CVD are a group of disorders of the heart and blood vessels accountable for approximately 31 % of mortality in Brazil(Reference Ribeiro, Duncan and Brant1) and worldwide(2). Besides the consequences of morbidity, CVD exerts socio-economic impacts such as increased expenditure on medications, medical care and social security(Reference Siqueira, De Siqueira-Filho and Land3).
Having a prior history of cardiovascular event is highlighted as a main risk factor for the recurrence of new cardiovascular events due to metabolic impairment(Reference Shah, Bain and Muehlbauer4). Also, chronic inflammation, characterised by elevated levels of pro-inflammatory markers in the bloodstream, is widely known to play a fundamental role in the development of CVD and related mortality(Reference Shivappa, Godos and Hébert5–Reference Golia, Limongelli and Natale7). Atherosclerosis, an inflammatory process, represents the most common pathological substrate of CHD(Reference Golia, Limongelli and Natale7). Lifestyle and environmental factors are usually the causes of chronic low-grade inflammation(Reference Galland8). Studies have suggested an association between inflammation, diet and some dietary components(Reference Shivappa, Godos and Hébert5). While fruit and vegetable consumption has been associated with low levels of inflammation, red meat and food sources of SFA have been shown to increase inflammation(Reference Santos, Oliveira and Lopes9–Reference Chai, Morimoto and Cooney11). In a recent clinical trial, coronary artery disease (CAD) participants on an 8-week vegan diet showed a significant reduction (32 %) in high-sensitivity C-reactive protein compared with the American Heart Association diet(Reference Shah, Newman and Woolf12). The consumption of unhealthy foods in the form of industrialised foods rich in Na, refined sugars, saturated fats and poor in fibre and antioxidants can trigger an inflammatory process(Reference Santos, Oliveira and Lopes9,Reference Ruiz-Núñez, Dijck-Brouwer and Muskiet13–Reference Hall, Ayuketah and Brychta15) . Thus, the evaluation of diet quality is relevant for the control and prevention of CVD.
The dietary inflammatory index (DII®) evaluates a diet according to its inflammatory potential(Reference Shivappa, Steck and Hurley16). Studies show that the consumption of pro-inflammatory foods such as processed meat is associated with a higher risk of CVD and mortality. In contrast, anti-inflammatory foods such as vegetables, fruits, and whole grains are inversely related to the risk of CVD occurrence(Reference Shivappa, Godos and Hébert5,Reference Almeida-de-Souza, Santos and Lopes10,Reference Chai, Morimoto and Cooney11) . The effect of pro-inflammatory and anti-inflammatory foods on CVD and its risk factors has been studied in the literature(Reference Shivappa, Godos and Hébert5,Reference Almeida-de-Souza, Santos and Lopes10,Reference Chai, Morimoto and Cooney11) ; however, no study has associated the number of cardiovascular events with dietary inflammatory potential.
The NOVA (a name, not an acronym) classification is a new concept where food is classified according to the nature, extent and purpose of industrial food processing(Reference Canella, Levy and Martins17,Reference Monteiro, Moubarac and Cannon18) . It highlights food sources and preparation methods, allowing consumers to make informed decisions. Studies show that the consumption of ultra-processed foods is associated with obesity(Reference Nardocci, Leclerc and Louzada19), risk of breast cancer and cancer in general(Reference Fiolet, Srour and Sellem20). Additionally, ultra-processed food consumption is directly associated with the higher intake of refined sugars, saturated fats and Na and inversely associated with fibre intake(Reference Rauber, Louzada and Steele21). Despite the benefits of these food classification schemes, few studies in the literature have explored the association between DII and the degree of food processing.
Our hypothesis is that a greater number of cardiovascular events, cardiometabolic risk factors, consumption of processed, ultra-processed foods and culinary ingredients are associated with a more pro-inflammatory diet. Thus, our primary objective was to estimate the association of DII with the number of cardiovascular events and cardiometabolic risk factors. As a secondary objective we evaluated the association between the DII and food groups classified according to the NOVA classification.
Materials and methods
Subjects and study design
The current study was a cross-sectional study that analysed the baseline data of a multicentre study – Brazilian Cardioprotective Nutritional Program Trial (BALANCE Program Trial), involving CVD patients with at least one established cardiovascular event in the previous 10 years. The BALANCE Program Trial is an intervention study aimed at evaluating the effect of a Brazilian cardioprotective diet on the reduction of cardiovascular events and risk factors in patients with established CVD. The study protocol, including criteria for inclusion, exclusion, ethical aspects, study characteristics, sample calculation and data collection, was previously described by WEBER et al. (Reference Weber, Bersch-Ferreira and Torreglosa22). A sample size of 1404 subjects was required for the effect studied in this work (a design effect of 1·0, 5 % absolute precision, 99·99 % CI and a prevalence of 63·4 % of subjects with ≥ 2 cardiovascular events in the third tertile of DII). The study is in accordance with the Helsinki Declaration principles(23) and was duly registered on ClinicalTrials.gov (NCT01620398).
Data collection
Socio-demographic, anthropometric and clinical data
Data on socio-demographic (age and sex), anthropometric (weight, height and waist circumference) and clinical characteristics (cardiovascular events, history of disease, use of medications, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol, LDL, HDL, TAG and fasting plasma glucose) were collected by professionals during the first consultation.
Older adult subjects were defined here as ≥ 60 years of age. Height was measured twice with wall-mounted stadiometers (0·5 cm precision) with the subject barefooted in a standing position. Weight was measured twice on calibrated scales (100 g precision) with the subject barefooted and wearing light clothes. Waist circumference was measured at the midpoint between the lower border of the costal arch and the iliac crest at the mid-axillary line(24), and BMI was calculated.
The medical diagnosis of cardiovascular events is described below. The following cardiovascular events were considered:
-
Asymptomatic CAD: History of angina (clinical diagnosis including diagnosis without complementary tests or history of positive stress test)
-
Symptomatic CAD: Angiography or coronary angiotomography, with atherosclerotic stenosis ≥ 70 % of the diameter of any coronary artery
-
Treated CAD: When the patient presented angioplasty/stent/revascularisation
-
Asymptomatic peripheral arterial disease: Ankle/arm ratio <0·9 of SBP in either leg at rest; angiographic or Doppler showing > 70 % stenosis in a cardiac artery
-
Symptomatic peripheral arterial disease: Intermittent claudication
-
Treated peripheral arterial disease: Vascular surgery for atherosclerotic disease
-
Heart attack: History of myocardial infarction or acute coronary syndrome; history of abnormality in the segmental movement of the cardiac wall based on echocardiography or a fixed segmental defect shown by scintigraphy
-
Abdominal aortic aneurysm
-
Amputation due to arterial cause
-
Stroke: When the patient presented a clinical diagnosis of stroke or evidence of previous stroke from computed tomography or MRI.
Furthermore, we grouped the patients according to the number (1, 2 or ≥ 3) of manifested cardiovascular events. Cardiometabolic risk factors (diabetes, hypertension, dyslipidaemia, metabolic syndrome and elevated waist circumference) were defined according to the I Diretriz Brasileira de Prevenção Cardiovascular(Reference Simão, Precoma and Andrade25,Reference Faludi, Izar and Saraiva26) . Blood pressure was taken by a trained nurse, following the recommendations of the American Heart Association(27). Medication data were obtained from medical prescriptions. Blood samples were collected after a fasting period of 12–14 h. LDL was determined by the Friedewald formula, and the other biochemical parameters were measured by the enzymatic colorimetric method (VITROS 5600; Johnsons & Johnsons).
Food consumption data
Food intake was estimated from the mean of two 24-h dietary recalls (with 15-d application interval) and the data were analysed by the Nutriquanti® software. The 24-h dietary recall was conducted according to the Automated Multiple-Pass Method(Reference Moshfegh, Rhodes and Baer28). Considering the wide variety of foods consumed in each region in Brazil, the utilisation of a 24-h dietary recall is ideal because it does not restrict answers to specific food options.
To evaluate the inflammatory potential of diet, the DII was used. This methodology classifies diet as more anti-inflammatory or more pro-inflammatory, based on food consumption data recorded in food surveys. Initially proposed by Cavicchia et al. (Reference Cavicchia, Steck and Hurley29), DII was updated by Shivappa et al. (Reference Shivappa, Steck and Hurley16). It was designed based on the potential of foods to induce the release of anti- or pro-inflammatory markers. Besides considering the inflammatory response to isolated nutrient or food ingested, DII also takes into account the type of study (with humans, animals or cell culture), model (experimental, cohort, case–control or cross-sectional), the number of articles with such type and model of study, and the mean daily intake of nutrient or food. The creators of DII assigned scores for each criterion mentioned above, and an overall score is attributed to each food or nutrient. For the present study, twenty-nine food parameters were considered: energy (kcal), protein (g), carbohydrate (g), total fat (g), saturated fat (g), cholesterol (mg), fibre (g), MUFA (g), PUFA (g), trans-fat (g), Fe (mg), Mg (mg), Zn (mg), selenium (µg), vitamin C (mg), vitamin E (mg), riboflavin (mg), vitamin A (RE), n-3 fatty acids (g), n-6 fatty acids (g), niacin (mg), vitamin B6 (mg), vitamin B12 (µg), thiamin (mg), vitamin D (µg), onion (g), garlic (g), ginger (g) and pepper (g). A lower DII indicates a higher consumption of anti-inflammatory foods, while a higher DII indicates a higher consumption of pro-inflammatory foods. Theoretically, the DII values range from −8 (more anti-inflammatory) to +8 (more pro-inflammatory)(Reference Shivappa, Steck and Hurley16).
The foods were further classified as unprocessed or minimally processed, culinary ingredients, processed and ultra-processed foods based on the NOVA classification(Reference Monteiro, Levy and Claro30). Mixed preparations were classified according to the proportion of the main ingredient. For instance, if a greater proportion of the ingredient came from ultra-processed foods, this preparation was classified as ultra-processed foods. Below, we describe some foods that make up each group proposed by the NOVA classification:
-
a. Unprocessed or minimally processed foods: vegetables, fruits, roots and tubers, cereals, nuts, dry fruits, eggs, meat, poultry, fish, seafood and others.
-
b. Culinary ingredients: sugar, honey, oils and fats of vegetable or animal origin, starches, salt, vinegar, alcoholic beverages and others.
-
c. Processed foods: preserved food, salted or sugary nuts, salted meats, cheeses, bread and others.
-
d. Ultra-processed foods: soft drinks, artificial juices, ice-creams, chocolates, candies and sweets in general, stuffed cookie, cakes, breakfast cereals, sausage, hamburger and other reconstituted meat products.
Statistical analyses
The distribution of the variables was analysed by the Kolmogorov–Smirnov test. Although the data did not follow a normal distribution, they were presented as mean (sd). Comparisons between groups were assessed by the Kruskal–Wallis test, Mann–Whitney U test and linear trend χ 2 test (categorical variables). The categorical data were presented as absolute and relative frequency. Multiple linear regression analysis was performed to explore associations between the quantitative variables, and the results were presented as β-values and 95 % CI. Residual analysis also was performed. The association between the number of cardiovascular events (dependent and categorical variable) and DII tertiles was evaluated by multinomial logistic regression. The same method was utilised to estimate the association between the DII tertiles (dependent and categorical variable) and the percentage of energies from each NOVA group. The regression results were presented as OR values and 95 % CI. The statistical analyses were performed using STATA® 13, considering α = 5 %. Assuming a prevalence of ≥2 cardiovascular events in the exposed (third DII tertile) and in non-exposed (first DII tertile) groups, the analysis had 80 % power to detect a difference of this magnitude or larger. OpenEpi online software was used for this calculation.
Results
A total of 2763 patients were assessed for eligibility, of which 2359 were included in the present study. The reasons for exclusions included not meeting eligibility criteria (n 37), refusal to participate (n 153), missing DII data (n 175) and other reasons (n 39) (Fig. 1). Patients from Southeast, South, Northeast, Midwest and North regions of Brazil accounted for 34·0, 27·3, 25·5, 7·2, and 6·1 %, respectively.
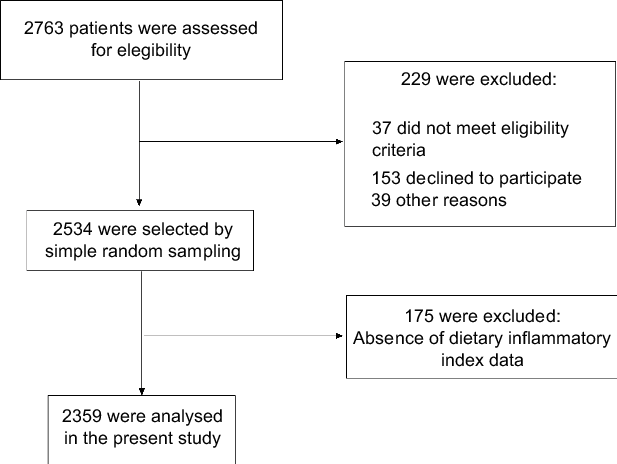
Fig. 1 Flow chart of study participants
Most of the patients were male (58·5 %), older adults (64·2 %), overweight (68·8 %), hypertensive (90·2 %) and dyslipidaemic (77·8 %). In addition, 40·0, 33·9 and 26·1 % of the patients were diagnosed with 1, 2 and ≥ 3 cardiovascular events, respectively.
After classifying the participants into groups according to the number of cardiovascular events, we observed that mean age, BMI, waist circumference and the prevalence of hypertension, diabetes, dyslipidaemia and metabolic syndrome were similar among them (Table 1). After stratifying the patients according to DII tertiles, we observed that those in the third DII tertile, indicative of a more pro-inflammatory diet, had higher waist circumference, SBP, DBP, total cholesterol and HDL than patients in the first tertile, characterised by a more anti-inflammatory diet (Table 2). We noted a trend of higher prevalence of treated CAD, asymptomatic peripheral arterial disease and a high number of patients with 2 and ≥ 3 cardiovascular events according to increase in DII tertiles (Table 2).
Table 1 Participants characteristics according to the number of cardiovascular events

CAD, coronary arterial disease; PAD, peripheral arterial disease.
*P-values obtained by linear trend χ 2 test or Kruskal–Wallis followed by Mann–Whitney U.
Table 2 Participants characteristics according to DII tertiles (n 2359)*
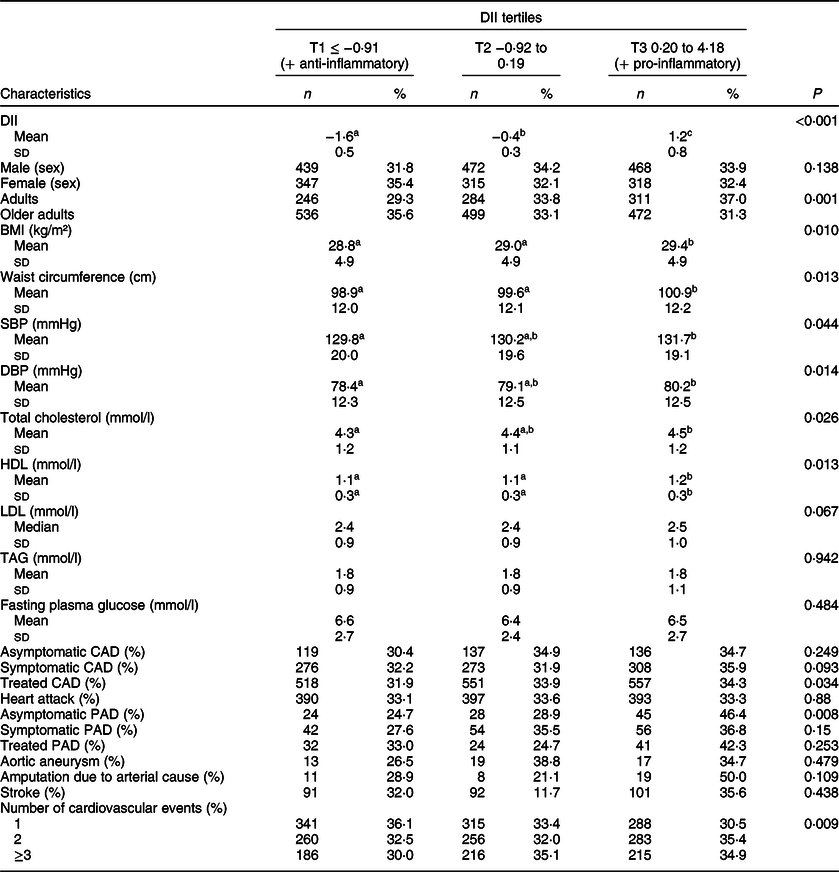
DII, dietary inflammatory index; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; TAG, triglycerides; CAD, coronary arterial disease; PAD, peripheral arterial disease.
Different letters show the presence of difference and equal letters show the absence of differences.
* P-values by Kruskal–Wallis test, Mann–Whitney U for quantitative variables, and linear trend χ 2 test for categorical variables.
A positive association was observed between DII, anthropometric data and clinical risk factors, such as BMI, waist circumference, SBP, DBP, total cholesterol, HDL and LDL (except for fasting plasma glucose and TAG), regardless of sex, age, use of medications and presence of comorbidities such as diabetes mellitus, dyslipidaemia or hypertension (Table 3). Moreover, patients in the third DII tertile (with a more pro-inflammatory diet) were 1·27 (95 % CI 1·01–1·61) and 1·39 (95 % CI 1·07–1·79) times more likely to have 2 and ≥3 cardiovascular events compared to patients with one CVD and in the first DII tertile (with a more anti-inflammatory diet) (Table 4).
Table 3 Association between cardiometabolic risk variables (dependent variables) and DII (n 2359)
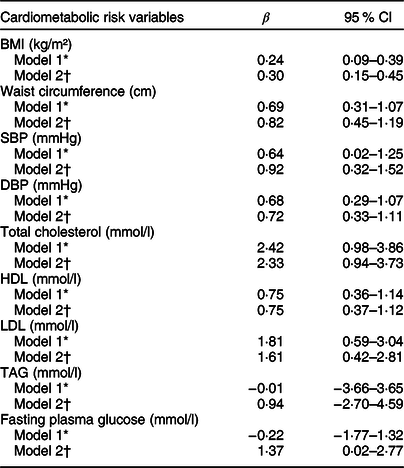
DII, dietary inflammatory index; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; TAG, triglycerides.
* Model 1: Crude.
† Model 2: Adjusted for sex, age, diabetes, hypertension, dyslipidaemia and use of medicaments.
Table 4 Associations between the number of cardiovascular events (dependent variable) and DII tertiles (n 2359)
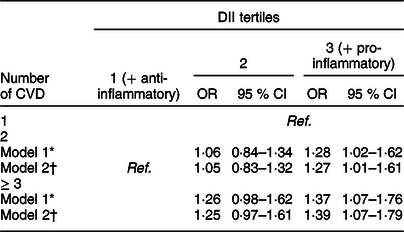
DII, dietary inflammatory index; CVD, cardiovascular diseases.
* Model 1: Crude;
† Model 2: Adjusted for sex, age, diabetes, hypertension, dyslipidaemias and use of medicaments.
Regarding the association between DII and NOVA food groups, we observed an inverse association with the consumption of unprocessed or minimally processed food and a positive association with the consumption of processed, ultra-processed food and culinary ingredients (Fig. 2).
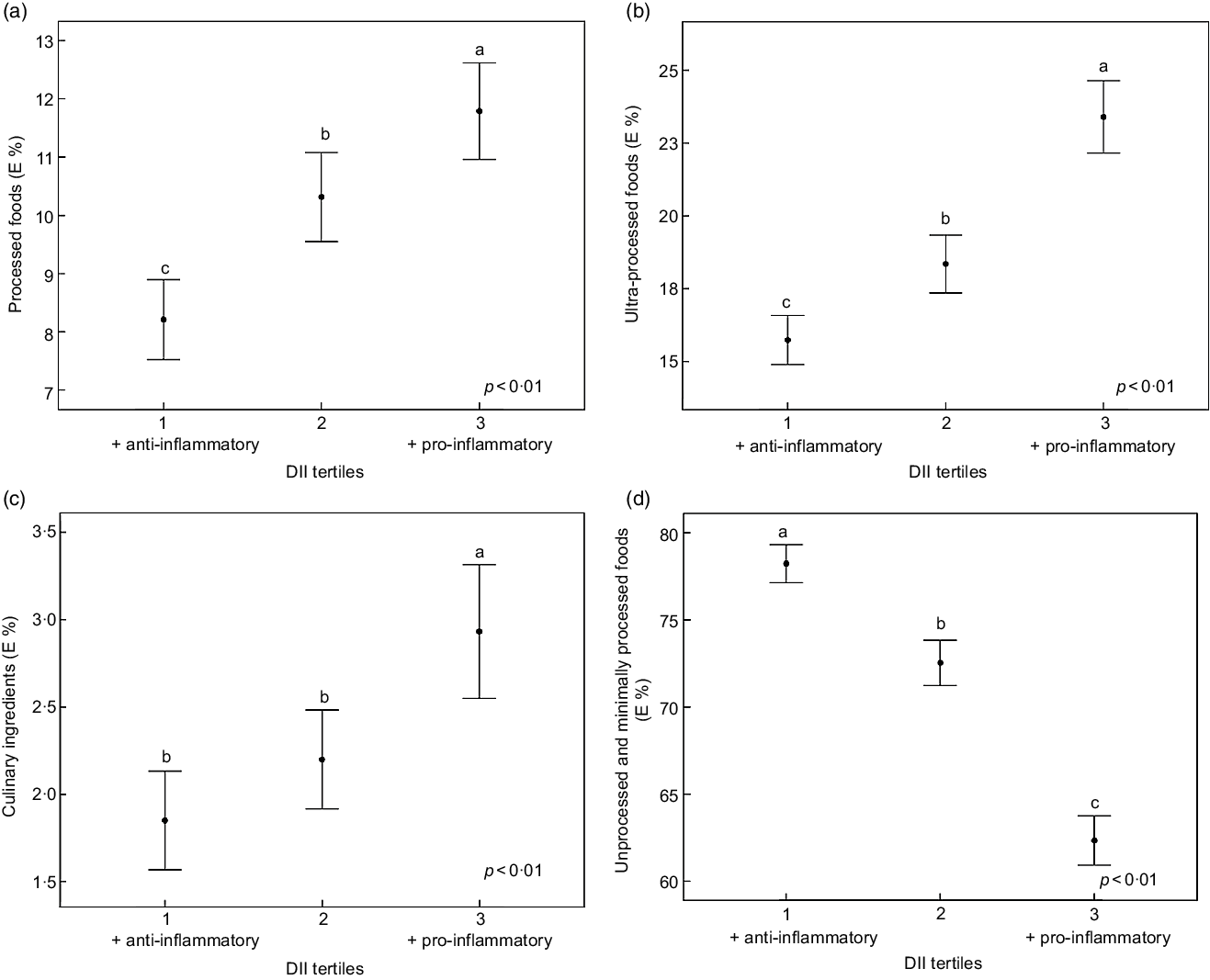
Fig. 2 Association between DII and the E % of food consumption according to the NOVA classification (a, b, c and d). Data are mean and error bars are 95 % CI. DII, dietary inflammatory index; E, energy. Different letters show presence of difference and equal letters show the absence of differences according to Kruskal–Wallis test and Mann–Whitney U. P-values by multinomial logistic regression, adjusted for sex, age, diabetes, hypertension, dyslipidaemia and use of medicaments
Discussion
We investigated the association between the DII and the number of cardiovascular events, cardiometabolic risk factors and the percentage consumption of food groups (NOVA classification) in cardiac patients. We verified that a more pro-inflammatory diet was associated with a high chance of having 2 (12·7 %) or ≥ 3 (13·9 %) cardiovascular events compared with a more anti-inflammatory diet. The observed association between pro-inflammatory diet and a higher chance of having 2 or ≥ 3 CVD events suggests that a pro-inflammatory diet can stimulate the release of pro-inflammatory markers, which, in turn, may aggravate the progression of atherosclerosis, a key factor of CVD. Studies have shown an association between a pro-inflammatory diet and multiple(Reference Wirth, Shivappa and Hurley31) and single CVD prevalence, such as congestive heart failure, heart attack and stroke(Reference Wirth, Shivappa and Hurley31–Reference Shivappa, Tavani and Hébert33). Furthermore, cohort studies have associated a pro-inflammatory diet with a high incidence of CVD(Reference O’Neil, Shivappa and Jacka34–Reference DeBoer, Garcia-Arellano and Ramallal36), CVD mortality and total mortality(Reference Shivappa, Blair and Prizment37–Reference Deng, Shivappa and Tang39) and the prevalence and incidence of cancer(Reference Shivappa, Godos and Hébert40–Reference Tyrovolas, Koyanagi and Kotsakis42). The Child Health CheckPoint Study, which evaluated adults, showed a consistent association between a more pro-inflammatory diet and adverse variations in vascular function and microvascular structure, preclinical phenotypes that favour CVD(Reference Davis, Liu and Kerr43). Despite evidence that some foods trigger anti- or pro-inflammatory response, the biological mechanisms underlying these effects are poorly understood(Reference Rocha, Lopes and da Silva44–Reference Khan, Khymenets and Urpí-Sardà46).
We verified that DII was positively associated with classic cardiometabolic risk markers, such as BMI, waist circumference, SBP, DBP, total cholesterol, LDL and paradoxically with higher HDL. Additionally, DII was associated with the consumption of processed, ultra-processed foods and culinary ingredients. On the other hand, DII was inversely associated with the consumption of unprocessed or minimally processed foods. The presence of risk factors in the patients was expected because they suffer from CVD. However, a high metabolic impairment expressed by high BMI, waist circumference, total cholesterol, SBP and DBP was observed among those who ate a more pro-inflammatory diet. In fact, apart from food quantity, food quality is also associated with weight gain and other cardiometabolic risk factors. In this sense, studies have associated a more pro-inflammatory diet with classic risk factors for CVD, such as obesity, hypercholesterolaemia, diabetes and arterial hypertension(Reference Tyrovolas, Koyanagi and Kotsakis42,Reference Phillips, Shivappa and Hébert47,Reference Mazidi, Shivappa and Wirth48) .
To the best of our knowledge, the present study is the first to associate the DII with the percentage consumption of food groups according to the NOVA classification. Studies show that the consumption of ultra-processed foods, known for being energy-dense and rich in refined sugars, dyes and preservatives, is associated with the risk of developing obesity and non-communicable diseases(Reference Nardocci, Leclerc and Louzada19,Reference Rauber, Louzada and Steele21,Reference Poti, Braga and Qin49,Reference Bielemann, Santos Motta and Minten50) . On the other hand, unprocessed or minimally processed foods, neither subjected to industrial processing nor containing added substances, are inversely associated with a more pro-inflammatory diet(Reference Almeida-de-Souza, Santos and Lopes10).
The Western dietary pattern, characterised by the high consumption of processed foods, red meats, soft drinks, confectionery products, among others, is rich in saturated fats, refined sugars, Na and poor in whole grains and natural foods. The ingestion of this type of food seems to activate an innate immune response due to the production of more pro-inflammatory cytokines than anti-inflammatory cytokines(Reference Giugliano, Ceriello and Esposito51). On the other hand, greater adherence to the Mediterranean diet is associated with a decrease in inflammatory markers such as IL-6 and C-reactive protein(Reference Salas-Salvadó, Garcia-Arellano and Estruch52–Reference Schwingshackl and Hoffmann54). Similarly, a higher DII, indicative of a more pro-inflammatory diet, is associated with a variety of inflammatory markers like C-reactive protein and IL(Reference Phillips, Shivappa and Hébert47,Reference Mayr, Itsiopoulos and Tierney55–Reference Shivappa, Wirth and Murphy60) . These results support the role of diet in the prevention and control of CVD because food serves as a vehicle of beneficial or detrimental nutrients to cardiovascular health.
It is important to highlight that we used the 24-h dietary recall to evaluate the food consumption of our patients with established cardiovascular events. This tool reflects food consumption in the last 24 h. Given the severity of CVD diagnosis among the participants, it is probable that many of them might have made dietary changes prior to the study because unhealthy diet is an important risk factor for CVD development and a healthy diet is one of the main strategies to treat, control and prevent more health complications. Despite the possibility that some participants may have changed their habitual diets based on dietary counselling, there is evidence of an association between a more pro-inflammatory diet and the presence of 2 and ≥3 cardiovascular events compared with a more anti-inflammatory diet.
The main strength of the present study is its design. The study is a multicentre study that evaluated a broad number of patients from all regions in Brazil. Patients with different eating habits were evaluated considering the great diversity of food cultures in Brazil. However, the cross-sectional design of the study is a limitation. In this sense, causality cannot be inferred, but the cross-sectional has its value in the scientific literature and most importantly, it served to answer our research question. Although we evaluated a large number of cardiac patients in our study, the sample size was calculated according to the aim of the intervention proposed and we analysed only baseline data. Despite this, the analysis power for the present study was approximately 80 %.
Conclusions
A more pro-inflammatory diet is associated with a poor metabolic profile and a high probability of 2 and ≥3 cardiovascular events in cardiac patients. The consumption of processed, ultra-processed and culinary ingredients foods is suggestive of a more pro-inflammatory diet. There is a clear need for public policies that raise awareness on the importance of healthy food choices, especially because the adoption of a healthy dietary pattern is a crucial step in reducing inflammation-associated chronic diseases.
Acknowledgements
Acknowledgements: The authors thank all the patients for participating in this project, the researchers B.W., A.C.B.F., C.R.T and all participating centres. Also, the authors thank the N.S. and J.R.H. for the partnership. Financial support: The current study was supported by the Hospital do Coração (HCor) as part of the ‘Programa de Apoio ao Desenvolvimento Institucional do Sistema Único de Sáude (PROADI-SUS)’, in partnership with the Brazilian Ministry of Health. The current study was also funded in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – Financial Code 001. J.B. and H.H.M.H. are research productivity fellows of CNPq (Ministry of Science and Technology, Brazil). Conflict of interest: Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company that has licensed the right to his invention of the dietary inflammatory index (DII®) from the University of South Carolina in order to develop computer and smartphone applications for patient counselling and dietary intervention in clinical settings. Dr. Nitin Shivappa is an employee of CHI. The subject matter of this paper will not have any direct bearing on that work, nor has that activity exerted any influence on this project. The authors have no other potential competing interest to disclose. Authorship: The conception and design of the study were performed by A.C.B.F., C.R.T. and B.W. The generation and data collection were performed by A.S., A.C.B.F., C.R.T., B.W., M.B.F., A.P.S.C., H.H.M.H. and J.B. The assembly and analysis and/or interpretation of the data were performed by A.S., M.B.F., A.P.S.C., H.H.M.H., N.S., J.R.H., A.C.B.F., C.R.T., B.W. and J.B. All authors read and approved the final manuscript. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Ethics Committee of the Hospital do Coração (parecer nº 1.171.748). Written informed consent was obtained from all subjects/patients.









