amaranth contains high amounts of Fe, the phytic acid content is also high (9·6 g/kg), resulting in a phytate:Fe molar ratio of 3:1 which is still within the inhibitory range(Reference Hurrell and Egli45). The high phytic acid concentration may therefore negatively affect Fe absorption even when the Fe intakes are increased, especially in the amaranth-enriched porridge group.
From an assumed linear relationship between Fe intake and status our simulated effects may suggest considerable improvement. However, the effects of absorbable Fe in real-life interventions may vary due to other factors that may be either internal/physiological or external(Reference Zimmermann, Harrington and Villalpando38). The presence of other micronutrients in the MNP which were not accounted for in the simulation and are thought to have a role in anaemia, particularly vitamin A(Reference Semba and Bloem46), vitamin B12 and folic acid(Reference Fishman, Christian and West47), may also contribute to an increased effect in the MNP group. In the present study we simulated an increment in SF concentration of 4·8 μg/l in the MNP group and 5·0 μg/l in the amaranth group. A study in South Africa among primary-school children showed an increment in SF concentration of 3·1 μg/l between baseline and post-intervention in the treatment group fed with MNP-fortified porridge after 6 months of intervention(Reference Troesch, van Stuijvenberg and Smuts48), while an increment of >10 μg/l in SF concentration was observed in another study in India(Reference Varma, Das and Sankar12). These varied observations in relation to the simulation could be due to different Fe requirements according to age group and the presence of infections and de-worming, thus reducing Fe loss due to parasitic infections(Reference Zimmermann and Hurrell1).
Conclusions
Our results suggest that addition of grain amaranth or MNP to existing maize meal porridges could increase Fe intake adequacy and decrease ID among children in rural Kenya. Validation of such simulations against actual impact on Fe status indicators is needed. However, when resources to do extensive intervention studies are limited, such theoretical simulations may guide the choice of appropriate interventions in the case of policy decisions.
Acknowledgements
Sources of funding: This work was supported by Clive West Micronutrient Fund, Foundation Van Dam Nutrition Plan and the Nestlé Foundation. Conflicts of interest: The authors declare to have no competing interests. Authors’ contributions: C.W.M.-M., A.M.M. and I.D.B. designed the research; C.W.M.-M., A.M.O. and J.M.M conducted the research; C.W.M.-M. and I.D.B refined the methodologies; C.W.M.-M. wrote the first draft of the manuscript; all authors read and approved the final manuscript. Acknowledgements: The authors thank the children and parents/caregivers who willingly participated in the study, and also the study field assistants who diligently collected the data.





