The primary role of vitamin D in the human body is to maintain extracellular Ca levels but recently it has been implicated to have a non-skeletal role including protection from infectious, inflammatory and neoplastic disease outcomes( Reference Holick 1 – Reference Holick 4 ). In man, vitamin D is primarily synthesized by the skin through exposure to sunlight (UV-B radiation, wavelength 290–315 nm) while only a small fraction (5–10 %) comes from the diet( Reference Holick 1 ). Recently a study estimated that 4 billion cases of bone disease (rickets, osteomalacia and osteoporosis) and 3·3 billion disability-adjusted life years are lost globally due to vitamin D deficiency that results from reduced UV exposure( Reference Lucas, McMichael and Armstrong 5 ).
The prevalence of vitamin D deficiency among children and adults varies significantly worldwide due to variation in sunlight exposure during the year and insufficient presence of corrective programmes( Reference Holick 1 , Reference Calvo, Whiting and Barton 6 – Reference van Schoor and Lips 9 ). Several small studies in the Indian subcontinent, including Bangladesh, have reported wide variation (as low as 2 % to 84 %) in the prevalence of vitamin D deficiency and insufficiency among pre-school children( Reference Agarwal, Mughal and Upadhyay 10 – Reference Wayse, Yousafzai and Mogale 17 ). Use of different serum cut-off points for vitamin D deficiency and insufficiency is also another important factor contributing to the wide range of variation in prevalence( Reference Arabi, El Rassi and El-Hajj Fuleihan 7 ). Several studies in rural Bangladesh have reported the vitamin D status of children under 5 years to 10 years of age although there is a high prevalence of rickets among the children( Reference Combs, Hassan and Dellagana 13 , Reference Fischer, Rahman and Cimma 18 ). Another study found a high prevalence of severe deficiency (<25 nmol/l) among children under 2 years old with pneumonia and matched healthy controls( Reference Roth, Shah and Black 14 ).
There have been few empirical studies aimed at identifying risk factors for vitamin D deficiency among pre-school children in the Indian subcontinent and Bangladesh( Reference Bhalala, Desai and Parekh 12 , Reference Roth, Shah and Black 14 – Reference Tiwari and Puliyel 16 , Reference Atiq, Suria and Nizami 19 , Reference Combs and Hassan 20 ). Studies conducted among other child populations have identified age, reduced intake of vitamin D-enriched foods, low sunshine exposure, skin covering, skin pigmentation, ethnicity, maternal vitamin D status, household crowding and air pollution as risk factors for vitamin D deficiency and insufficiency( Reference Bhalala, Desai and Parekh 12 , Reference Roth, Shah and Black 15 , Reference Tiwari and Puliyel 16 , Reference Andiran, Celik and Akca 21 – Reference Zhu, Zhan and Shao 28 ). A study from rural Bangladesh among children aged under 2 years found that vitamin D-deficient children were more likely to live in households of lower socio-economic status and were more stunted than vitamin D-sufficient children( Reference Roth, Shah and Black 14 ). However, a study in Pakistani infants found significantly lower vitamin D concentration among children from upper socio-economic strata and among infants of educated mothers( Reference Atiq, Suria and Nizami 19 ). Thus, risk indicators for vitamin D deficiency need to be explored more carefully among children under 2 years of age.
The important role of vitamin D in health and disease has led to increased interest in measuring vitamin D status among children under 2 years old. There is a lack of evidence about the prevalence of vitamin D deficiency in children under 2 years of age in Bangladesh. Most of the studies in Bangladesh reported prevalence of vitamin D deficiency from rural areas. We were unable to identify studies reporting vitamin D deficiency among urban Bangladeshi children or what risk factors are associated with deficiency and insufficiency. Additionally, most of the studies did not consider nutritional status, which may play role in the prevalence rate as well as risk factors for insufficiency and deficiency among children under 2 years old. In the present study we aimed to determine the prevalence of vitamin D insufficiency and deficiency among 6–24-month-old underweight and normal-weight urban slum children as well as examine the socio-economic and dietary risk indicators for vitamin D deficiency and insufficiency among these children.
Methods
Study design, setting and participants
We used data from the Bangladesh component of the Malnutrition & Enteric Diseases (MAL-ED) consortium( 29 ), which is a multisite research project concerned with malnutrition and diarrhoeal diseases in early childhood. One of the components of the MAL-ED study was an intervention study carried out at the urban Mirpur field site in Dhaka. The coordinates of Mirpur are 23·8042°N, 90·3667°E. Children aged 6–24 months with severe to moderate underweight (weight-for-age Z-score <−2·00) were selected as cases for enrolment in the study through biannual household demographic surveillance of the community. Controls were well-nourished, normal-weight children (weight-for-age Z-score ≥−1·00) matched for area of residence only. Details of the study design and site have been reported elsewhere( Reference Ahmed, Mahfuz and Islam 30 ). Five hundred cases and 480 controls were enrolled during the period of November 2009 to February 2012. Children were assigned to receive one of two different intervention packages according to their nutritional status (underweight or normal weight) for 5 months while enrolled in the study. To achieve our proposed objectives, only the baseline data from the cross-sectional study design were analysed. Thus intervention packages and follow-up procedures are not described herein.
Data collection
Trained field workers collected household socio-economic information and qualitative dietary intake information from mothers through a structured questionnaire at the time of enrolment. Demographic and Health Survey (DHS) questionnaires on household socio-economic and demographic status were adapted for data collection( 31 ). Similarly, dietary intake data were also collected using an FFQ based upon the DHS questionnaires which had previously been adapted (local names for common examples of the food items) and field tested by our team prior to data collection( 31 ). Field staff received standard training for data and sample collection before the implementation of the study at the field site. Trained field workers measured children’s weight using a digital baby or toddler scale (Seca 354) at the time of enrolment and on a monthly basis throughout the enrolment period. Biannual refresher training courses were also conducted for quality assurance of data collection.
Laboratory procedure
A 5 ml sample of venous blood was collected from each child at the time of enrolment. Samples were collected in trace-element-free containers for micronutrient assay. All assays for micronutrients, including vitamin D, were performed at the nutritional biochemistry laboratory of the International Centre for Diarrheal Disease Research, Bangladesh (icddr,b). Serum vitamin D was measured using the IDS 25-Hydroxy Vitamin D EIA (enzyme immunoassay) Kit( Reference Wallace, Gibson and de la Hunty 32 ) (IDS Ltd, Boldon, UK). Two levels of controls (REF AC-5705A, AC-5705B) were included in each kit. These two controls were run in each plate/run for monitoring accuracy and precision. The CV was 3·8–11·8 % for control 1 and 5·2–10·7 % for control 2. Serum retinol was measured using the HPLC method described elsewhere( Reference Wahed, Alvarez and Khaled 33 ). Serum/plasma Zn concentration was determined using an air–acetylene flame atomic absorption spectrophotometer at 213·9 nm following dilution of the sample twelve times with deionized water. Accuracy and precision of analyses were ensured by using a bi-level serum trace element control provided by UTAK Laboratories Inc. (Valencia, CA, USA).
Sample size and measurements
The study profile is described in Fig. 1. Complete data of 468 underweight and 445 normal-weight children were available for the final analysis. The primary outcome of the study is the prevalence of vitamin D insufficiency and deficiency among 6–24-month-old children. Serum vitamin D was recoded using standard serum cut-off points: severe deficiency (<25 nmol/l), deficiency (25–49·99 nmol/l), insufficiency (50–74·99 nmol/l) and sufficiency (≥75 nmol/l). The available exposure variables comprised personal characteristics, measures of socio-economic status, environmental factors, seasonality, and vitamin A and Zn status. A household asset index was constructed from the household asset information with principal component analysis as described for the DHS( Reference Tomkins 34 ). The UV index is usually high in Dhaka during April to September and lowest from November to January( 35 ). As UV-B radiation is essential for synthesis of vitamin D in human skin, four seasons, i.e. summer (May to July), autumn (August to October), winter (November to January) and spring (February to April), were created from the date of blood sample collection to measure the seasonal variation. Child’s age (6–11, 12–17 and 18–24 months), maternal education (illiterate, 1–5 years and ≥6 years of institutional education) and other relevant variables were created with recoding of information in the database. Vitamin A deficiency was defined as serum retinol level of <0·7 µmol/l( 36 ) and serum Zn deficiency was defined as serum Zn level of <9·9 µmol/l in pre-school children( Reference Brown, Rivera and Bhutta 37 ).
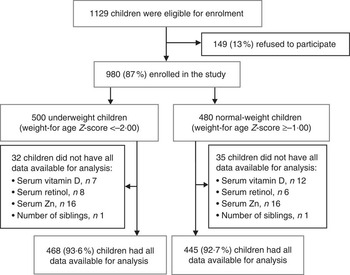
Fig. 1 Study profile
Statistical analysis
Socio-economic and demographic variables, qualitative dietary intake, and vitamin A and Zn status were compared among individuals with vitamin D sufficiency, insufficiency and deficiency using ANOVA for continuous variables and the χ 2 test of independence for categorical variables. For multivariable analyses, we combined both severe deficiency and deficiency into one category of deficient status (<50 nmol/l) to increase the statistical precision. A probability of less than 0·05 was considered a statistically significant association. Strength of association was measured by the estimating odds ratio and 95 % confidence interval. Multinomial logistic regression was used to estimate the odds of being vitamin D deficient or insufficient with the reference being vitamin D sufficient. Analyses were then done separately for underweight and normal-weight children. The variables that were statistically significant in the univariate analysis or deemed physiologically important factors or reported risk indicators in published literature were subsequently included in multivariable models to determine their independent association with the outcome variable. Analyses were carried out using the statistical software package STATA version 12·0.
Ethical considerations
The study (proposal # 2008–020) was approved by the Research Review Committee and the Ethical Review Committee of icddr,b. Informed voluntary written consent was obtained from the parents or guardian for the participation of their child in the study. Parents or caregivers were assured about the non-disclosure of information collected from them, and were also informed about the use of data for analysis and use of results for improving health and nutritional care activities as well as publication, without disclosing the name or identity of their children.
Results
Vitamin D status
Vitamin D status of underweight and normal-weight children is presented in Table 1. The median (interquartile range) vitamin D concentration was 57·5 (45·7–73·6) nmol/l among underweight children and 51·8 (39·9–65·9) nmol/l among normal-weight children. Only 23·1 % of underweight children and 14·8 % of normal-weight children were vitamin D sufficient.
Table 1 Serum vitamin D status among 6–24-month-old underweight and normal-weight children living in an urban slum of Dhaka, Bangladesh, November 2009–February 2012

WAZ, weight-for-age Z-score; IQR, interquartile range.
Factors associated with vitamin D status in underweight children
In the unadjusted analysis, compared with being serum vitamin D sufficient, the odds of being serum vitamin D deficient were significantly increased among underweight children aged 12–17 and 18–24 months compared with children 6–11 months of age (Table 2). Similarly, underweight children were at a significantly higher risk of vitamin D deficiency during winter and spring compared with summer. Underweight children who did not consume any animal protein in last 24 h had 40 % lower risk of vitamin D deficiency than children who consumed animal protein. There was a 50 % lower risk of vitamin D deficiency among the underweight children who were serum Zn sufficient than in those who were serum Zn deficient.
Table 2 Characteristics and factors associated with vitamin D deficiency and insufficiency among 6–24-month-old underweight children living in an urban slum of Dhaka, Bangladesh, November 2009–February 2012; multinomial logistic regression analysis with sufficient serum vitamin D status as reference (n 468)
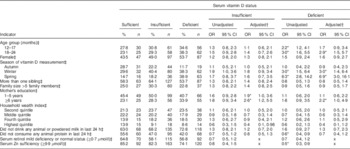
*P<0·05.
† Adjusted for child age group, child sex, season of vitamin D measurement, number of siblings, family size, mother’s education, household wealth index, consumption of any diary product and animal protein.
‡ Reference values for independent variables: 6–11 months; male; summer; number of siblings ≤1, illiterate mother; family size ≤5 members, lowest asset quintile; consumption of animal or powered milk; consumption of any animal protein; serum retinol moderate to severe deficiency; and serum Zn deficiency.
In the adjusted model, compared with being serum vitamin D sufficient, children who were ≥18 months of age were found to have a significantly greater risk of being vitamin D deficient than the younger group (6–11 months). Similarly, children had 3·0 times greater risk of vitamin D deficiency in winter than in summer. Likewise, in spring the risk increased up to 6·9 times compared with summer (Table 2). The probability of being vitamin D sufficient or insufficient among the underweight children was lowest during the spring time. On the other hand, the probability of being vitamin D deficient was highest among the underweight children during winter and spring (Fig. 2). Children whose mothers had ≥6 years of institutional education were found to be at 2·2 times greater risk of vitamin D deficiency and 2·6 times greater risk of vitamin D insufficiency than the children of illiterate mothers. Children whose mothers had 1–5 years of institutional education were found to be at 1·9 times greater risk of vitamin D insufficiency v. vitamin D sufficiency than the children of illiterate mothers after adjustment for other variables (Table 2).

Fig. 2 Adjusted predictions of vitamin D sufficiency, insufficiency and deficiency by season, with 95 % CI indicated by vertical bars, among underweight children living in an urban slum of Dhaka, Bangladesh, November 2009–February 2012
Factors associated with vitamin D status in normal-weight children
Compared with being serum vitamin D sufficient, the risk of being serum vitamin D insufficient was greater among normal-weight children aged 18–24 months than among 6–11-month-old children in the unadjusted analysis (Table 3). However, there was no association of season with vitamin D insufficiency or deficiency found in the unadjusted analyses. Normal-weight children whose mothers had ≥6 years of institutional education were found to have approximately threefold greater risk of vitamin D deficiency compared with illiterate mothers’ children. A similar result was found with children who were from the highest quintile of the household wealth index compared with those from the lowest quintile. There was respectively 2·4 and 2·2 times greater risk of vitamin D insufficiency and deficiency, compared with vitamin D sufficiency, among normal-weight children with serum Zn sufficiency than among normal-weight children with serum Zn insufficiency (Table 3).
Table 3 Characteristics and factors associated with vitamin D deficiency and insufficiency among 6–24-month-old normal-weight children living in an urban slum of Dhaka, Bangladesh, November 2009–February 2012; multinomial logistic regression analysis with sufficient serum vitamin D status as reference (n 445)
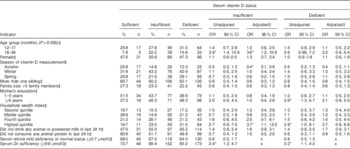
*P<0·05.
† Adjusted for child age group, child sex, season of vitamin D measurement, number of siblings, family size, mother’s education, household wealth index, consumption of any diary product and animal protein.
‡ Reference values for independent variables: 6–11 months; male; summer; number of siblings ≤1, illiterate mother; family size ≤5 members, lowest asset quintile; consumption of animal or powered milk; consumption of any animal protein; serum retinol moderate to severe deficiency; and serum Zn deficiency.
In the adjusted model, compared with being serum vitamin D sufficient, children aged 18–24 months were found to be 3·6 times more vitamin D insufficient than the younger age group (6–11 months old). Autumn was found to be associated with a significantly lower risk of vitamin D insufficiency among children than the summer months. Winter and spring were not associated with either deficiency or insufficiency of serum vitamin D status among the normal-weight children (Table 3). Among the normal-weight children the probabilities of vitamin D deficiency and insufficiency were high (40–50 %) even during the summer (Fig. 3). Maternal education was not associated with vitamin D deficiency or insufficiency after adjusting for other variables. On the other hand, normal-weight children from the richest quintile were 3·7 times more likely to be vitamin D insufficient v. vitamin D sufficient than those in the lower quintile of the household wealth index (Table 3).
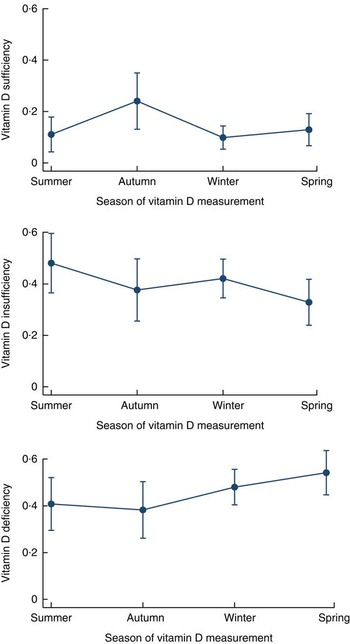
Fig. 3 Adjusted predictions of vitamin D sufficiency, insufficiency and deficiency by season, with 95 % CI indicated by vertical bars, among normal-weight children living in an urban slum of Dhaka, Bangladesh, November 2009–February 2012
A detailed description of the consumption of vitamin D-rich foods in the last 24 h by vitamin D status among underweight and normal-weight children is presented in Table 4.
Table 4 Consumption of vitamin D-rich foods in the last 24 h by vitamin D status among 6–24-month-old underweight and normal-weight children living in an urban slum of Dhaka, Bangladesh, November 2009–February 2012

† Number of underweight children: sufficient, n 108; insufficient, n 198; deficient, n 162.
‡ Number of normal-weight children: sufficient, n 66; insufficient, n 176; deficient, n 203.
§ Number of underweight children: sufficient, n 106; insufficient, n 196, deficient, n 157.
|| Number of normal-weight children: sufficient, n 65; insufficient, n 167; deficient, n 200.
Discussion
We have found remarkable prevalence of vitamin D insufficiency and deficiency among 6–24-month-old underweight as well as normal-weight children from an urban slum, indicating that vitamin D insufficiency and deficiency are important health problems especially among young children living in Bangladesh. Importantly, the factors associated with vitamin D insufficiency and deficiency were different between normal-weight children and underweight children.
One of the important drawbacks of available studies reporting prevalence of vitamin D status is the use of different cut-off levels by researchers. Most clinicians define vitamin D deficiency using a cut-off point of serum vitamin D <25 nmol/l, which is the cut-off point associated with the occurrence of rickets and osteomalacia( Reference Lips 38 , Reference Need, O’Loughlin and Morris 39 ). One study has proposed a cut-off point of >50 nmol/l for optimal bone mineral density, bone turnover and muscle strength, while a cut-off point of >75 nmol/l for maintaining an adequate immune response has been proposed by another( Reference Bischoff-Ferrari, Giovannucci and Willett 40 , Reference Kuchuk, Pluijm and van Schoor 41 ). The difference in these recommendations may reflect the different actions of vitamin D in physiological processes. Regardless of the different cut-off levels used for reporting status of serum vitamin D, all studies( Reference Combs, Hassan and Dellagana 13 – Reference Roth, Shah and Black 15 , Reference Fischer, Rahman and Cimma 18 ) including our study showed a significant burden of vitamin D deficiency and insufficiency among children under 2 years old in Bangladesh.
The tropical geographical location of Bangladesh means that vitamin D synthesis is possible year-round due to the intense UV-B radiation in the country compared with other zones of the globe( 35 ). Our study shows that despite geographical setting, vitamin D insufficiency and deficiency is quite prevalent in Bangladeshi children 6–24 months of age. Our study demonstrates that this is a significant issue in normal-weight children since it has reported a high prevalence of vitamin D insufficiency and deficiency among 6–24-month-old normal-weight children. Studies carried out in Chakaria, Coxesbazar found that 11 % of children had active rickets and that vitamin D deficiency ranged from 6 to 21 % among the children under 5 years old in that community( Reference Fischer, Rahman and Cimma 18 , Reference Combs and Hassan 20 ). Additionally a study from same area reported that only 6 % of children (ten out of 158 participants) were suffering from severe vitamin D deficiency( Reference Combs, Hassan and Dellagana 13 ), which supports our finding (Table 1). Recently a case–control study( Reference Roth, Shah and Black 15 ) was carried out among 1–24-month-old children of north-eastern rural Bangladesh and found that 32 % of all children (including cases and controls) were severely vitamin D deficient (<25 nmol/l), with 70 % having serum vitamin D less than 40 nmol/l. However, that study was conducted during the winter season (January–February) when children had a significant risk of severe deficiency of vitamin D. Unreported results of our study also correlate with the finding of high prevalence of severe deficiency during winter and spring seasons.
Several studies from India also have demonstrated low serum vitamin D levels among pre-school children. A longitudinal study conducted among pregnant women found that 36 and 62 % of neonates were vitamin D deficient or insufficient, respectively( Reference Bhalala, Desai and Parekh 12 ). Another study in impoverished areas of Delhi, India found that the prevalence of low serum vitamin D status among children ranged from 2 to 84 % but could not explain this wide variation in prevalence( Reference Tiwari and Puliyel 16 ). In Karachi, Pakistan, severe vitamin D deficiency was found among 52 % of healthy breast-fed infants( Reference Atiq, Suria and Nizami 11 ). Thus, our findings from urban Bangladesh in combination with earlier findings from infants in urban Pakistan and India demonstrate that there is remarkable vitamin D deficiency and insufficiency among young infants in South Asia and that a tropical climate with year-round adequate sunshine does not necessarily protect against low vitamin D status in the first 2 years of life. In regions such as Bangladesh, vitamin D levels can be low due to skin pigmentation( Reference Hintzpeter, Scheidt-Nave and Muller 25 , Reference Clemens, Adams and Henderson 42 ), air pollution (by preventing the penetration of UV-B radiation)( Reference Nair and Maseeh 43 ), clothing covering practices of children( Reference Matsuoka, Wortsman and Dannenberg 44 ), less physical and outdoor activities, maternal vitamin D deficiency( Reference Roth, Al Mahmud and Raqib 45 ), inadequate or very little intake of liver, eggs, dairy products, sea fish or fish oil (which are rich sources of vitamin D), and also the absence of any supplementary or fortification programme in this vulnerable population group.
Our study demonstrates that the vitamin D deficiency and insufficiency in young children increases with age, which is in agreement with previous studies( Reference Oren, Shapira and Agmon-Levin 8 , Reference Andiran, Celik and Akca 21 , Reference Mansbach, Ginde and Camargo 26 ). The high level of vitamin D in our youngest age group can partially be explained by the consumption of dairy products and almost universal rate of breast-feeding during the younger childhood period compared with the older childhood period. Our underweight children had significant risk of vitamin D deficiency during winter and spring, which was also observed in the studies conducted among the general child population elsewhere( Reference Arabi, El Rassi and El-Hajj Fuleihan 7 , Reference van Schoor and Lips 9 , Reference Agarwal, Mughal and Upadhyay 10 , Reference Bhalala, Desai and Parekh 12 , Reference Tiwari and Puliyel 16 , Reference Andiran, Celik and Akca 21 , Reference Andiran, Yordam and Ozon 22 , Reference Grant, Wall and Crengle 24 , Reference Kazemi, Sharifi and Jafari 46 ). However in our study, autumn was found to be protective for vitamin D insufficiency in normal-weight children. The higher probabilities (40–50 %) of normal-weight children being vitamin D insufficient and deficient in summer could influence the results in later periods – autumn, winter and spring (Fig. 3). Perhaps for this reason autumn was found to be protective and no significant differences were seen in vitamin D deficiency or insufficiency during winter and spring among normal-weight children.
In our study, underweight children whose mothers had ≥6 years of school instruction were significantly more vitamin D insufficient and deficient than children of illiterate mothers. Studies from Pakistan, Jordan and Saudi Arabia have reported similar findings in the general child population( Reference Atiq, Suria and Nizami 11 , Reference Gharaibeh and Stoecker 23 , Reference Sedrani, Abanmy and Slaman 47 ). Educated mothers may confine their infants indoors due to the polluted, congested and highly populated slum environment, which ultimately leads to reduced exposure to sunlight, and this could explain the high prevalence of vitamin D deficiency among them. Maternal education did not predict vitamin D deficiency in normal-weight children. In an unreported analysis of our study we found that mothers of normal-weight children are significantly more literate than underweight children’s mothers. Homogeneity in the higher educational status of the mothers of normal-weight children probably plays a role in this finding among normal-weight children.
In our study we did not find any association between dietary intake of milk products or animal protein and vitamin D deficiency or insufficiency. Breast-fed infants are often at a greater risk of developing deficiency, which might be due to low vitamin D status of women of childbearing age( Reference Roth, Al Mahmud and Raqib 45 , Reference Islam, Lamberg-Allardt and Karkkainen 48 ). However, we did not collect breast milk or blood samples from mothers for estimation of serum vitamin D status. Moreover, the breast-feeding rate was almost universal in our study participants; thus we are unable to explore the role of breast-feeding on serum vitamin D status.
Vitamin D and Zn both play important roles in human health and do not interact directly. Both play an important role in immune function. In our study we found a positive relationship between insufficient serum Zn status and deficient status of vitamin D in underweight children but an inverse relationship in normal-weight children in unadjusted analysis. A possible explanation for this finding is that underweight children were suffering from multiple micronutrient deficiencies( Reference Bhaskaram 49 , Reference Winichagoon 50 ), especially coexisting serum Zn and vitamin D deficiencies.
Limitations
The results of the present study need to be interpreted in light of its limitations. First, we used data from the MAL-ED community-based study with prospective case–control design in an urban setting; results do not represent the general child population as well as the rural population. Second, the main source of vitamin D in man is exposure of bare skin to sunlight. We did not collect any information about the frequency and duration of sunlight exposure, clothing practices of children, cultural beliefs and outdoor activities of the children, which could be weaknesses of the study. Third, supporting information related to vitamin D and Ca homeostasis, such as serum intact parathyroid hormone levels, alkaline phosphatase, bone markers or bone parameters, which could add more strength to the study, was not measured.
Conclusions
The present study provides important information about the significant burden of vitamin D insufficiency and deficiency in both underweight and normal-weight children and associated risk factors in urban Bangladesh. Our study demonstrates that the risk factors for vitamin D deficiency and insufficiency differ between normal-weight and underweight children, and highlight the need for interventions, including nutritional education regarding spending time outdoors in sunshine for 10–16 min at least three or four times weekly and supplementation, to be tailored to the specific needs of particular subgroups. Importantly, the burden and risk factors of vitamin D deficiency in both underweight and normal-weight 6–24-month-old children identified in the study warrant the design and implementation of a vitamin D-specific health and nutritional programme in Bangladesh.
Acknowledgements
Financial support: The Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) is carried out as a collaborative project supported by the Bill & Melinda Gates Foundation, the Foundation for the National Institutes of Health, and the National Institutes of Health, Fogarty International Center. The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: A.M.S.A., T.A., K.Z.L., R.J.S.M. and A.A.M. conceived and designed the study; A.M.S.A., T.A., M.M., M.M.I., M.I.H., S.M.A.G., A.S., R.H., R.L.G. and W.A.P. oversaw data collection; A.M.S.A., K.Z.L., R.J.S.M. and A.A.M. analysed the data; A.M.S.A., T.A., K.Z.L., R.J.S.M., M.M., M.M.I., M.I.H., S.M.A.G., A.S., R.H., R.L.G., W.A.P. and A.A.M. interpreted the data; A.M.S.A. drafted the manuscript; A.M.S.A., T.A., K.Z.L., R.J.S.M. and A.A.M. contributed to redrafting the manuscript. Ethics of human subject participation: The present analysis of data of children aged less than 2 years was part of a Bangladesh, MAL-ED Network study that aimed to understand burden of vitamin D deficiency and its predictors. The study (proposal # 2008–020) was approved by the Research Review Committee and the Ethical Review Committee of icddr,b in 2008. The caregiver of eligible children provided informed written consent before collection of data and all biological samples at the time of enrolment. The written consent was documented by keeping a check mark in the questionnaire which was again shown to the parents. Parents or caregivers were assured about the non-disclosure of information collected from them, and were informed about the use of data for analysis and use of results for improving health and nutritional care activities as well as publication, without disclosing the name or identity of their children. The Ethical Review Committee of icddr,b was satisfied with the written participation, maintenance of all rights to participate in the study or to withdraw the child from the study at any time without depriving from usual services.










