The intake of salt (NaCl) with food is a matter of public health concern worldwide because a high salt intake is associated with hypertension, which is recognized as the leading cause of CVD and stroke(Reference Stamler1–Reference Cook, Cutler, Obarzanek, Buring, Rexrode, Kumanyika, Appel and Whelton4). Health authorities and scientific societies recommend that salt intake should be at most 5–6 g/d (85–102 mmol Na/d) whether being hypertensive or not(Reference Fodor, Whitmore, Leenen and Larochelle5–8). In Sweden, data based on dietary calculations indicate that the salt intake of middle-aged men and women is substantially greater, but reliable measurements of the actual intake are lacking(Reference Becker and Pearson9). Moreover, there are very few data on the salt intake in younger age groups. In a rapidly changing society where processed and often salty fast food is served in canteens, worksite restaurants and schools, new and reliable data on salt intake are therefore urgently needed.
We have investigated the salt intake in a group of young men, using the 24 h urinary excretion of Na as the measure of salt intake. We also measured the excretion of K and calculated the total energy intake from the diet and Na intake from various foods.
Subjects and methods
Subjects
The subjects were participants in an epidemiological study of environmental and genetic factors of importance for fat and bone mass, the Gothenburg Obesity and Osteoporosis Determinants (GOOD) study(Reference Lorentzon, Mellström and Ohlsson10, Reference Klingberg, Hallenberg, Lorentzon, Mellström, Ohlsson and Hulthén11). Study subjects were randomly identified using national population registers, contacted by telephone, and asked to participate in the study. In total 1068 Caucasian men, mean age 18·9 (sd 0·6) years, from the greater Gothenburg area, were included. To be included in the GOOD study, subjects had to be >18 and <20 years of age and willing to participate in the study. There were no other exclusion criteria. A standardized questionnaire was used to collect information about current and previous medication, amount of weight-bearing physical activity (hours per week, duration in years), smoking and history of fracture, as well as fracture history in the subjects’ families. Data were collected between February 2005 and December 2005. The GOOD study was approved by the ethics committee at the University of Gothenburg. Written and oral informed consent was obtained from all study participants.
A subset of eighty-six randomly selected subjects in the GOOD study was investigated for renal Na and K excretion and dietary habitual intake. Every tenth subject involved in the study was selected for this sub-sample.
Methods
All subjects collected one 24 h urine sample. The urine samples were collected in acid-washed containers. The start (after the first morning urine) and the end (right after the morning urine the next day) of the urine collection, as well as any lost specimens, were reported. We used the p-aminobenzoic acid (PABA) check method to verify that the urine collection was carried out properly. Three 80 mg tablets of PABA were ingested with morning, midday and evening meals for the evaluation of urine collection completeness(Reference Bingham and Cummings12). A collection containing less than 85 % of PABA was excluded, being regarded as incomplete. The volume of the 24 h collection was determined and a 10 ml aliquot was stored at −20°C until analysis. Na and K in urine were determined by flame atomic absorption spectrophotometry (Perkin–Elmer model 5000; Norwalk, CT, USA) according to routine clinical laboratory procedures, and the Na:K ratio in the 24 h urine samples was calculated.
All data on diet evaluation were collected at Sahlgrenska University Hospital. The dietary assessment consisted of a detailed FFQ and an individual interview by a trained nutritionist(Reference Klingberg, Hallenberg, Lorentzon, Mellström, Ohlsson and Hulthén11). Each respondent was asked about customary food intake. Portion sizes of foods were described in terms of household measures, standard weights of food items and portion photographs of known weights. This dietary assessment method was previously validated against the doubly labelled water technique and the diet history was found to be valid to assess habitual energy intake(Reference Sjöberg, Slinde, Arvidsson, Ellegård, Gramatkovski, Hallberg and Hulthén13). Dietary Na and K intake was measured as the 24 h urinary Na and K excretion.
Height and weight were measured with subjects in underwear, without shoes, using standardized equipment. The criterion validation values were below 1 % for these measurements.
Blood pressure was measured in the recumbent position after a 10 min rest, using the Omron (Santa Clara, CA, USA) 705 CP blood pressure monitor, to the nearest mmHg.
Calculations and statistical analysis
BMI was calculated from weight (kg) divided by the square of height (m2).
Nutrient calculations were performed with the Diet 32 software package (Aivo, Solna, Sweden), which utilizes the Swedish Food Data Base (updated 2001) of the Swedish National Food Administration(14). Snack foods included sweets, nuts, potato crisps, popcorn and cheese doodles. Fast foods included hamburgers, kebab, pizza, hot dogs and chips.
The ratio of energy intake to BMR was used to define under-reporters, acceptable reporters and under-reporters among the participants in the GOOD study(Reference Klingberg, Hallenberg, Lorentzon, Mellström, Ohlsson and Hulthén11). The energy intake and food choices of the acceptable reporters are used in the present analysis.
Data are expressed as means and standard deviations. Differences between measurements and groups were analysed with the use of the Mann–Whitney U test. A two-tailed P value of <0·05 was considered statistically significant. All statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS for Windows) statistical software package version 11·5 (SPSS Inc., Chicago, IL, USA).
Results
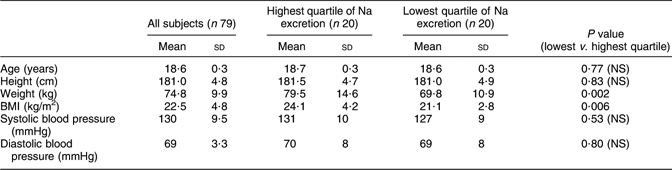
Seven urine collections were excluded because of insufficient recovery (see Methods). This left seventy-nine collections acceptable for the study. Demographic data on this group of subjects are given in Table 1.
Table 1 Anthropometry and blood pressure in a random sample of seventy-nine young men in Sweden
Salt excretion
The percentage distributions in the 24 h urinary Na and K excretions and the Na:K ratio are given in Fig. 1. There was a considerable variation. Na excretion varied from 60 to 420 mmol/24 h, K from 30 to 170 mmol/24 h and the Na:K ratio from 0·75 to 6·75.
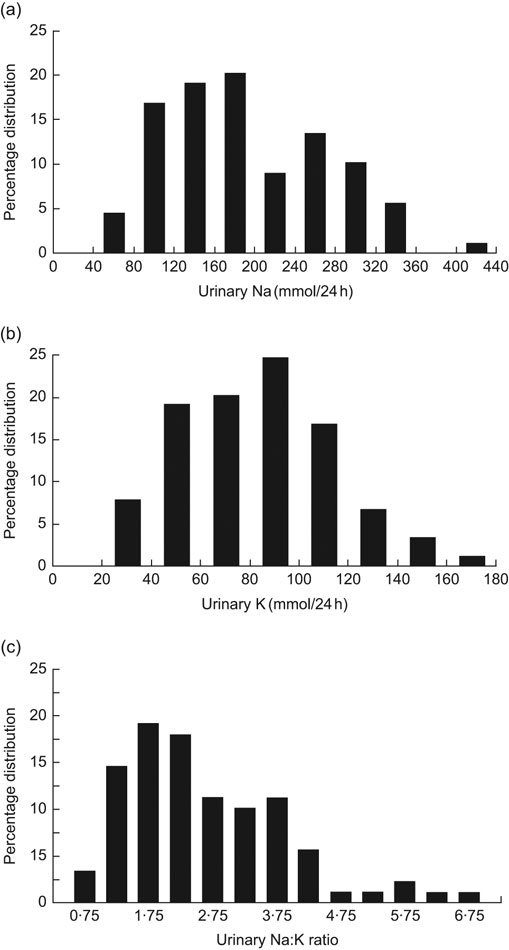
Fig. 1 Distribution of (a) urinary sodium excretion (mmol/24 h), urinary potassium excretion (mmol/24 h) and (c) urinary sodium:potassium ratio in young Swedish men (n 79)
Mean values for anthropometric and blood pressure measurements were calculated for the whole group and for subjects in the highest and lowest quartiles of Na excretion. Data in Table 1 show that subjects in the highest quartile were heavier and had higher BMI than subjects in the lowest quartile, but that systolic and diastolic blood pressure did not differ.
Table 2 gives the mean values of salt excretion. Na excretion in the whole group was 198 mmol/24 h, which corresponds to 11·5 g NaCl; in the highest and lowest quartiles, Na excretion was 297 and 100 mmol/24 h, respectively, corresponding to 17·2 and 5·8 g NaCl.
Table 2 Urinary excretion of sodium and potassium in a random sample of seventy-nine young men in Sweden
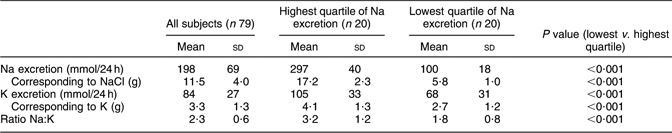
Mean K excretion was 84 mmol/24 h in the whole group, corresponding to 3·3 g K; in the highest and lowest Na excretion quartiles, K excretion was 105 and 68 mmol/24 h, respectively, corresponding to 4·1 and 2·7 g K.
Mean Na:K ratio was 2·3 in the whole group, and respectively 3·2 and 1·8 in the highest and lowest quartiles of Na excretion.
Blood pressure
The young men in the present study were all normotensive; the mean blood pressure is given in Table 1. There was no difference in systolic or diastolic blood pressure between subjects when comparing means across quartiles of Na excretion. Also, a comparison between the highest and lowest quartiles of Na and K excretion and Na:K ratio showed no significant association (data not shown).
Energy intake and diet analysis
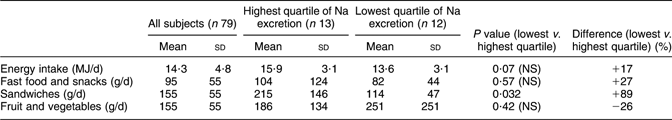
Data on energy intake and some foods are given in Table 3. Energy intake tended to be higher (P = 0·07) in the highest compared with the lowest quartile of Na excretion. The intake of fast food and snacks was higher and the intake of fruit and vegetables was lower in the high Na excretion quartile compared with the low quartile, but none of these differences were statistically significant. The intake of sandwiches, however, was significantly higher in the highest Na excretion quartile compared with the lowest quartile by no less than 89 %.
Table 3 Intake of energy and certain foods in a random sample of seventy-nine young men in Sweden with high and low sodium excretion
Discussion
The present study indicates that the intake of Na is very high in a representative group of young Swedish men in an urban population. It is in fact double the recommended intake of not more than 80–110 mmol Na/d (5–6 g salt/d) issued by WHO in 1982(15) and since then adopted in the Nordic Nutrition Recommendations(8) and by all other major international and scientific societies. The K intake is, however, only slightly below the recommended intake. The resulting Na:K ratio in urine is therefore due to the excessively high Na intake and indicates a substantial overconsumption of Na from salt.
Previous data from two small Swedish sub-populations, studies in adolescents, males and females vegans(Reference Larsson and Johansson16) and middle-aged men and women(Reference Rosell, Hellenius and de Faire17), on urinary Na excretion show values corresponding to 8–10 g salt/d.
The results of the present study also indicate that Na excretion is positively correlated to body weight and BMI, but almost significantly correlated to energy intake only. This may indicate that subjects with high Na intake are heavier due to a more sedentary lifestyle and not spending energy on physical activities; the results from the GOOD questionnaire pointed in that direction although the difference was not statistically significant. These subjects obviously augmented their energy intake with foods known to be salty, which explains the higher Na intake. The subjects in the low Na excretion quartile reported fewer snacks, fast foods and sandwiches than the subjects in the high quartile. The young men in the present study have thus made a food selection more neutral to energy than to Na intake.
Blood pressure is not significantly related to salt excretion in the present study. As high blood pressure is a disease generally starting in the third and fourth decades of life, it may not be surprising that there was no correlation between Na excretion and blood pressure in this relatively small study in young men. One may, however, speculate what will happen with blood pressure in the next decades if the high Na intake is maintained, as the kidneys’ ability to excrete excess Na is reduced with age(Reference Grim, Luft, Miller, Brown, Gannon and Weinberger18, Reference Gudmundsson, Berglund, Herlitz, Andersson and Jonsson19). It is well known that reducing salt intake reduces blood pressure moderately but significantly in hyper- as well as normotensive middle-aged men and women(Reference Stamler1, Reference He and MacGregor3, Reference Cook, Cutler, Obarzanek, Buring, Rexrode, Kumanyika, Appel and Whelton4, Reference Melander, von Wowern, Frandsen, Burri, Willsteen, Aurell and Hulthén20). This fact is underlined in the extensively controlled meta-analysis by He and MacGregor(Reference He and MacGregor3), based on twenty-eight carefully selected studies with in total 734 hypertensive and 2220 normotensive individuals. Moderately reduced salt intake has also been shown to substantially reduce cardiovascular morbidity and mortality(Reference Cook, Cutler, Obarzanek, Buring, Rexrode, Kumanyika, Appel and Whelton4).
The main sources of Na in the diet are processed foods, e.g. bread, cheese, meat spread and fish products. The contribution of Na from added salt and salt-containing spice mixtures and condiments varies. In a recent study from Denmark the total salt intake was assessed to be 10·6 g/d in men and 7·1 g/d in women(Reference Andersen, Rasmussen, Larsen and Jakobsen21). The study also suggested that household salt constituted a minor part of the total salt intake, 10 % in men and 9 % in women. The other sources, primarily salt added during processing but also salt added in the preparation of meals in canteens and restaurants, are the most significant sources.
Various ways to reduce salt intake in the population have therefore been advocated. Most of the salt we ingest, in fact 75–80 %, derives from processed food such as bread, cheese, meat and fish products, breakfast cereals and fast food. Labelling the salt content in foods has been shown to be a useful tool to reduce Na intake in Finland, by giving consumers the possibility to choose products with less salt(Reference Pietinen, Valsta, Hirvonen and Sinkko22). Campaigns to change lifestyle factors regrettably often fail to reach out and are often ineffective. Regulation by the authorities of the salt content in various foods is far more effective, as discussed in an important WHO paper(Reference Murray, Lauer, Hutubessy, Niessen, Tomijima, Rodgers, Lawes and Evans23).
The conclusion is therefore that salt intake in young Swedish men is alarmingly high at present and steps should be taken to reduce it. Regulation of the salt content in processed and fast food and in snacks is advocated to curtail the salt burden on society imposed by the food industry.
Acknowledgements
Sources of funding: The study was supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, the ALF/LUA Research Grant in Gothenburg, the Lundberg Foundation, the Torsten and Ragnar Söderberg’s Foundation, the Petrus and Augusta Hedlund’s Foundation, and the Novo Nordisk Foundation. Conflict of interest: The authors have no conflict of interest. Author contributions: M.L. and C.O. were responsible for the design and performance of the GOOD study, M.A and L.H. for the salt part of the study. S.K., E.H. and L.H. were responsible for the dietary assessment. S.K. and E.H. administered the dietary assessment. L.H. and M.A. wrote the paper with contributions from the other authors.






