Vitamin A deficiency (VAD) is a major public health problem in low- and middle-income countries. On the basis of biochemical criteria (serum retinol <0·70 μmol/l), about 19·1 million pregnant women and 190 million children under the age of 5 years are estimated to have VAD, and in 122 countries this condition is a moderate-to-severe public health problem( 1 ). Infants and children have increased vitamin A requirements to promote rapid growth and to help combat infections. The recent WHO guidelines therefore recommend high-dose vitamin A supplementation in infants aged 6–11 months (30 mg retinol equivalent once) and children aged 12–59 months (60 mg retinol equivalent every 4–6 months) in settings where VAD is a public health problem( 2 ).
Most population surveys assess VAD prevalence in young children and, with increasing frequency, in pregnant or lactating women( 1 ). Two sets of indicators are commonly used for this purpose: (i) clinically assessed eye features (night blindness and Bitot's spots (BS)); and (ii) biochemically determined retinol concentrations( 1 ). Although night blindness and BS are considered to be mild stages of eye disease, both are believed to represent moderate-to-severe systemic VAD, as evidenced by low serum retinol concentrations( 1 , Reference Sommer, Hussaini and Muhilal 3 ).
In view of economic and logistical constraints, the prevalence of BS is still used in India to quantify the VAD burden, both before and after mega-dose vitamin A supplementation (MVAS) programmes. Also in young children, BS is believed to be a more valid clinical indicator than night blindness because it: (i) can be reliably detected by trained paramedical personnel; (ii) is a specific sign for VAD( Reference Sommer 4 ); and (iii) is responsive to vitamin A therapy. However, in three small studies in pre-school children conducted two to three decades ago( Reference Sinha and Bang 5 – Reference Sovani, Humphrey and Kuntinalibronto 7 ), overall 20% (range: 6–36%) of BS did not respond completely to 60 mg retinol equivalent of MVAS. Thus in settings relying on BS to assess VAD prevalence and the success of MVAS programmes, it is important to realize that a fifth of individuals may not be cured of all BS after intervention. Because of altering socio-economic status, community development and nutrition intervention programmes, the prevalence rate of BS as well as the proportion of non-responsive BS may change over time( Reference Semba, Wirasasmita and Natadisastra 6 ). We therefore undertook a prospective cohort study in contemporary times in rural India to evaluate the responsiveness of BS over 1 year to MVAS administered as per the national programme.
Methods
The present community-based cohort study was conducted in an area endemic for VAD, namely Jasra rural block of Allahabad District, Uttar Pradesh, India. The inclusion criteria were: (i) confirmed BS in one or both eyes; (ii) age between 12 and 71 months (verified by local events calendar, birth certificate or pulse polio immunization records); (iii) permanent resident of that area; (iv) unlikely to migrate within the next year; and (v) informed written consent from the parent or caregiver. Exclusion criteria comprised: (i) any illness requiring hospitalization; (ii) associated corneal lesion or keratomalacia; and (iii) history of receiving MVAS within the past 6 months.
We adopted a purposive sampling approach. Children with BS were identified by a two-step process through (i) the existing government health and nutrition functionaries in the block (accredited social health activists or community health workers, anganwadi workers from the Integrated Child Development Services Scheme, and auxiliary nurse midwives) and (ii) the research team comprising four field investigators and one research supervisor. In the preparatory phase, government functionaries and the research team were briefed about the study objectives and familiarized with the local terminology for VAD (‘Rataundhi’ for night blindness, ‘Phuli’ or ‘Kamal’ for BS). They were demonstrated clinical cases and photographs of xerophthalmia (VAD). Subsequently, the research team was given an intensive three days training by the first author. This included a one day didactic lecture followed by two days of field training. The research team members were given photographs of BS and other conditions requiring differentiation (pterygium and pinguecula) to facilitate an accurate diagnosis. They were also taught to perform the confirmatory Kajal test, wherein Kajal (carbon black) is applied to the eyelids and a mild massage of the upper bulbar area is done. The Kajal sticks to BS, staining it black( Reference Chouhan 8 ).
The government functionaries, through a house-to-house survey, evaluated children to make a provisional diagnosis of BS. These children were taken to research team members for further detailed evaluation. The research team confirmed the diagnosis of BS and performed the Kajal test in doubtful cases (117 children). The government functionaries were offered a small incentive (∼$US 1) for referring a confirmed case of BS.
Each child with BS was provided an identity card, in which details like identification number, name, age, sex, father's name and name of the village were recorded. Other information recorded at baseline on a pre-tested questionnaire included socio-economic status, education and occupation of parents, immunization status, breast-feeding, consumption of green leafy vegetables, current morbidity status and any morbidity in the last 15 d. Digital photographs of both eyes were then taken.
Children with suspected or confirmed BS were administered MVAS as per the protocol recommended under the National Program for Prevention of Nutritional Blindness due to VAD, Ministry of Health and Family Welfare, Government of India( 9 ). Under this protocol, each child suffering from clinical VAD is given 60 mg (retinol equivalent) of vitamin A on the day of diagnosis, and this dose is repeated after 1 month. Subsequently another dose was repeated 6 months after the initial recruitment. Vitamin A supplementation was always done under direct supervision.
Children with BS were followed up at monthly intervals for 1 year. In cases where a child was not available at the scheduled time of the monthly visit, a repeat visit was made within the next week and another one, if required, within the subsequent 3–7 d. At each follow-up visit, relevant clinical data and digital photographs of both eyes were recorded. An independent ophthalmologic validation was done by the research supervisor in 20% and by the first author in 5% of children at all time points. The baseline and follow-up digital photographs were blinded and given to three nutritionists for their individual diagnosis regarding absence or presence of BS. The agreed diagnosis between at least two of them was recorded as the final valid diagnosis. In cases of ambiguity, a repeat field visit was undertaken by the research team within a week.
We employed definitions that would replicate programmatic conditions. If no BS lesion was detected in either eye, the child was classified as cured or a responder. A child with any detectable BS lesion even in one eye was classified as a non-responder at that follow-up visit. Thus children with a reduction in size of BS lesions or with disappearance of BS lesions from one eye in the case of bilateral BS lesions were categorized as non-responders.
A sample size of 250 was estimated for evaluating 20% non-responsiveness during 1 year of follow-up after diagnosis and administration of MVAS, with 5% absolute precision and 95% confidence with an attrition rate of 5%. The Kaplan–Meier survival curve was utilized to determine the time to cure (resolution) of BS in the children at monthly intervals until 1 year of follow-up. The log rank test was performed to determine the statistical significance of various baseline characteristics for predicting cure at 6 and 12 months of follow-up.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the institutional ethics committee of the All India Institute of Medical Sciences. Written informed consent was obtained from the parents of all children.
Results
We enrolled 262 children in the study, seventy-five (29%) with BS in one eye and 187 (71%) with BS in both eyes. During 1 year, three children were lost to follow-up (one child died due to a road traffic accident in the first month, two children migrated from the study area in months 7 and 10). Table 1 summarizes the baseline information of recruited children. The majority were from a nuclear family (82%) with illiterate mothers (91%) and fathers (57%), were above 3 years of age (90%), were boys (59%), did not receive colostrum at birth (87%), were not immunized for measles (66%), were breast-fed for 12–24 months (97%), did not consume green leafy vegetables (80%) or extra fat (93%) in the diet, and did not have morbidity at the time of examination or within the past 15 d (85%). Sixty-three per cent of the enrolments were done in the rainy season (August to October).
Table 1 Association of baseline characteristics with cure of Bitot's spots (BS) at 6 and 12 months of follow-up among 262 children aged between 1 and 5 years, rural Uttar Pradesh, India
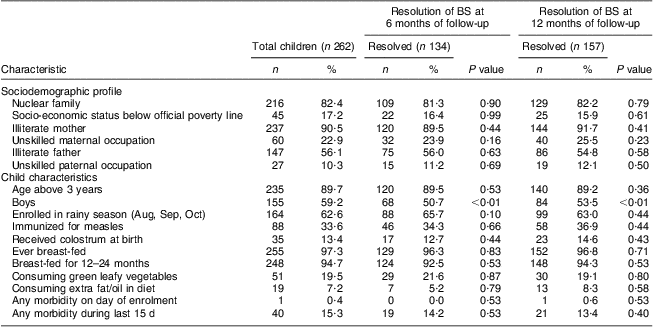
Data for a total of one to eight children were missing for individual baseline characteristics.
P values were calculated by the log rank test, to compare baseline characteristics between children with resolved and unresolved BS.
Figure 1 shows the Kaplan–Meier curve for responsiveness of BS to MVAS. At 6 months of follow-up (MVAS at baseline and 1 month later), 51·1 (95% CI 45·3, 57·3) % were classified as cured (responders). The corresponding figure at 1 year of follow-up (MVAS at baseline, at 1 month and at 6 months) was 59·9 (95% CI 54·1, 65·9) %. Among the children responding to MVAS at 6 months of follow-up, about half and three-quarters had resolved at 2 and 3 months, respectively. However, the response at 1 month was only 4·5%.
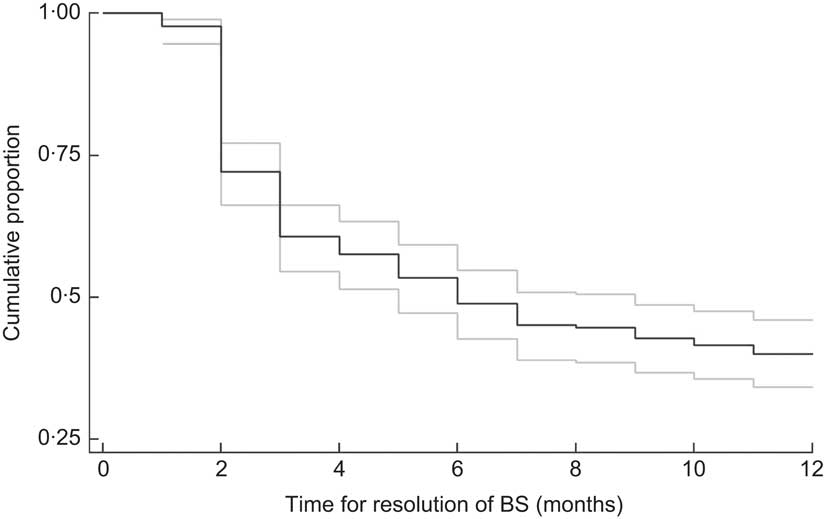
Fig. 1 Kaplan–Meier curve for resolution of Bitot's spots (BS) during 12 months of follow-up among 262 children aged between 1 and 5 years, rural Uttar Pradesh, India (——, survivor (resolution) function; ![]() , 95% confidence interval)
, 95% confidence interval)
Among the variables recorded at baseline, apart from gender (better response in girls; P < 0·01 at 6 months and P < 0·01 at 12 months), there were no significant predictors of response to MVAS at 6 and 12 months of follow-up (Table 1).
Discussion
Our study design permits inferences only about the effect of MVAS on resolution of BS; the effect on biomarkers and functional consequences of VAD cannot be commented upon. In contemporary times in rural India, a substantial proportion of children were not cured of BS after two doses of MVAS at 6 months (49%) or even three doses at 1 year (40%). Apart from male gender, there were no significant sociodemographic or clinical predictors of cure.
Strengths and limitations
The current study from contemporary times was conducted on a fairly large cohort of children with BS. MVAS was administered under directly supervised conditions. Follow-up was done at monthly intervals and 98·9% of children were available during 1 year of follow up. Validity of the clinical diagnosis of BS was ensured and sequential digital photographs of both eyes were recorded.
The following limitations merit consideration. We did not evaluate serum retinol for correlating responsiveness. This decision was based on economic constraints, sacrificing blood drawing for ensuring cooperation in the community setting and avoiding potential issues with ethical clearance. Additional investigations could not be performed to determine the aetiology of non-responsive BS. Ethical considerations precluded the inclusion of a placebo control group. Consequently, we may have overestimated the effect of MVAS on resolution of BS because of the theoretical possibility of spontaneous resolution in a proportion of controls.
Comparison with earlier data
There is paucity of contemporary prospective cohort studies for comparison. However, in three early and relatively small studies in pre-school children, overall 20% of BS did not respond completely to MVAS (60 mg retinol equivalent); the proportion of non-responsive BS was 9/25 (36%) after three 4-monthly doses in Kolkata, India( Reference Sinha and Bang 5 ), 12/45 (27%) after 5 weeks of a single dose in Indonesia( Reference Semba, Wirasasmita and Natadisastra 6 ) and 3/52 (6%) after 6 months of a single dose in Indonesia( Reference Sovani, Humphrey and Kuntinalibronto 7 ). Even accounting for differences in dosing frequency and follow-up duration, non-response was considerably greater in the current study than in these Indonesian studies( Reference Semba, Wirasasmita and Natadisastra 6 , Reference Sovani, Humphrey and Kuntinalibronto 7 ). However, non-response was broadly comparable with the Indian study( Reference Sinha and Bang 5 ) after three doses at 1 year.
The cure proportion at 6 months (51%) is also plausible according to the recent meta-analysis of vitamin A supplementation trials( Reference Imdad, Herzer and Mayo-Wilson 10 ); among 63 278 recruited subjects from four trials, after a mean intervention of 80·72 weeks, the relative risk of detecting BS was 0·45 (95% CI 0·33, 0·61). In conformity with an Indonesian trial( Reference Semba, Wirasasmita and Natadisastra 6 ), the predominant proportion of responders at 6 months of follow-up had resolved by 3 months.
In conformity with earlier reports( Reference Semba, Wirasasmita and Natadisastra 6 , Reference Sovani, Humphrey and Kuntinalibronto 7 , Reference Sommer, Green and Kenyon 11 ), we documented no consistent clinical predictor of non-responsive BS. Minor histopathological differences have however been reported( Reference Sommer, Green and Kenyon 11 ). In non-responsive cases, the pathological process is sharply limited to the area of the lesion itself, the transition between abnormal and surrounding normal conjunctiva is abrupt. However, the greater degree of subepithelial inflammatory infiltration and the higher concentration of surface bacteria may simply reflect their chronicity( Reference Sommer, Green and Kenyon 11 ). It has been posited that non-responsive BS may be caused by irreversible squamous metaplasia( Reference Semba, Wirasasmita and Natadisastra 6 ) because after excision these never recur( Reference Wittpenn and Sommer 12 ). Biochemically, non-responsive subjects have higher pretreatment serum retinol concentrations( Reference Semba, Wirasasmita and Natadisastra 6 , Reference Sovani, Humphrey and Kuntinalibronto 7 , Reference Emran and Tjakrasudjatma 13 ) and hepatic retinyl stores( Reference Sovani, Humphrey and Kuntinalibronto 7 ). It was therefore proposed that non-responsive BS represent sequelae of past deficiency rather than severe current VAD( Reference Semba, Wirasasmita and Natadisastra 6 , Reference Sovani, Humphrey and Kuntinalibronto 7 , Reference Emran and Tjakrasudjatma 13 ). The period of time before the conjunctival lesion becomes unresponsive to vitamin A treatment is undetermined( Reference Semba, Wirasasmita and Natadisastra 6 ). There are probably no data exploring the aetiological role of other potential nutritional deficiencies, infections and environmental factors.
Definition of Bitot's spot resolution
The justification for the universal vitamin A supplementation programme in India and other low- and middle-income countries is fiercely debated( Reference Kapil and Sachdev 14 – Reference Kapil and Sachdev 20 ). A part of the confusion may arise from the use of BS as an important indicator of VAD and the programmatic success of MVAS; our study was intended to provide relevant information in this context. We therefore employed definitions that closely replicated programmatic assessment of VAD through BS. Cure or resolution was defined if there was no discernible BS in either eye. Cured subjects can relapse during sequential follow-ups, as was also documented in the present study. However, we categorized such cases as resolved because MVAS was initially associated with the disappearance of lesions. A reduction in size of BS or disappearance from one eye for bilateral lesions was labelled as non-response because assessment of size is subjective and during cross-sectional surveys, despite partial response, either of these cases would be considered BS positive.
Implications for policy
On the basis of available evidence, it would be reasonable to concur with McLaren et al.'s conclusion( Reference McLaren, Oomen and Escapini 21 ) that ‘Bitot's spot is a useful indicator of vitamin A deficiency, especially in young children, but is not pathognomonic’. Substantial non-response to MVAS at 6 and 12 months of follow-up suggests that presently in the Indian subcontinent, BS is a relatively crude indicator of severe current VAD. For programmatic decisions and evaluation, the public health burden of VAD should not be assessed solely through BS; it would be desirable to consider other important health consequences. The findings may also partially explain: (i) the recorded discrepancy between BS and low serum retinol prevalences at population level in India; and (ii) the fact that the predominant decline in BS prevalence antedated a functioning universal vitamin A supplementation programme in India( Reference Shah and Sachdev 22 ). This decline has, however, now stagnated for a considerable period despite an improvement in MVAS coverage, suggesting that factors other than severe VAD may be operating. It would be useful to revisit the guidelines for management of individual children with BS.
Implications for future research
Similar studies of adequate sample size from varied settings are desirable to draw robust inferences for policy. Future investigations should simultaneously evaluate biomarkers for VAD, other nutritional deficiencies, and microbiological and environmental factors; however, related histopathological investigations may be difficult to justify ethically. The relative efficacy of frequent low-dose supplementation, fortification and other food-based approaches in curing BS and other consequences of VAD also deserve exploration.
Conclusions
Substantial non-response to MVAS at 6 months (49%) and 1 year (40%) of follow-up suggests that, presently in the Indian subcontinent, BS is a relatively crude indicator of severe current VAD. For programmatic decisions and evaluation, the public health burden of VAD should not be assessed solely through BS.
Acknowledgements
Sources of funding: This work was supported by the Indian Council of Medical Research (grant number 5/9/9/2003-RHN). Conflicts of interest: None declared by any author. Authors’ contributions: U.K., S.D., H.S.S., S.N.D. and R.M.P. designed the study. R.M.P., A.U., H.S.S. and S.N.D. were responsible for statistical analysis; H.H. did data management under their supervision. U.K., H.S.S. and H.H. co-drafted the initial manuscript. All authors provided input on the preliminary draft and approved the final manuscript.




